Abstract
The number of breast cancer incidences reported worldwide has increased tremendously over the years. Scoping down to Asia, in 2020, the reported incidences of breast cancer are appalling, comprising 1,026,171 cases, occupying up to 45.4% of cases across the globe. Breast cancer is a non-communicable disease, that emerges in variegated forms, self-subsistent, and the etiology is observed to be multifactorial, dependent on the individual reproductive pattern, hormonal factors, diet, physical activity, lifestyle, and exposure to certain advent procedures. Given this complexity, breast cancer is expected to undergo a persistent increment in the number of incidences in near future, exacerbating the public health quality, regardless of race, ethnicity, geographical subgroups, and socioeconomic. In this review article, the authors examine breast cancer in multiple facets, comprising the updated statistics on breast cancer, typically in Asia; etiology of breast cancer; diagnosis of breast cancer; grading system; and challenges in breast cancer from the country’s income perspective. Realizing the ever-increasing demand for quality treatment, here, the article also contemplates common therapies in breast cancer, such as breast-conserving therapy, mastectomy, postmastectomy radiation therapy, neoadjuvant chemotherapy, axillary surgery, chemotherapy, adjuvant medical therapies, biological and targeted therapies, and endocrine therapy. This review article intended to provide a brief yet broad panoramic view of breast cancer, to readers, ranging from newcomers, existing researchers, and relevant stakeholders in the topic of interest.
graphical abstract

Introduction
Asia is the largest continent that comprises 60.0% of the human population in the world, consisting of five regions (i.e., Western Asia, Southern Asia, South-Eastern Asia, Eastern Asia, and Central Asia), and 48 countries with huge diversity in cultures and ethnicities. Most of these countries are low-to middle-income countries with different healthcare systems and policies. Overall, the estimated number of new cases of breast cancer in 2020, for all ages, Asia constitutes 1,026,171 new cases which are 45.4% across the globe, contrive of the world’s largest number of new cases, followed by Europe (531,086 cases, 23.5%), Northern America (281,591 cases, 12.5%), and others (422,571 cases, 18.7%). The number of new cases of breast cancer in Asia was found to increasing rapidly in the last decade due to urbanization, rapid growth in the economy (as most of the countries are from low-to middle-income countries), and a steady increase in socioeconomic status in recent years.
In light of the aggravation of breast cancer cases, this article aims to provide an updated narrative review on six main facets concerning breast cancer, focusing on Asia: (1) statistics of breast cancer in Asia; (2) etiology of breast cancer; (3) diagnosis of breast cancer; (4) grading system; (5) therapies; and (6) challenges in breast cancer from the country income perspective. Over the years, an increasing number of breast cancer cases has captured the attention of numerous researchers in surveying and reviewing literature in a specific domain. Nonetheless, a brief yet broad review alongside the updated statistic on breast cancer focusing on Asia is limited. In this article, the main purpose is to provide a panoramic overview to readers, ranging from newcomers to existing researchers and relevant stakeholders, on the topic of interest. The narrative review serves different purposes for different audiences. For those who are new to the field, it offers a comprehensive and up-to-date overview of breast cancer, providing a broad understanding of the topic. Experienced researchers can use the review as a tool to stay abreast of the latest developments and keep their knowledge current. Meanwhile, relevant stakeholders can leverage the narrative review to identify priority areas for research and project funding, directing resources toward solutions that can have an immediate and meaningful impact.
Breast cancer in Asia
Breast cancer is a disease in which cells in the breast start to grow out of control. The mutated cells usually start in lobules or ducts forming a lump and often can be seen via mammogram. Deterioration of a benign tumour could result in metastasizing in which the tumour cells invade surrounding tissues (e.g., blood and lymph vessels).
Breast cancer is the most prevalent carcinoma in women where the estimated number of new cases is more than twice the estimated number of other cancers. In 2020, 2,261,419 new cases were reported worldwide [1]. Breast cancer is currently the most common cancer in women, contributing 24.5% of the total number of new cases diagnosed in 2020 across the globe [1].
Developing countries in Asia were found to have a notable increase in the number of incidences and mortality [1], [2], [3]. In 2020, 1,026,171 new cases of breast cancer were reported in Asia, contributing 22.9% of the total number of new cases diagnosed [1]. 346,009 (33.7%) deaths were recorded from the reported cases in the same year [1]. The Global Cancer Incidence, Mortality, and Prevalence (Globocan) have characterized breast cancer as a Rank one cancer (i.e., top priority) [1, 3]. The number of cases in 5-year prevalence (all ages) of breast cancer was 3,218,496 cases and this number is estimated to undergo persistent deterioration in the near future. The estimation is based on the number of reported cases in Asia for the past five consecutive years (i.e., 2016–2020).
The incidence age-standardized rates (ASR) of breast cancer in Asia are of huge variation, from the lowest 5.0/100,000 (i.e., Bhutan) to the highest 82.9/100,000 (i.e., Cyprus). The incidence ASRs for the world and Asia are 47.8/100,000 and 36.8/100,000, respectively. Table 1 shows the incidence ASR and mortality ASR for respective countries in Asia categorized based on regions. For each region, the country with the highest incidence of ASR is highlighted.
Incidence ASR and mortality ASR of breast cancer in 2020 [1].
| Incidence ASRa | Mortality ASRa | |
|---|---|---|
| World | 47.8 | 13.6 |
| Asia | 36.8 | 11.9 |
|
|
||
| Western Asia | ||
|
|
||
| Cyprus | 82.0 | 17.8 |
| Bahrain | 44.2 | 13.6 |
| Qatar | 42.7 | 13.2 |
| Armenia | 49.6 | 19.2 |
| Georgia | 57.5 | 3.5 |
| Kuwait | 50.3 | 17.0 |
| State of Palestine | – | – |
| Oman | 38.5 | 14.5 |
| Lebanon | 54.8 | 19.9 |
| Israel | 78.3 | 16.7 |
| United Arab Emirates | 58.5 | 16.6 |
| Azerbaijan | 34.7 | 13.6 |
| Jordan | 59.5 | 19.7 |
| Syria | 57.1 | 26.2 |
| Yemen | – | – |
| Saudi Arabia | 28.8 | 8.9 |
| Iraq | 54.5 | 23.3 |
| Turkey | 46.6 | 12.9 |
|
|
||
| Southern Asia | ||
|
|
||
| Maldives | 43.5 | 14.9 |
| Bhutan | 5.0 | 2.6 |
| Sri Lanka | 27.3 | 11.0 |
| Nepal | 13.9 | 7.6 |
| Afghanistan | 28.9 | 17.9 |
| Iran | 35.8 | 10.8 |
| Bangladesh | 17.0 | 9.3 |
| Pakistan | 34.4 | 18.8 |
| India | 25.8 | 13.3 |
|
|
||
| South-Eastern Asia | ||
|
|
||
| Indonesia | 44.0 | 15.3 |
| Philippines | 52.7 | 19.3 |
| Vietnam | 34.2 | 13.8 |
| Thailand | 37.8 | 12.7 |
| Myanmar | 22.0 | 9.6 |
| Malaysia | 49.3 | 20.7 |
| Cambodia | 23.5 | 10.3 |
| Laos | 36.7 | 15.8 |
| Singapore | 77.9 | 17.8 |
| Timor-Leste | 25.6 | 10.8 |
| Brunei | 55.9 | 12.5 |
|
|
||
| Eastern Asia | ||
|
|
||
| Mongolia | 11.1 | 3.9 |
| North Korea | 32.9 | 10.0 |
| South Korea | 64.2 | 6.4 |
| Japan | 76.3 | 9.9 |
| China | 39.1 | 10.0 |
|
|
||
| Central Asia | ||
|
|
||
| Turkmenistan | 32.9 | 15.1 |
| Kyrgyzstan | 24.3 | 8.1 |
| Tajikistan | 19.5 | 8.0 |
| Uzbekistan | 26.4 | 12.8 |
-
aAge-standardized rates per 100,000, females, all ages. Highest values of incidence ASR and mortality ASR in each region in Asia.
Based on statistics from the World Health Organization (WHO), it is found that countries with significant urbanization, rapid growth in the economy, and population socioeconomic status show a remarkable increase in new incidences [4], [5], [6], [7], [8]. The incidence of ASR in Asia in 2012 is 29.1/100,000. The incidence of ASR has increased by 26.5% (7.7) to 36.8/100,000 in 2020. On the contrary, developed country such as the United States of America shows a slight decrease in incidence ASR from 92.9/100,000 in 2012 to 90.3/100,000 in 2020 (decreased by 2.8% (2.6)). The total number of incidences and mortality in 2020 for all countries in Asia is depicted in Figure 1.
![Figure 1:
Number of incidences and mortality in Asia for the year 2020 [1], such that the y-axis shows the countries in Asia and the x-axis shows the number of incidences/mortalities.](/document/doi/10.1515/oncologie-2022-1011/asset/graphic/j_oncologie-2022-1011_fig_001.jpg)
Number of incidences and mortality in Asia for the year 2020 [1], such that the y-axis shows the countries in Asia and the x-axis shows the number of incidences/mortalities.
Etiology of breast cancer
The etiology of breast cancer starts in utero and continues throughout the development of one’s lifespan. The origin of breast cancer is likely to be multifactorial, dependent on the individual reproductive pattern, hormonal factors, diet, physical activity, lifestyle, and exposure to certain advent procedures [2, 9]. Based on the descriptive epidemiological data, breast cancer is a disease of an affluent society that acquires a high-calorie diet rich in animal protein and saturated fats, is associated with an inactive lifestyle [2, 9]. Developed countries such as Northern Europe, North America, and Australia that adapted to the lifestyle have attained a plateau in the number of incidences (i.e., 70.0 to 90.0 per 100,000 populations) [2, 9]. Developing and industrialized countries such as South Korea, India, and Malaysia show a notable increase in incidence and mortality.
The reproduction pattern has been reported as one of the main risk factors that contribute to breast cancer. Women having early menarche, nulliparous, giving birth at late age at first delivery, reduced number of pregnancies, shorter breastfeeding time, and late menopause were found to have an increased risk of breast cancer [10], [11], [12], [13].
Various studies have shown that the hormonal factor has a notable role in breast cancer [9, 14], [15], [16], [17], [18], [19], [20]. Endogenous and exogenous hormones that alter the level of androgen, estrogen, and progestogen hormones have an important role in the development of breast cancer. For endogenous hormones, women with a high concentration of estrogen and testosterone in post-menopause and a high concentration of prolactin in both pre-and post-menopause are associated with an increased risk of breast cancer [13, 18, 21]. In terms of the exogenous hormone, the users of oral contraceptives for at least 10 years were found to have a 24.0% increase in risk as compared to never-users in the development of breast cancer [22], [23], [24]. The International Agency for Research on Cancer (IARC) has characterized the estrogen-progesterone oral contraceptive as a class 1 carcinogen. Besides, the long duration and current users of post-menopausal hormone replacement therapy that involved the alteration of estrogen and/or estrogen-progesterone were found to have increased risk of breast cancer. Users of unopposed estrogen in post-menopausal therapy have been reported with a 10.0% increase in risk for a duration of use of 1–4 years, 30.0% increase in risk for 5–9 years, 20.0% increase in risk for 10–14 years, and 60.0% increase in risk for at least 15 years as compared to a never-users [9, 25]. Some studies found the use of estrogen-progesterone during post-menopausal therapy could increase the risk of breast cancer [26], [27], [28]. These findings are consistent with a study by the Women’s Health Initiative (WHI) and Samson K. [27]. The combination of estrogen–progesterone has shown no protective effect on breast cancer [2, 29].
Body adiposity (i.e., typically measured using Body Mass Index (BMI)) is another risk factor for breast cancer and is highly dependent on the menopausal status. Interestingly, the population with a higher BMI was found to have a lower risk in the development of pre-menopausal breast cancer but positively relates to post-menopausal breast cancer [2, 9]. The lower risk of pre-menopausal breast cancer in a high-body adiposity population is closely related to a reduced ovulatory cycle and exposure to hormones such as estrogen and progesterone. Studies found that increasing body adiposity promotes irregular menstrual cycles [2, 9, 30].
Physical activity is another important factor in the development of breast cancer [2, 9, 31, 32]. Population with a high level of physical activity from menarche through adulthood have been consistently associated with a reduced risk of breast cancer [33], [34], [35]. On the contrary, unhealthy lifestyles such as high dietary in animal protein, saturated animal fat, animal meat specifically in red or fried (i.e., browned) meat, consistent alcohol consumption, and passive smoker are associated with the development of breast cancer [36], [37], [38], [39], [40].
The emerging method of scanning the whole genome for genetic changes (GWAS) [41, 42] is found possible to convey an increased risk of breast cancer development [43, 44]. Demarcation and strategies are essential to offer prevention to patients who are subjected to this method.
Diagnosis of breast cancer
Patients with breast symptoms are highly recommended to have a full diagnostic test using triple assessment [45]. The triple assessment consists of clinical examination, mammography and/or ultrasonography, and pathologist examination on cytology and/or histology. A clinical examination of the breast is performed by a health professional or doctor to find lumps or abnormalities that may be missed by the patients during self-examination. Imaging procedure via mammography and ultrasonography is typically used for patients aged 40 years and above. For younger patients, mammography may not be helpful to detect mass as the breast tissue is very dense. Magnetic resonance imaging (MRI) is another imaging alternative but it is only used for high-risk patients. The imaging procedure is usually followed by the fine needle aspiration or core biopsy procedure. Fine needle aspiration collects sample cells using a fine needle whereas core biopsy collects lump tissue samples using a large-bore needle. Hematoxylin and Eosin (H&E) stain is performed on the tissue slide and a diagnosis is given depending on the morphology characteristic of the tissue sample. Histopathological confirmation is required before any definitive surgical procedure can be done [45]. In the case of breast cancer, a standard grading procedure known as Nottingham Histopathological Grading (NHG) system is used to grade cancer. The NHG system has a paramount role in diagnosis and prognosis, especially in treatment planning before any adjuvant therapy is done.
Grading system for breast cancer
A patient confined with breast cancer is required to perform a grading procedure. Breast cancer grading provides a strong prognosis value and is well recognized as one of the key components of clinical decision-making tools [2, 9, 46]. The breast cancer grading assessment was introduced by Patey and Scarff [47] and Bloom and Richardson [48]. The grading system was modified by Elston and Ellis [49] and became more semi-quantitative as compared to the previous methods. The modified grading system by Elston and Ellis is known as the NHG system [9]. Nowadays, the NHG system is accepted worldwide and is used as a gold standard in performing the grading procedure [9].
In the NHG system, the overall output grade is dependent on the total score obtained from three important breast features. The features are tubule formation, nucleus pleomorphism, and mitotic count. For each feature, score 1 for low, score 2 for moderate, and score 3 for high scores (Table 2).
The semi-quantitative histology grading system: NHG system [9].
| Feature | Remark | Score |
|---|---|---|
| Tubule formation | Majority of tumour (>75.0%) | 1 |
|
|
||
| Moderate degree (10.0–75.0%) | 2 | |
| Little or none (<10.0%) | 3 | |
|
|
||
| Nucleus pleomorphism | Small nuclei with little increase in size respect to normal breast epithelial cells, regular outlines, uniform nuclear chromatin and low degree of variation in size | 1 |
|
|
||
| Increase in cell size with open vesicular nuclei, visible nucleoli, and moderate variability in term of shape and size | 2 | |
| Vesicular nuclei, often with prominent nucleoli, present significant variation in shape and size, occasionally with very large and eccentric forms | 3 | |
|
|
||
| Mitotic counts | Depend on microscope field area | 1–3 (Table 3) |
|
|
||
| Final grading | Grade 1 (well-differentiated) | Total score: 3–5 |
| Grade 2 (moderately differentiated) | Total score: 6 or 7 | |
| Grade 3 (poorly differentiated) | Total score: 8 or 9 | |
The assessment of tubule formation is performed at low magnification, typically at 10× magnification on the entire histopathological slide. Two important criteria considered in the assessment of tubule formation are tumour region areas and tubule areas. Tumour regions or relevant regions provide prognosis value and meaningful information for the tubule assessment. Non-tumour regions or irrelevant regions refer to the areas that contain fat tissue, stroma tissue, epithelial cells, and the background. These areas are not important and are always ignored during the assessment. Figure 2 shows a sample of images with tumour, non-tumour regions, and background. The tubule is a structure that exhibits a clear central lumen surrounded by polarized neoplastic cells (Figure 3) [9]. Tubule formation can be measured using the following equation [9]:

Samples of tumor, non-tumor regions (irrelevant regions), and the background areas in breast histopathological images. In each image, tumor regions (i.e., dark purple regions), non-tumor regions (i.e., light purple/pinkish regions), and background areas (i.e., white regions) are shown by green, red and black arrows, respectively.
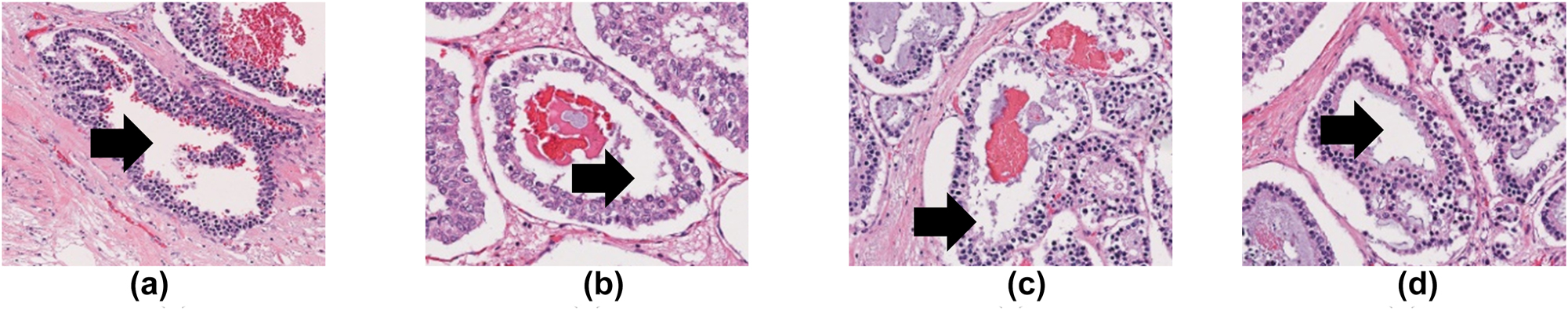
The central lumen of the tubule in different breast histopathological images are shown by the black arrows.
Nucleus pleomorphism focuses on the appearance, regularity, and size of the tumour nuclei with regard to normal cells (Figure 4). An increase in irregularity is positively related to a higher score in the NHG system (Table 2). When comparing normal and abnormal nuclei size, scores 1, 2, and 3 show an increase in size deviation: low (<1.5), moderate (1.5–2.0), and high (>2), respectively [9]. For instance, a bizarre nucleus in Figure 4d presents a score of 3.
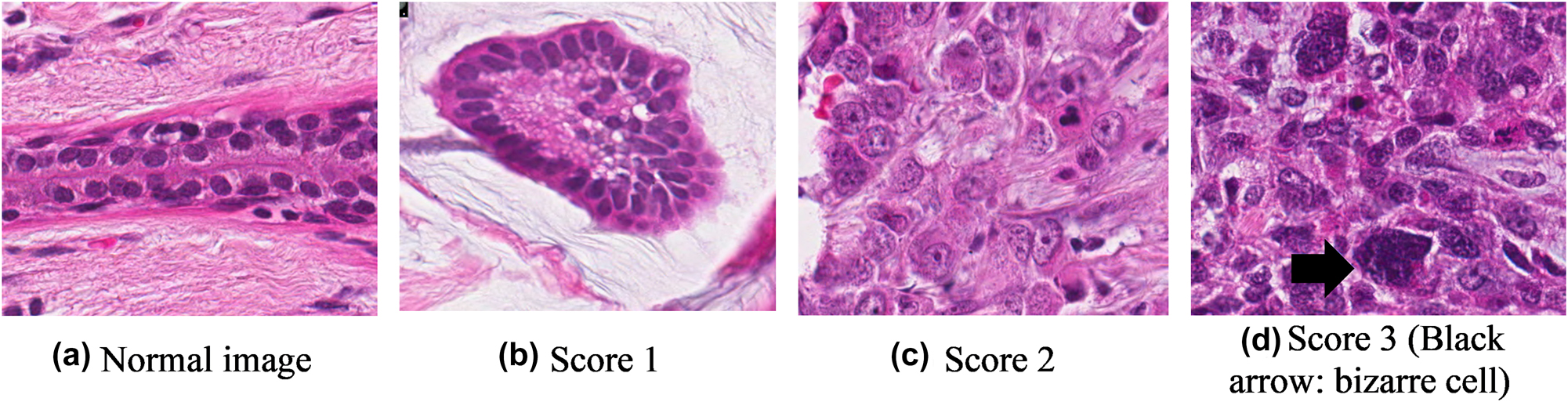
Nucleus pleomorphism at different scores.
Assessment of mitotic count is time-consuming, tedious, requires careful examination, and requires the experience [50], [51], [52]. The assessment highly relies on tissue fixation and slide preparation procedures. The observers must only include clear and definite mitotic cells (Figure 5). Karyorrhectic and apoptotic nuclei that shared a similar appearance with the mitotic cells are excluded during the manual inspection. To perform a mitotic count, the observers should first delimit the area with the highest mitotic activity. The total number of mitotic cells in the defined area expressed in mm2 is then recorded. The defined area is meant for standardization in the true area where the mitotic cells are enumerated. This guideline is released by WHO in 2019 [9] and is especially helpful for grading procedures using digital systems. The cut-off thresholds for mitotic counts on the field diameter of different high-power fields and their corresponding defined area (in mm2) are presented in Table 3.
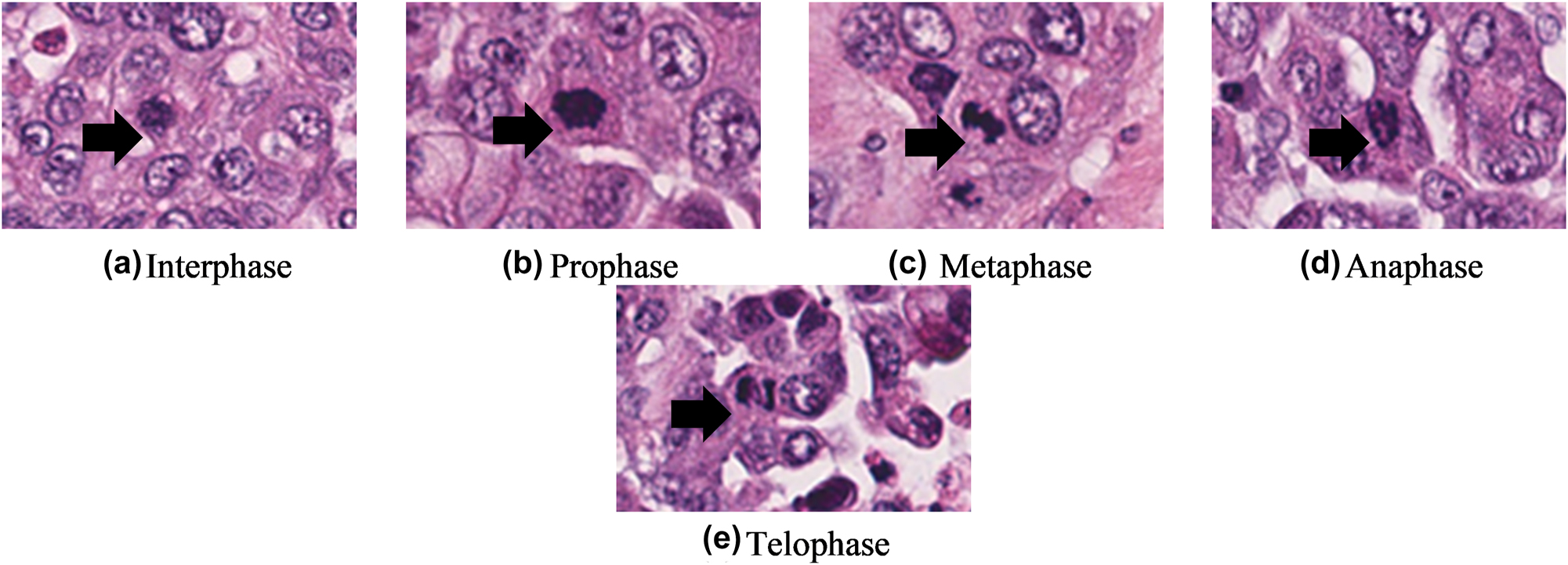
Different phases of mitotic cell are shown by the black arrows.
Score threshold for mitotic counts [9].
| Field diameter, mm | Field area, mm2 | Mitotic count (score) | ||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| 0.40 | 0.126 | ≤4 | 5–9 | ≥10 |
| 0.41 | 0.132 | ≤4 | 5–9 | ≥10 |
| 0.42 | 0.138 | ≤5 | 6–10 | ≥11 |
| 0.43 | 0.145 | ≤5 | 6–10 | ≥11 |
| 0.44 | 0.152 | ≤5 | 6–11 | ≥12 |
| 0.45 | 0.159 | ≤5 | 6–11 | ≥12 |
| 0.46 | 0.166 | ≤6 | 7–12 | ≥13 |
| 0.47 | 0.173 | ≤6 | 7–12 | ≥13 |
| 0.48 | 0.181 | ≤6 | 7–13 | ≥14 |
| 0.90 | 0.188 | ≤6 | 7–13 | ≥14 |
| 0.50 | 0.196 | ≤7 | 8–14 | ≥15 |
| 0.51 | 0.204 | ≤7 | 8–14 | ≥15 |
| 0.52 | 0.212 | ≤7 | 8–15 | ≥16 |
| 0.53 | 0.221 | ≤8 | 9–16 | ≥17 |
| 0.54 | 0.229 | ≤8 | 9–16 | ≥17 |
| 0.55 | 0.237 | ≤8 | 9–17 | ≥18 |
| 0.56 | 0.246 | ≤8 | 9–17 | ≥18 |
| 0.57 | 0.255 | ≤9 | 10–18 | ≥19 |
| 0.58 | 0.264 | ≤9 | 10–19 | ≥20 |
| 0.59 | 0.273 | ≤9 | 10–19 | ≥20 |
| 0.60 | 0.283 | ≤10 | 11–20 | ≥21 |
| 0.61 | 0.292 | ≤10 | 11–21 | ≥22 |
| 0.62 | 0.302 | ≤11 | 12–22 | ≥23 |
| 0.63 | 0.312 | ≤11 | 12–22 | ≥23 |
| 0.64 | 0.322 | ≤11 | 12–23 | ≥24 |
| 0.65 | 0.332 | ≤12 | 13–24 | ≥25 |
| 0.66 | 0.342 | ≤12 | 13–24 | ≥25 |
| 0.67 | 0.352 | ≤12 | 13–25 | ≥26 |
| 0.68 | 0.363 | ≤13 | 14–26 | ≥27 |
| 0.69 | 0.374 | ≤13 | 14–27 | ≥28 |
-
Adapted from: WHO Classification of Tumors Editorial Board. Breast tumors. Lyon (France): International Agency for Research on Cancer; 2019. (WHO classification of tumors series, 5th ed.; vol. 2). https://publications.iarc.fr/581.
The total score obtained from the three important breast features, ranging from 3 to 9 is used to determine the overall grade of breast cancer (Table 2). Grade 1 (total score: 3 to 5) denotes a well-differentiated tumour, Grade 2 (total score: 6 to 7) denotes a moderately differentiated tumour, and Grade 3 (total score: 8 to 9) denotes a poorly differentiated tumour. For reporting, the overall grade of breast cancer should be accompanied by the individual score from each feature to precisely describe the tumour condition.
Therapies in breast cancer
After a diagnosis is confirmed, a comprehensive evaluation of the disease is conducted to determine the necessity of preoperative (neoadjuvant) systemic therapy. Breast cancer treatment required concerted efforts from multi-subspecialties. To date, breast-conserving therapy, mastectomy, postmastectomy radiation therapy, neoadjuvant chemotherapy, axillary surgery, chemotherapy, adjuvant medical therapies, biological and targeted therapies, and endocrine therapy are the therapy options depending on the grade of the diagnosed breast cancer. The main focus of this section is meant to highlight the currently available therapies for breast cancer. It is important to note that the recommendation of specific therapy across different grading is not included herein.
Breast-conserving therapy included an excision procedure followed by adjuvant whole breast irradiation [53]. To opt for this therapy, the tumor must be able to excise to a negative margin with a definite cosmetic outcome decided by the medical experts. A negative margin is referred to the edge of a tissue where no cancer cells are found [54]. Ultrasound, mammography, and physical examination are the common modalities used in the imaging stage to validate patients for breast-conserving therapy.
Mastectomy comprises total mastectomy, nipple-areola-complex sparing mastectomy, and skin-sparing mastectomy [55]. Total mastectomy involves the removal of the breast parenchyma, nipple-areolar complex, and excess skin from the chest wall while preserving a sufficient amount of skin for wound closure [55]. Nipple-areola-complex sparing mastectomy is a surgical technique that enables complete glandular dissection while conserving the skin envelope and nipple-areola complex [56], whereas, skin-sparing mastectomy is commonly recommended for patients with immediate breast reconstruction [56]. This procedure removes the breast parenchyma and nipple-areolar complex. The skin remains a natural envelope for the subsequent implant procedure.
Postmastectomy radiation therapy is often recommended to patients with high-risk pathologic features and/or with locally advanced breast cancer after a mastectomy [57]. Postmastectomy radiation therapy after a breast reconstruction procedure is found associated with an increased rate of reconstructive failure [58]. The type of implant and timing of breast reconstruction are vital considerations before the reconstruction surgery.
Neoadjuvant chemotherapy aims to convert patients with locally advanced or inoperable breast cancer into operable disease [59]. Neoadjuvant chemotherapy has the advantages of surgical downstaging purposes, preventing complete axillary dissections in patients with good response toward the mentioned therapy, and promoting the succession rate in breast conservation. Neoadjuvant chemotherapy is now considered by the National Comprehensive Cancer Network and St. Gallen International Expert Consensus Panel as the preferred therapy as well as the new protocol of care for triple-negative breast cancer (TNBC) or Her2 positive (Her2+) breast cancer patients [60].
Axillary surgery is well recognized as one of the core tenets for staging and treatment of breast cancer as the axillary lymph nodes have been observed as the systematic route for breast cancer to metastasis. Axillary surgery serves two primary purposes: firstly, it offers anatomic staging information that is crucial for predicting prognosis and guiding decisions on adjuvant therapy; secondly, it may be utilized as a treatment option for managing axillary disease [61]. Patients with newly diagnosed invasive breast cancer alongside clinically negative axilla are recommended to perform axillary staging for prognostic and treatment planning purposes.
Chemotherapy is observed as the primary option for high-risk metastatic breast cancer patients. In brief, there are several standard options in chemotherapy that commonly contain anthracycline and taxane [62, 63]. Initial therapy for TNBC patients usually involves single-agent chemotherapy, although combination chemotherapy may be recommended in cases of rapidly progressing visceral disease.
Systemic adjuvant medical therapies for operable breast cancer are capable to eradicate the clinical micrometastatic disease that may progress into metastatic disease if remained untreated. Given this succession, adjuvant medical therapies have become the standard treatment for breast cancer [64]. Adjuvant medical therapy is selected based on the recurrence risk, such that low-risk patients receive de-escalated treatment while high-risk patients are given aggressive systemic treatment with an appropriate dose and duration.
With the breakthroughs in immunotherapy and molecular biology, specific biological and targeted therapies can be tailored to specifically target the pathophysiology of different breast cancer, aiming to inhibit the function of specific molecules that are responsible for cancer proliferation [65]. For example, a combination of chemotherapy with Her2 targeted therapy is given to patients diagnosed with Her2+ breast cancer, which was later found to have a significant impact on the prognostic value for patients with Her2+ breast cancer [63].
The introduction of endocrine therapies such as tamoxifen has brought about a revolutionary change in breast cancer treatment, leading to substantial reductions in cancer-related deaths [66]. Aromatase inhibitors like anastrozole and letrozole have added to this progress, enhancing the chances of survival for breast cancer patients [67, 68]. As a result of the widespread use of these therapies and the resulting decrease in mortality rates, breast cancer patients are now experiencing longer lifespans than ever before.
Over the past decades, with rousing advancements in technology and knowledge across a wide spectrum in medically relevant domains, therapies available for breast cancer are expected to continue to gain momentum towards betterment in both prognostic and diagnostic values. Advancement in imaging modalities could greatly improve diagnostic accuracy, improving precision in medical diagnostics [2]. Challenges such as drug resistance could be addressed by drug repurposing [67, 69]; effective cancer inhibitors (e.g., anti-breast cancer compounds) could be derived from marine composites [70]; and innovative in multimodal breast cancer management could be the solution for better surgical outcome [71]. Concerted efforts from multidisciplinary are expected to improve the therapies for breast cancer.
Challenges in breast cancer
The healthcare resources required for breast cancer can be costly. Therefore, the treatment protocol available to the patients can be varied from the country, typically based on socioeconomic development [4], [5], [6], [7]. The countries in Asia can be characterized into three different groups, namely high-resource, moderate-resource, and low-resource.
Countries with low resources in the healthcare system (e.g., Nepal, Pakistan, and Iraq) are found to have low prognostic value with extremely poor cancer diagnostic [72, 73] and treatment resources [4, 74]. In general, the main challenges in low-resource countries are the community having low awareness of breast cancer, inadequate/poor health care system, and shortage of essential diagnostic and treatment facilities. Low-resources countries such as Nepal have a very limited number of radiation equipment (e.g., mammogram) with a fragmented health care system. More than 80.0% of cancer patients in Nepal were found at the metastatic stage where palliation care became the only goal of treatment [73].
Countries with moderate-resource such as Malaysia, Turkey, and Iran have fragmented care systems, typically in prioritization of breast cancer, poor data registry, inadequate multidisciplinary coordination, and lack of standardization in health care management [75]. Costly medical procedures and drugs which are not covered by insurance are other challenges in moderate-resource countries. Breast cancer patients in the metastatic stage may not have many options and can only opt for affordable solutions (e.g., 40.0 and 15.0% of breast cancer patients opt for second-line and third-line breast cancer treatments, respectively) [4]. These challenges impinge the overall diagnostic and prognostic stages for breast cancer and therefore, the survival rate of breast cancer patients in moderate-resource countries is lower than that of high-resource countries.
High-resource countries such as Japan and Singapore are found to have a high number of incidences with low mortality rates (e.g., Japan: incidence ASR of 76.3/100,000 and mortality ASR of 9.9/100,000; Singapore: incidence ASR of 77.9/100,000 and mortality ASR of 17.8/100,000). The high number of incidence ASR is highly correlated to the significant urbanization and increasing socioeconomic status in these countries, whereas, the low mortality ASR is highly correlated to a well-established healthcare system and breast cancer protocol. On the contrary to moderate-resource countries, 80.0% of breast cancer patients in Japan received second-line breast cancer treatment which greatly increases the survival rate of breast cancer patients.
Conclusions
Over the years, breast cancer has become the apex of the cancer cumulative risk ranking for women in Asia. In 2020, 2.3 million breast cancer cases were reported worldwide, notably, Asia remarked 1,026,171 cases with a percentage up to 45.4%. The etiology of breast cancer is observed to be multifactorial, ranging from the individual reproductive pattern, hormonal factors, diet, physical activity, lifestyle, and exposure to certain advent procedures. To date, in breast cancer, the NHG system remained the mainstay for grading purposes, considering three main features: mitotic count, tubule formation, and nucleus pleomorphism. In light of rapid economic growth, significant urbanization, and fast pace development in socioeconomic status, Asia is anticipated to have a persistent increment in the number of breast cancer incidences in near future. In terms of therapies, common therapy options such as breast-conserving therapy, mastectomy, postmastectomy radiation therapy, neoadjuvant chemotherapy, axillary surgery, chemotherapy, adjuvant medical therapies, biological and targeted therapies, and endocrine therapy are included in the article. With the emergence of advent technology, for example, artificial intelligence could probably offer betterment across cancer screening, cancer detection, cancer diagnosis, disease monitoring, and data management. Advent technologies in molecular biology, pharmaceutical, and innovative surgical procedures could further improve diagnostic and prognostic outcomes. From a broader view, government plays a pivotal role in establishing a standard healthcare system, research funding, cancer control protocol, cancer diagnostic and prognostic database, well-equipped radiative equipment, better healthcare insurance, and public health awareness. In this article, the authors contemplate statistics of breast cancer in Asia; the etiology of breast cancer; diagnosis of breast cancer; grading system; therapies; and challenges in breast cancer from the country’s income perspective, aiming to provide a brief yet broad overview of breast cancer from a multifaceted perspective. The narrative review acts as an updated scientific communication to newcomers, existing researcher, and relevant stakeholders, on the topic of interest.
Funding source: Tunku Abdul Rahman University of Management and Technology
Award Identifier / Grant number: UC/I/G2021-00078
-
Research funding: This research was funded by internal grant from Tunku Abdul Rahman University of Management and Technology (TAR UMT) with grant number: UC/I/G2021-00078. The APC was funded in part by the Centre for Multimodal Signal Processing, Department of Electrical and Electronics Engineering, TAR UMT.
-
Author contributions: X.J. Tan: Author; study conception and design; data collection; draft manuscript preparation, W.L. Cheor: data collection and study conception and design, E.M. Cheng: data collection; study conception and design, K.S. Ab Rahman: study conception and design; interpretation of results, W.Z.A. Wan Muhamad: study conception and design; interpretation of results, W.Z. Leow: interpretation of results. All authors reviewed the results and approved the final version of the manuscript.
-
Competing interests: The authors declare that they have no conflicts of interest to report regarding the present study.
-
Informed consent: Not applicable.
-
Ethical approval: The protocol of this study has been approved by the Medical Research and Committee of National Medical Research Register (NMRR) Malaysia (NMRR-21-949-58903).
References
1. Sung, H, Ferlay, J, Siegel, RL, Laversanne, M, Soerjomataram, I, Jemal, A, et al.. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J Clin 2021;71:1–41. https://doi.org/10.3322/caac.21660.Search in Google Scholar PubMed
2. Tan, XJ, Cheor, WL, Lim, LL, Ab-Rahman, KS, Hisyam, IB. Artificial intelligence (ai) in breast imaging: a scientometric umbrella review. Diagnostics 2022;12:1–35. https://doi.org/10.3390/diagnostics12123111.Search in Google Scholar PubMed PubMed Central
3. Bray, F, Ferlay, J, Soerjomataram, I, Siegel, RL, Torre, LA, Jemal, A. Global cancer statistics 2018: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J Clin 2018;68:394–424. https://doi.org/10.3322/caac.21492.Search in Google Scholar PubMed
4. Wen, DG, Wen, XD, Yang, Y, Chen, YT, Wei, LZ, He, YT, et al.. Urban rural disparity in female breast cancer incidence rate in China and the increasing trend in parallel with socioeconomic development and urbanization in a rural setting. Thorac. Cancer 2018;9:262–72. https://doi.org/10.1111/1759-7714.12575.Search in Google Scholar PubMed PubMed Central
5. LeBlanc, G, Lee, I, Carretta, H, Luo, Y, Sinha, D, Rust, G. Rural-urban differences in breast cancer stage at diagnosis. Wom Health Rep 2022;3:207–14. https://doi.org/10.1089/whr.2021.0082.Search in Google Scholar PubMed PubMed Central
6. Dreyer, MS, Nattinger, AB, McGinley, EL, Pezzin, LE. Socioeconomic status and breast cancer treatment. Breast Cancer Res Treat 2018;167:1–8. https://doi.org/10.1007/s10549-017-4490-3.Search in Google Scholar PubMed PubMed Central
7. Ji, P, Gong, Y, Jiang, CC, Hu, X, Di, GH, Shao, ZM. Association between socioeconomic factors at diagnosis and survival in breast cancer: a population-based study. Cancer Med 2020;9:1922–36. https://doi.org/10.1002/cam4.2842.Search in Google Scholar PubMed PubMed Central
8. WHO. Cancer Today. World health Organization; 2021. Available from: https://www.who.int/.Search in Google Scholar
9. WHO. Classification of Tumours Editorial Board. WHO classification of tumors series: Breast tumors, 5th ed. Lyon, France: International Agency for Research on Cancer (IARC); 2019.Search in Google Scholar
10. Goldberg, M, D’Aloisio, AA, O’Brien, KM, Zhao, S, Sandler, DP. Pubertal timing and breast cancer risk in the Sister Study cohort. Breast Cancer Res 2020;22:1–11. https://doi.org/10.1186/s13058-020-01326-2.Search in Google Scholar PubMed PubMed Central
11. Husby, A, Wohlfahrt, J, Øyen, N, Melbye, M. Pregnancy duration and breast cancer risk. Nat Commun 2018;9:1–7. https://doi.org/10.1038/s41467-018-06748-3.Search in Google Scholar PubMed PubMed Central
12. Anstey, EH, Shoemaker, ML, Barrera, CM, O’Neil, ME, Verma, AB, Holman, DM. Breastfeeding and breast cancer risk reduction: implications for black mothers. Am J Prev Med 2017;53:40–6. https://doi.org/10.1016/j.amepre.2017.04.024.Search in Google Scholar PubMed PubMed Central
13. Houghton, LC, Jung, SY, Troisi, R, LeBlanc, ES, Snetselaar, LG, Hylton, NM, et al.. Pubertal timing and breast density in young women: a prospective cohort study. Breast Cancer Res 2019;21:1–8. https://doi.org/10.1186/s13058-019-1209-x.Search in Google Scholar PubMed PubMed Central
14. Momenimovahed, Z, Salehiniya, H. Epidemiological characteristics of and risk factors for breast cancer in the world. Breast Cancer 2019;11:151–64. https://doi.org/10.2147/bctt.s176070.Search in Google Scholar PubMed PubMed Central
15. Tin-Tin, S, Reeves, GK, Key, TJ. Endogenous hormones and risk of invasive breast cancer in pre- and post-menopausal women: findings from the UK Biobank. Br J Cancer 2021;125:126–34. https://doi.org/10.1038/s41416-021-01392-z.Search in Google Scholar PubMed PubMed Central
16. Khan, SA. Progesterone exposure and breast cancer risk-addressing barriers. JAMA Netw Open 2020;3:1–3. https://doi.org/10.1001/jamanetworkopen.2020.3608.Search in Google Scholar PubMed
17. Valko-Rokytovská, M, Očenáš, P, Salayová, A, Kostecká, Z. Breast cancer: Targeting of steroid hormones in cancerogenesis and diagnostics. Int J Mol Sci 2021;22:1–15. https://doi.org/10.3390/ijms22115878.Search in Google Scholar PubMed PubMed Central
18. Schuler, LA, O’Leary, KA. Prolactin: the third hormone in breast cancer. Front Endocrinol 2022;13:1–14. https://doi.org/10.3389/fendo.2022.910978.Search in Google Scholar PubMed PubMed Central
19. Hada, M, Oh, H, Pfeiffer, RM, Falk, RT, Fan, S, Mullooly, M, et al.. Relationship of circulating insulin-like growth factor-I and binding proteins 1–7 with mammographic density among women undergoing image-guided diagnostic breast biopsy. Breast Cancer Res 2019;21:1–16. https://doi.org/10.1186/s13058-019-1162-8.Search in Google Scholar PubMed PubMed Central
20. Horn, J, Vatten, LJ. Reproductive and hormonal risk factors of breast cancer: a historical perspective. Int J Wom Health 2017;9:265–72. https://doi.org/10.2147/ijwh.s129017.Search in Google Scholar PubMed PubMed Central
21. Yang, TYO, Cairns, BJ, Pirie, K, Green, J, Beral, V, Floud, S, et al.. Body size in early life and the risk of postmenopausal breast cancer. BMC Cancer 2022;22:1–9. https://doi.org/10.1186/s12885-022-09233-9.Search in Google Scholar PubMed PubMed Central
22. Hultstrand, JN, Gemzell-Danielsson, K, Kallner, HK, Lindman, H, Wikman, P, Sundstrom-Poromaa, I. Hormonal contraception and risk of breast cancer and breast cancer in situ among Swedish women 15–34 years of age: a nationwide register-based study. Lancet Reg. Health – Eur 2022;21:1–13.10.1016/j.lanepe.2022.100470Search in Google Scholar PubMed PubMed Central
23. White, ND. Hormonal contraception and breast cancer risk. Am J Lifestyle Med 2018;12:224–6. https://doi.org/10.1177/1559827618754833.Search in Google Scholar PubMed PubMed Central
24. Bardaweel, SK, Akour, AA, Al-Muhaissen, S, Alsalamat, HA, Ammar, K. Oral contraceptive and breast cancer: do benefits outweigh the risks? A case – control study from Jordan. BMC Wom Health 2019;19:1–7. https://doi.org/10.1186/s12905-019-0770-x.Search in Google Scholar PubMed PubMed Central
25. Lakhani, SR, Ellis, IO, Schnitt, SJ, Tan, PH, Van-de-Vijver, MJ. WHO Classification of Tumours of the Breast, 4th ed. Lyon, France: International Agency for Research on Cancer (IARC); 2012.Search in Google Scholar
26. Wang, SM, Pfeiffer, RM, Gierach, GL, Falk, RT. Use of postmenopausal hormone therapies and risk of histology- and hormone receptor-defined breast cancer: results from a 15-year prospective analysis of NIH-AARP cohort. Breast Cancer Res 2020;22:1–10. https://doi.org/10.1186/s13058-020-01365-9.Search in Google Scholar PubMed PubMed Central
27. Samson, K. Estrogen-Progestin therapy raises breast cancer risk. Oncol Times 2020;42:32. https://doi.org/10.1097/01.cot.0000656004.63036.93.Search in Google Scholar
28. Syal, A, Aggarwal, N. Postmenopausal hormone therapy and its association with breast cancer. J Midlife Health 2020;11:187–95. https://doi.org/10.4103/jmh.jmh_284_20.Search in Google Scholar PubMed PubMed Central
29. Shetty, V, Kundapur, R, Chandramohan, S, Baisil, S, Saxena, D. Physical violence against doctors: a content analysis from online Indian newspapers. Indian J Community Med 2017;42:147–50. https://doi.org/10.4103/ijcm.ijcm_41_16.Search in Google Scholar
30. He, Y, Tian, J, Blizzard, L, Oddy, WH, Dwyer, T, Bazzano, LA, et al.. Associations of childhood adiposity with menstrual irregularity and polycystic ovary syndrome in adulthood: the childhood determinants of adult health study and the Bogalusa heart study. Hum Reprod 2020;35:1185–98. https://doi.org/10.1093/humrep/deaa069.Search in Google Scholar PubMed PubMed Central
31. Ortega, MA, Fraile-Martínez, O, García-Montero, C, Pekarek, L, Guijarro, LG, Castellanos, AJ, et al.. Physical activity as an imperative support in breast cancer management. Cancers 2021;13:1–30. https://doi.org/10.3390/cancers13010055.Search in Google Scholar PubMed PubMed Central
32. Jia, T, Liu, Y, Fan, Y, Wang, L, Jiang, E. Association of healthy diet and physical activity with breast cancer: lifestyle interventions and oncology education. Front Public Health 2022;10:1–12. https://doi.org/10.3389/fpubh.2022.797794.Search in Google Scholar PubMed PubMed Central
33. Ma, HY, Xu, XX, Clague, J, Lu, Y, Togawa, K, Wang, SS, et al.. Recreational physical activity and risk of triple negative breast cancer in the California teachers study. Breast Cancer Res 2016;18:1–16. https://doi.org/10.1186/s13058-016-0723-3.Search in Google Scholar PubMed PubMed Central
34. Guo, W, Fensom, GK, Reeves, GK, Key, TJ. Physical activity and breast cancer risk: results from the UK Biobank prospective cohort. Br J Cancer 2020;122:726–32. https://doi.org/10.1038/s41416-019-0700-6.Search in Google Scholar PubMed PubMed Central
35. Jung, AY, Behrens, S, Schmidt, M, Thoene, K, Obi, N, Hüsing, A, et al.. Pre- to postdiagnosis leisure-time physical activity and prognosis in postmenopausal breast cancer survivors. Breast Cancer Res 2019;21:1–11. https://doi.org/10.1186/s13058-019-1206-0.Search in Google Scholar PubMed PubMed Central
36. Freudenheim, JL. Alcohol’s effects on breast cancer in women. Alcohol Res Curr Rev 2020;40:1–12. https://doi.org/10.35946/arcr.v40.2.11.Search in Google Scholar PubMed PubMed Central
37. Zeinomar, N, Knight, JA, Genkinger, JM, Phillips, KA, Daly, MB, Milne, RL, et al.. Alcohol consumption, cigarette smoking, and familial breast cancer risk: findings from the prospective family study cohort (ProF-SC). Breast Cancer Res 2019;21:1–14. https://doi.org/10.1186/s13058-019-1213-1.Search in Google Scholar PubMed PubMed Central
38. Kispert, S, McHowat, J. Recent insights into cigarette smoking as a lifestyle risk factor for breast cancer. Breast Cancer 2017;9:127–32. https://doi.org/10.2147/bctt.s129746.Search in Google Scholar
39. Lo, JJ, Park, MM, Sinha, R, Sandler, DP. Association between meat consumption and risk of breast cancer: findings from the Sister Study. Int J Cancer 2020;146:2156–65. https://doi.org/10.1002/ijc.32547.Search in Google Scholar PubMed PubMed Central
40. Rumgay, H, Shield, K, Charvat, H, Ferrari, P, Sornpaisarn, B, Obot, I, et al.. Global burden of cancer in 2020 attributable to alcohol consumption: a population-based study. Lancet Oncol 2021;22:1071–80. https://doi.org/10.1016/s1470-2045(21)00279-5.Search in Google Scholar
41. Rivandi, M, Martens, JWM, Hollestelle, A. Elucidating the underlying functional mechanisms of breast cancer susceptibility through post-GWAS analyses. Front Genet 2018;9:1–18. https://doi.org/10.3389/fgene.2018.00280.Search in Google Scholar PubMed PubMed Central
42. Fanfani, V, Zatopkova, M, Harris, AL, Pezzella, F, Stracquadanio, G. Dissecting the heritable risk of breast cancer: from statistical methods to susceptibility genes. Semin Cancer Biol 2020:1–10.10.1016/j.semcancer.2020.06.001Search in Google Scholar PubMed
43. Läll, K, Lepamets, M, Palover, M, Esko, T, Metspalu, A, Tõnisson, N, et al.. Polygenic prediction of breast cancer: comparison of genetic predictors and implications for risk stratification. BMC Cancer 2019;19:1–9. https://doi.org/10.1186/s12885-019-5783-1.Search in Google Scholar PubMed PubMed Central
44. Ou-Yang, F, Da-Lin, Y, Chuang, LY, Chang, HW, Yang, CH, Hou, MF. The combinational polymorphisms of ORAI1 gene are associated with preventive models of breast cancer in the Taiwanese. BioMed Res Int 2015;2015:1–8. https://doi.org/10.1155/2015/281263.Search in Google Scholar PubMed PubMed Central
45. Karim, MO, Khan, KA, Khan, AJ, Javed, A, Fazid, S, Aslam, MI. Triple assessment of breast lump: should we perform core biopsy for every patient? Cureus 2020;107:1–7. https://doi.org/10.7759/cureus.7479.Search in Google Scholar PubMed PubMed Central
46. Htay, MNN, Donnelly, M, Schliemann, D, Loh, SY, Dahlui, M, Somasundaram, S, et al.. Breast cancer screening in Malaysia: a policy review. Asian Pac J Cancer Prev APJCP 2021;22:1685–93. https://doi.org/10.31557/apjcp.2021.22.6.1685.Search in Google Scholar
47. Patey, DH, Scarff, RW. The position of histology in the prognosis of carcinoma of the breast. The Lancet 1928;211:801–4. https://doi.org/10.1016/S0140-6736(00)76762-6.Search in Google Scholar
48. Bloom, HJG, Richardson, WW. Histological grading and prognosis of breast cancer. Br J Cancer 1957;22:36–7.10.1038/bjc.1957.43Search in Google Scholar PubMed PubMed Central
49. Elston, C, Ellis, I. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology 1991;19:403–10. https://doi.org/10.1111/j.1365-2559.1991.tb00229.x.Search in Google Scholar PubMed
50. Li, C, Wang, X, Liu, W, Latecki, LJ. DeepMitosis: mitosis detection via deep detection, verification and segmentation networks. Med Image Anal 2018;45:121–33. https://doi.org/10.1016/j.media.2017.12.002.Search in Google Scholar PubMed
51. Tan, XJ, Mustafa, N, Mashor, MY, Ab-Rahman, KS. Automated knowledge-assisted mitosis cells detection framework in breast histopathology images. Math Biosci Eng 2022;19:1721–45. https://doi.org/10.3934/mbe.2022081.Search in Google Scholar PubMed
52. Saha, M, Chakraborty, C, Racoceanu, D. Efficient deep learning model for mitosis detection using breast histopathology images. Comput Med Imag Graph 2018;64:29–40. https://doi.org/10.1016/j.compmedimag.2017.12.001.Search in Google Scholar PubMed
53. Ji, JL, Yuan, SS, He, JW, Liu, H, Yang, L, He, XX. Breast-conserving therapy is associated with better survival than mastectomy in early-stage breast cancer: a propensity score analysis. Cancer Med 2021;11:1646–58. https://doi.org/10.1002/cam4.4510.Search in Google Scholar PubMed PubMed Central
54. Pilewskie, M, Morrow, M. Margins in breast cancer: how much is enough? Cancer 2018;124:1335–41. https://doi.org/10.1002/cncr.31221.Search in Google Scholar PubMed PubMed Central
55. Sun, ZH, Chen, C, Kuang, XW, Song, JL, Sun, SR, Wang, WX. Breast surgery for young women with early-stage breast cancer: mastectomy or breast-conserving therapy? Medicine 2021;100:E25880. https://doi.org/10.1097/md.0000000000025880.Search in Google Scholar PubMed PubMed Central
56. Rossi, C, Mingozzi, M, Curcio, A, Buggi, F, Folli, S. Nipple areola complex sparing mastectomy. Gland Surg 2015;4:528–40. https://doi.org/10.3978/j.issn.2227-684X.2015.04.12.Search in Google Scholar PubMed PubMed Central
57. Luo, C, Zhong, X, Fan, Y, Wang, C, Wang, Y, Luo, T. The effect of postmastectomy radiation therapy on high-risk patients with T1-2N0 breast cancer. Breast 2021;60:1–5. https://doi.org/10.1016/j.breast.2021.08.006.Search in Google Scholar PubMed PubMed Central
58. Remick, J, Amin, NP. Postmastectomy breast cancer radiation therapy. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2022. Available from: https://www.ncbi.nlm.nih.gov/books/NBK519034/.Search in Google Scholar
59. Tse, T, Sehdev, S, Seely, J, Gravel, DH, Clemons, M, Cordeiro, E, et al.. Neoadjuvant chemotherapy in breast cancer: review of the evidence and conditions that facilitated its use during the global pandemic. Curr Oncol 2021;28:1338–47. https://doi.org/10.3390/curroncol28020127.Search in Google Scholar PubMed PubMed Central
60. Lüftner, D, Bauerfeind, I, Braun, M, Brucker, SY, Fasching, PA, Felberbaum, R, et al.. Treatment of early breast cancer patients: evidence, controversies, consensus: focusing on systemic therapy – German experts’ opinions for the 16th international St. Gallen consensus conference (Vienna 2019). Breast Care 2019;14:315–24. https://doi.org/10.1159/000502603.Search in Google Scholar PubMed PubMed Central
61. Chen, MY, Gillanders, WE. Staging of the axilla in breast cancer and the evolving role of axillary ultrasound. Breast Cancer 2021;13:311–23. https://doi.org/10.2147/bctt.s273039.Search in Google Scholar PubMed PubMed Central
62. Hanna, K, Mayden, K. Chemotherapy treatment considerations in metastatic breast cancer. J Adv Pract Oncol 2021;12:6–12. https://doi.org/10.6004/jadpro.2021.12.2.11.Search in Google Scholar PubMed PubMed Central
63. Moo, TA, Sanford, R, Dang, C, Morrow, M. Overview of breast cancer therapy. Pet Clin 2018;13:339–54. https://doi.org/10.1016/j.cpet.2018.02.006.Search in Google Scholar PubMed PubMed Central
64. Shien, T, Iwata, H. Adjuvant and neoadjuvant therapy for breast cancer. Jpn J Clin Oncol 2020;50:225–9. https://doi.org/10.1093/jjco/hyz213.Search in Google Scholar PubMed
65. Masoud, V, Pagès, G. Targeted therapies in breast cancer: new challenges to fight against resistance. World J Clin Oncol 2017;8:120–34. https://doi.org/10.5306/wjco.v8.i2.120.Search in Google Scholar PubMed PubMed Central
66. Krauss, K, Stickeler, E. Endocrine therapy in early breast cancer. Breast Care 2020;15:337–46. https://doi.org/10.1159/000509362.Search in Google Scholar PubMed PubMed Central
67. Malik, JA, Ahmed, S, Jan, B, Bender, O, Hagbani, TA, Alqarni, A, et al.. Drugs repurposed: an advanced step towards the treatment of breast cancer and associated challenges. Biomed Pharmacother 2022;145:112375. https://doi.org/10.1016/j.biopha.2021.112375.Search in Google Scholar PubMed
68. Heery, M, Farley, S, Sparkman, R, Eighmy, W, Zahrah, G, Zelkowitz, R, et al.. Precautions for patients taking aromatase inhibitors. J Adv Pract Oncol 2020;11:184–9. https://doi.org/10.6004/jadpro.2020.11.2.6.Search in Google Scholar PubMed PubMed Central
69. Malik, JA, Jan, R, Ahmed, S, Anwar, S. Breast cancer drug repurposing a tool for a challenging disease. London, UK: IntechOpen; 2021.Search in Google Scholar
70. Alamri, A, Rauf, A, Khalil, AA, Alghamdi, A, Alafnan, A, Alshammari, A, et al.. In Silico screening of marine compounds as an emerging and promising approach against estrogen receptor alpha-positive breast cancer. BioMed Res Int 2021;2021:1–7. https://doi.org/10.1155/2021/9734279.InSearch in Google Scholar PubMed PubMed Central
71. Vidya, R, Leff, DR, Green, M, McIntosh, SA, John, ES, Kirwan, CC, et al.. Innovations for the future of breast surgery. Br J Surg 2021;108:908–16. https://doi.org/10.1093/bjs/znab147.Search in Google Scholar PubMed
72. Francies, FZ, Hull, R, Khanyile, R, Dlamini, Z. Breast cancer in low-middle income countries: abnormality in splicing and lack of targeted treatment options. Am. J. Cancer Res. 2020;10:1568–91.Search in Google Scholar
73. Karn, A. Current status of availability of radiotherapy machines in Nepal. J Clin Oncol 2017;35:18042. https://doi.org/10.1200/jco.2017.35.15_suppl.e18042.Search in Google Scholar
74. Martei, YM, Pace, LE, Brock, JE, Shulman, LN. Breast cancer in low- and middle-income countries: why we need pathology capability to solve this challenge. Clin Lab Med 2018;38:161–73. https://doi.org/10.1016/j.cll.2017.10.013.Search in Google Scholar PubMed PubMed Central
75. Nichols, K, Girdwood, SJ, Inglis, A, Ondoa, P, Sy, KTL, Benade, M, et al.. Bringing data analytics to the design of optimized diagnostic networks in low-and middle-income countries: process, terms and definitions. Diagnostics 2021;11:1–11. https://doi.org/10.3390/diagnostics11010022.Search in Google Scholar PubMed PubMed Central
© 2023 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Review Articles
- Breast cancer status, grading system, etiology, and challenges in Asia: an updated review
- The role of acetylation of histone H3 and H4 in oral squamous cell carcinoma
- Research Articles
- Temporal trends of cervical cancer between 1990 and 2019, in Asian countries by geographical region and socio-demographic index, and comparison with global data
- Human EVI2B acts as a Janus-faced oncogene/antioncogene by differently affecting as per cancer type neoplastic cells growth and immune infiltration
- Wnt/β-catenin signaling activation by TIMP1 confers cisplatin-resistant gastric cancer cells to malignant behaviors and epithelial–mesenchymal transition
- Hsa_circ_0079598 acts as a potential diagnostic and prognostic biomarker for gastric cancer
- The inhibitory function of GDF11/BMP11 in liver cancer by inducing apoptosis and ROS–JNK pathway
- Identification of cancer stem cells derived from U118MG and the involvement of LncRNA-DC and STAT3 in promoting their malignant transformation
Articles in the same Issue
- Frontmatter
- Review Articles
- Breast cancer status, grading system, etiology, and challenges in Asia: an updated review
- The role of acetylation of histone H3 and H4 in oral squamous cell carcinoma
- Research Articles
- Temporal trends of cervical cancer between 1990 and 2019, in Asian countries by geographical region and socio-demographic index, and comparison with global data
- Human EVI2B acts as a Janus-faced oncogene/antioncogene by differently affecting as per cancer type neoplastic cells growth and immune infiltration
- Wnt/β-catenin signaling activation by TIMP1 confers cisplatin-resistant gastric cancer cells to malignant behaviors and epithelial–mesenchymal transition
- Hsa_circ_0079598 acts as a potential diagnostic and prognostic biomarker for gastric cancer
- The inhibitory function of GDF11/BMP11 in liver cancer by inducing apoptosis and ROS–JNK pathway
- Identification of cancer stem cells derived from U118MG and the involvement of LncRNA-DC and STAT3 in promoting their malignant transformation

