Abstract
Background
Neurofibromatosis (NF) is a group of rare genetic disorders characterized by the development of tumours that may affect the brain, spinal cord, and the nerves that send signals between the brain and spinal cord and all other parts of the body. The disease burden on Greek patients and caregivers is unknown.
Objective
The aim of this study is to determine the societal economic burden and health-related quality of life (HRQoL) of families with neurofibromatosis type 1 (NF1) in Greece.
Methods
A cross-sectional study was conducted in which an online questionnaire was completed by NF1 patients and their caregivers. A cost of illness model was developed in which costs per NF1 patient were calculated from a societal perspective and extrapolated to the total affected population in Greece. Finally, the 36-item PedsQL™ Family Impact Module was used to measure the HRQoL of families with NF1 members.
Results
The economic burden of NF1 was estimated at €21,594 per patient in 2022. When extrapolating this outcome across all affected persons, the average annual cost was €56,319,583. Out-of-pocket expenses accounted for 10% of the total. Results of the analysis on HRQoL indicated a significant burden on family members representing the caregivers of NF1 patients with the majority of scores falling below 50 and a mean total score of 45.
Conclusions
The results highlight the considerable burden of NF1 not only in terms of the high costs but also in terms of reduced HRQoL for patients’ families.
1 Introduction
Neurofibromatosis (NF) is a group of rare genetic disorders characterized by the development of tumours that may affect the brain, spinal cord, and the nerves that send signals between the brain and spinal cord as well as all other parts of the body. There are three different types of NF: neurofibromatosis type 1 (NF1), neurofibromatosis type 2-related schwannomatosis (NF2), and schwannomatosis (SWN). NF1 is the most common form of NF, also known as von Recklinghausen disease. The most prominent symptoms of this condition include café-au-lait spots, freckling, and the development of neurofibromas, which are slow-growing, non-cancerous tumours [1]. NF2 is characterized by the development of multiple benign nerve sheath tumours called schwannomas, particularly affecting the vestibular nerve. Persons with NF2 usually present with bilateral vestibular schwannomas but can have schwannomas on other cranial, spinal, and peripheral/cutaneous nerves [2,3]. SWN could be termed “SMARCB1-related SWN” (for patients with germline P variants [PV] in SMARCB1), “LZTR1-related SWN” (for patients with germline PV in LZTR1), “22q-related SWN” (for patients with multiple schwannomas with common molecular findings on chromosome 22q), “SWN-not otherwise specified” (for patients who have clinical features of NF2/SWN but have not had molecular analysis), or “SWN is not elsewhere classified” (for patients in whom molecular analysis of blood and tumours has failed to detect a PV) [3]. All NF types occur in both biological sexes and in all races and ethnic groups [4]. The global prevalence of NF1 and NF2 have been estimated to 1:2,052 [5] and 1:60,000, respectively [6], whereas no data are available regarding SWN.
Humanistic burden of NF1 is considerably high with a recent systematic literature review showing that NF1 patients with plexiform neurofibromas (PNs) experience pain, decreased social functioning, physical function impact, stigma, and emotional distress [7,8]. Further, studies conducted in the United States and France indicated the significant economic burden that NF1 imposes on paediatric patients and patients of all ages, respectively [9,10].
Despite its impact, the economic and societal burden of NF1 has not been explored in Greece. The objective of this study was to estimate the economic burden of NF1 to society and quantify how the disease affects caregivers’ health-related quality of life (HRQoL) and the functioning of the family. Our aim was to generate evidence to help policymakers devise appropriate intervention programs for patients with NF and their families.
2 Methodology
A cross-sectional study was conducted between October and December 2022. An online questionnaire with 21 queries about patients’ healthcare resource use (HCRU) was developed to capture patients’ sociodemographic parameters and HCRU such as public healthcare, non-healthcare resource use, costs of professional private care, informal care, issues regarding equipment, and services necessary for patients’ daily activities during the last year.
Participants were recruited through the Panhellenic Association of Neurofibromatosis Patients & Friends “Life with NF” and those eligible to participate were patients diagnosed with NF1. The main caregiver was responsible for the completion of the self-administered questionnaire in the case of adolescent patients. Patients and legal guardians/parents were informed about the study objectives as well as the confidentiality and anonymity of the data and gave their written consent to participate in the study, with the option of data withdrawal. Final questionnaires were provided by Health Care Professionals in hard copy or completed online via the Computer Assisted Web Interviewing method. The present study was conducted in accordance with the Declaration of Helsinki and the Greek legislation (Law 2328/1995, Presidential Decree 310/1996, Law 3603/2007, Law 2472/1997, Law 3471/2006), stating that there is no need for ethics approval in telephone and internet surveys such the one presented here.
The questionnaire was divided into two sections, one devoted to HCRU and one to HRQoL. Overall, 28 patients with NF1 returned a completed HCRU questionnaire. The HCRU as reported by respondents with NF1 was leveraged as input in the cost of illness (COI) model. Cost inputs were retrieved from official sources and inserted into the model to be combined with HCRU and produce the results.
Following the queries focused on assessing the economic burden, the 36-item PedsQL™ Family Impact Module (FIM) was used to assess the impact on the family’s HRQoL. This section of the questionnaire was completed by all 79 patients. The 36-item PedsQL™ FIM Scales encompasses six scales’ measuring parent self-reported functioning: (1) physical functioning (six items), (2) emotional functioning (five items), (3) social functioning (four items), (4) cognitive functioning (five items), (5) communication (three items), (6) worry (five items), and two scales measuring parent-reported family functioning: (7) daily activities (three items) and (8) family relationships (five items) [11]. Each item is scored on a five-point response scale and then converted to a 0–100 scale. A five-point response scale is utilized (0 = never a problem; 4 = always a problem). Items are reverse scored and linearly transformed to a 0–100 scale (0 = 100, 1 = 75, 2 = 50, 3 = 25, and 4 = 0), so that higher scores indicate better functioning (less negative impact). Scale scores are computed as the sum of the items divided by the number of items answered (this accounts for missing data).
2.1 COI analysis
A COI model was developed to estimate the average annual economic burden of NF1 in the Greek population from a societal perspective. The model adopted a prevalence approach, and as such all prevalent cases of NF1 in Greece were considered. To estimate the number of NF1 patients, the prevalence value reported by Orphanet (i.e., 2.5/10,000) [5] was utilized. Applying this to the total Greek population (10,432,481) [12], it was estimated that there are currently 2,608 patients with NF1 in Greece.
The resources used, as reported in the questionnaires, were multiplied by the unit costs [13] to calculate the annual cost per patient and per total NF1 population using 2022 as the reference year. An overview of the model structure is illustrated in Figure 1.
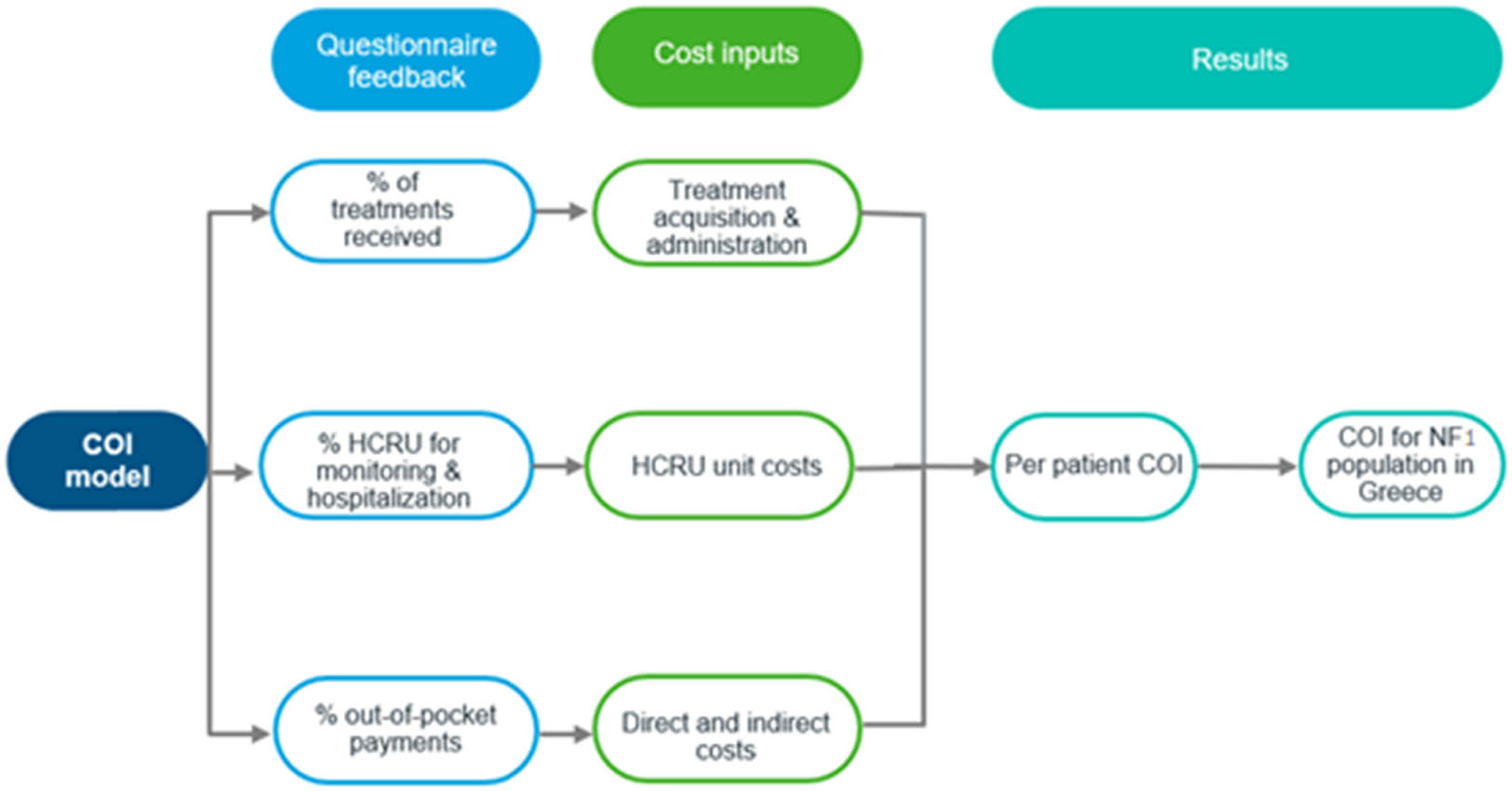
Model structure.
2.1.1 Healthcare resource use inputs
HCRU of patients with NF1 as reported by respondents is presented in Table 1. Overall, the healthcare resources with the highest usage from patients were visits to a healthcare practitioner, physiotherapy, and complete blood count testing. In the previous year, one-third of the patients required approximately one hospitalization (including hospitalizations for the treatment administration) lasting approximately 2 days. Moreover, 25% of patients required physiotherapy, 30% required speech and language therapy, 45% required occupational therapy, and 22% required psychotherapy. Furthermore, a number of patients required tumour resection surgery (32%), auditory brainstem implants and cochlear implants (4%), chemotherapy (14%), and radiotherapy (7%).
Yearly healthcare resource use of patients with NF1
| Average HCRU | Proportion of patients (%) | |
|---|---|---|
| Medical visit | 3.04 | 89.3 |
| Complete blood count | 1.67 | 96.4 |
| Genetic tests | 0.75 | 21.4 |
| X-ray | 0.63 | 39.3 |
| CT scan | 1.06 | 21.4 |
| MRI scan | 1.63 | 89.3 |
| Hospitalization | 1.05 | 28.6 |
| Otorhinolaryngologist | 1.17 | 35.7 |
| Dermatologist | 1.94 | 46.4 |
| Eye test | 1.61 | 82.1 |
| Physiotherapy | 8.17 | 28.6 |
| Speech and language therapist | 1.46 | 35.9 |
| Occupational therapy | 1.29 | 37.3 |
| Psychotherapy | 2.27 | 32.1 |
CT – computed tomography; HCRU – healthcare resource use; MRI – magnetic resonance imaging. Source: Responses on the questionnaire.
According to the responses on questionnaires, physiotherapy, speech and language therapy, occupational therapy, and psychotherapy are not fully reimbursed in Greece (Table 2).
Percentage of reimbursement of rehabilitation therapies
| Physiotherapy | Speech and language therapist | Occupational therapy | Psychotherapy | |
|---|---|---|---|---|
| % of expenditure that is not reimbursed | 25 | 30 | 45 | 22 |
Source: Responses on the questionnaire.
Moreover, half of NF1 patients required additional resources to manage the disease on a daily basis, which were only required purchasing once, such as an elevator, a ventilator, or a vehicle. It is also common for patients to visit hospitals and clinics at regular intervals for the treatment administration, which contributes to the already high out-of-pocket costs due to the transportation costs that burden the patients and their caregivers. Finally, approximately 32% of the responders stated that they were unable to work due to the occurrence of NF1 symptoms, which led to their absence from the work for approximately 5 days.
2.1.2 Cost inputs
Cost inputs comprise treatment acquisition and administration, monitoring and hospitalization, as well as out-of-pocket expenses and indirect costs (e.g., loss of productivity).
Treatment acquisition unit costs were based on the recommended dosing schemes as sourced from each treatment’s summary of product characteristics issued by the European Medicines Agency [14,15,16,17], a randomized study [18], and the published unit prices as sourced from the latest Drug price bulletin issued by the Greek Ministry of Health [19]. The price of selumetinib could not be identified in the official Greek databases, and thus, the price in the National Institute for Health and Care Excellence (NICE) Highly specialized technologies guidance [20] was used after being converted to euros and without considering any confidential price discounts. In treatments administered via intravenous infusion, an administration cost of €80 [21] was applied. The drug acquisition costs are presented in Table S1.
Unit costs of the resources accounted for the monitoring and hospitalization were based on official National Organization for Healthcare Provision (EOPYY) reimbursed prices and Diagnosis Related Groups [22] and are presented in Table S2. Resources such as tumour resection surgery, stereotactic radiosurgery, auditory brainstem implants and cochlear implants, and radiotherapy were assumed to only occur once in a patient’s lifetime.
In addition to reimbursed costs, patients and their families are required to make significant out-of-pocket payments. More specifically, physiotherapy, speech therapy, occupational therapy, and psychotherapy are partially reimbursed by the Social Security Fund, so patients do have to cover a portion of them. Regarding the out-of-pocket expenses, the average cost of the home adjustments was estimated at €941, and it was assumed that takes place once in lifetime. Out-of-pocket transportation costs contributed on average an additional €326 per year per patient. An overview of out-of-pocket unit cost inputs is illustrated in Table S3. Furthermore, a breakdown of activities comprising out-of-pocket costs for NF1 patients is shown in Figure 2.
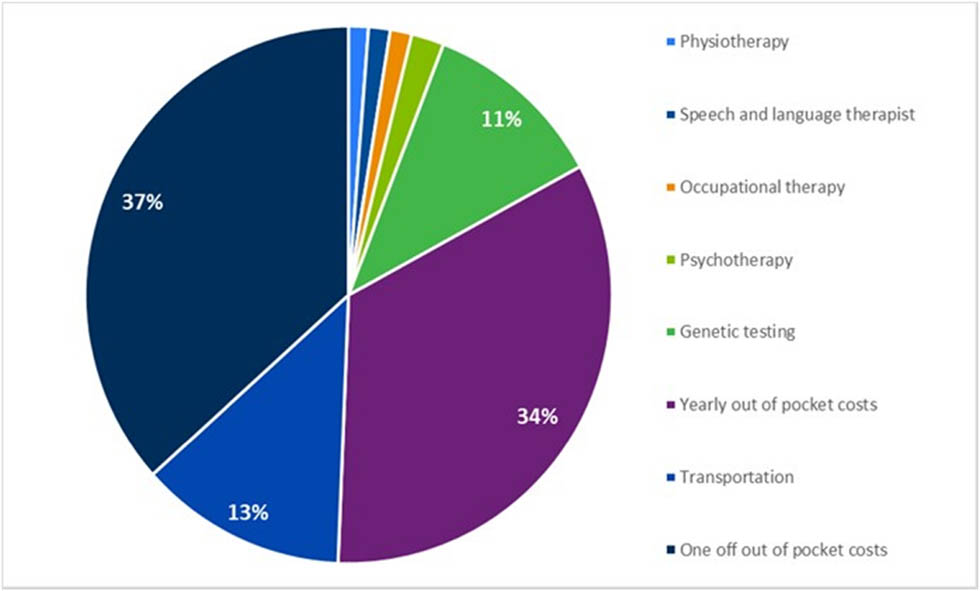
Breakdown of out-of-pocket payments.
Finally, indirect costs were calculated based on caregivers’ reported absence days from work (5 days per month) and the average daily wage (€56.7) as sourced from Hellenic Statistical Authority [23]. The annual productivity loss cost was estimated at €312.
3 Results
3.1 COI
Responses about patients’ HCRU were received from 28 participants. The main characteristics of the participants are shown in Table 3. The average age of the participants was 34.8 years, and 43% were males.
Demographic characteristic of the study participants
| Characteristics of NF1 patients | n = 28 |
|---|---|
| Age | |
| Mean (SD) | 34.8 (13.7) |
| Minimum–maximum | 9–54 |
| Gender | |
| Female | 12 (42.9%) |
| Male | 16 (57.1%) |
| % Disability | |
| <67% | 21 (75.0%) |
| >67% | 7 (25.0%) |
| Treatment | |
| Selumetinib | 1 (3.6%) |
| Magnesium | 2 (7.1%) |
| Acetylsalicylic acid | 1 (3.6%) |
| Levetiracetam | 1 (3.6%) |
| Brivaracetam | 1 (3.6%) |
NF1 – neurofibromatosis type 1.
Source: Responses on the questionnaire.
Seventy-five percent of the 28 patients reported to have a disability rate equal or greater than 67%, representing the cutoff point of severe disability according to the Disability Committee classifications. Time to diagnosis from the appearance of the first symptoms ranged from 4 weeks to 22 months with a median time to diagnosis of 17.2 weeks.
COI comprise the summary of treatment acquisition and administration costs, monitoring and hospitalization, out-of-pocket expenditure, as well as indirect costs.
Overall, the average annual cost of NF1 was estimated at €21,594 per patient in 2022. Subsequently, the average annual out-of-pocket expenditure per patient was €2,097 which represents 10% of the average annual cost. Extrapolating the results to the whole affected population with NF1 indicates that average annual economic burden was estimated at €56,319,583, with drug acquisition costs representing the majority of the costs (78%) followed by out-of-pocket costs. The COI results are presented in Table 4.
COI results breakdown
| Cost category | Average annual cost (€) | |
|---|---|---|
| Per patient | For total NF1 population (N = 2,608) | |
| Drug acquisition | 16,875 | 44,011,700 |
| Drug administration | 720 | 1,877,847 |
| Direct HCRU | 1,590 | 4,146,934 |
| Out of pocket | 2,097 | 5,468,325 |
| Indirect | 312 | 814,777 |
| Total | 21,594 | 56,319,583 |
HCRU – healthcare resource use.
Source: Calculations – COI model.
3.2 HRQoL
Responses about HRQoL were received from all 79 participants. Results of the analysis indicate that the impact of NF1 on the family’s HRQoL was high with the scores in the majority of the scales falling below 50 (Table 5). The scales of “Worry” and “Daily activities” are the ones with the lowest scores, while the scale of “Cognitive functioning” is the one with the highest.
PedsQL FIM scores in different functioning categories
| Scale | Mean | Median |
|---|---|---|
| Total scores | 44.6 | 44.6 |
| Parent HRQoL summary Score | 51.0 | 49.0 |
| Family functioning summary score | 44.7 | 37.5 |
| Physical functioning | 44.6 | 42.0 |
| Emotional functioning | 39.2 | 40.0 |
| Social functioning | 45.4 | 43.7 |
| Cognitive functioning | 75.0 | 75.0 |
| Communication | 45.2 | 41.7 |
| Worry | 18.4 | 20.0 |
| Daily activities | 30.7 | 16.7 |
| Family relationships | 53.2 | 50.0 |
FIM – family impact module; HRQoL – health-related quality of life; PedsQL – paediatric quality of life.
Source: Calculations based on questionnaire responses.
The mean HRQoL score was estimated at 44.6, which is considered low when compared with a score of 100 representing perfect health. It is evident from these results that NF1 constitutes a burden on the HRQoL of the families.
4 Discussion
Our study showed that both economic and humanistic burden of patients with NF1, which is the most common NF condition, is considerable. According to this study, NF1 is estimated to cost 21,594 per patient in 2022, while the average annual economic burden across 2,608 NF1 patients was estimated at €56,319,583.
Drug acquisition costs represented approximately 78% of the total costs followed by out-of-pocket costs (10%), HCRU costs (7%), administration costs (3%), and indirect costs (1%). To our knowledge, this is the first attempt to measure the total economic burden for NF1 in Greece from a societal perspective as well as the HRQoL of caregivers of patients. This is also the most recent European study published since the last evaluation of HCRU, which was conducted in 2000 in 201 adult NF1 patients who received care at a French hospital [9].
The French study showed that the annual cost per patient was £810, which is considerably lower compared to our results. This can be potentially attributed to the inclusion of different resources in our study such as drug, rehabilitation and operation costs (e.g., tumour resection surgery, auditory brainstem implants, and cochlear implants), as well as the inclusion of one-off costs, such as home adjustments, and non-healthcare costs such as transportation.
Other studies in the literature conducted in the United States [10,24] have focused on the estimation of HCRU of paediatric patients with NF1 and PNs, which is a different population than the one examined in this study. Furthermore, in contrast to these studies [10,24] where a high proportion of patients reported receiving pain medication, this was not the case in our study.
A limitation of our COI study relates to the sample size, which consisted of 28 patients. This is a relatively small sample size, which does not allow us to make safe conclusions regarding the actual COI. However, in Greece, a single system or database capturing all patients suffering from rare diseases is missing, thus making the identification of these patients very difficult.
Another limitation concerns the cross-sectional study design and the possibility of recall bias, which could have impacted the self-reporting of resource use. Our results were based on information collected from the administration of a single questionnaire at one point in time and concerned the previous year. As a result, some patients might have forgotten some of the healthcare resources that they used, and thus, the overall COI and total economic burden might have been underestimated. Furthermore, due to recall bias, some of the patients are more likely to remember larger expenses such as the cost of formal care rather than smaller costs.
Since NF1 is characterized by high clinical variability, it may be more likely that severely affected patients will be active in patients’ organizations, leading to a potential risk of selection bias for our sample, as all of the respondents were members of the Panhellenic Association of Neurofibromatosis Patients & Friends “Life with NF.” However, to minimize bias, we collected data from patients of different ages and the level of disease severity.
In addition to the limitations of our study, it is important to mention that there is an absence of comprehensive national data on NF1 prevalence in Greece. To overcome this, we utilized data reported in the Orphanet database [5,6].
Moreover, selumetinib is not currently reimbursed in Greece through the official reimbursement channels (i.e., EOPYY), and thus, a standard pack price is not available. For that reason, we retrieved the pack cost of selumetinib as presented in the NICE highly specialized technologies guidance [20] and converted it to euros without considering any confidential price discounts. This means that the drug acquisition costs might be lower if and when it gets reimbursed through EOPYY considering that a discount will also be applied to the original price of the drug.
Our study provides insights into potential policy recommendations and directives that can be adopted by stakeholders. According to the questionnaire answers, only 4 of the 28 patients with NF1 received treatment. Among these, two received levetiracetam or brivaracetam, which are symptomatic treatments to control seizures, one received analgesic, and one received targeted therapy. The targeted therapy pertains to selumetinib, which was not reimbursed at the time this article was written. Considering the aforementioned discussion, reimbursement of newly introduced targeted therapies is required. Aside from this, our study indicates that NF1 patients often face the burden of purchasing additional equipment, such as an elevator, ventilator, or means of transportation. Providing full or partial reimbursement for these resources would be a positive step forward.
Finally, it is widely recognized that patient registries offer important sources of information concerning healthcare practices, medicine consumption, and clinical outcomes. The establishment of a national patient registry in Greece may assist in meeting these challenges with respect to NF by providing clinical and patient community information on epidemiology, standards of care and treatment patterns, as well as supporting regulatory decisions concerning medicinal products.
Research in the future in Greece can focus on the use of targeted therapies, which could lead to a reduction in HCRU and consequently to a decrease in disease management costs.
5 Conclusions
This is the first study in Greece to highlight the considerable economic burden among patients with NF1 as well as the impact on the HRQoL of families. Our results also demonstrate how important the reimbursement of innovative targeted therapies and the creation of patient registries is for the accurate estimation of the economic burden of rare diseases and the financing of the healthcare system.
Abbreviations
- NF
-
Neurofibromatosis
- HRQoL
-
Health-Related Quality of Life
- PedsQL FIM
-
Pediatric Quality of Life Family Impact Module
- NF1
-
Neurofibromatosis type 1
- NF2
-
Neurofibromatosis type 2 – related schwannomatosis
- SWN
-
Schwannomatosis
- HCRU
-
Health Care Resource Use
- COI
-
Cost of Illness
- EMA
-
European Medicines Agency
- NICE
-
National Institute of Health Care Excellence
- HST
-
Highly Specialized Technologies
- EOPYY
-
National Organization for Health Care Services (Greece)
- DRG
-
Diagnostic Related Group
-
Funding information: The data collection for this study was supported by unconditional grants from Astrazeneca Greece, Genesis Pharma, and Takeda Hellas. No funding was received for the preparation of this article.
-
Author contributions: Mary Adamopoulou, Dimitris Athanasiou, and Maria Kalogeropoulou report receiving financial support for the conduct of this study from Astrazeneca Greece, Genesis Pharma, and Takeda Hellas. Maria Kalogeropoulou, Mary Adamopoulou, and Dimitris Athanasiou conceived and designed the study. Maria Kalogeropoulou, Mary Adamopoulou, Dimitris Athanasiou, and Lamprini Sotiropoulou designed the study questionnaires. Maria Kalogeropoulou and Marios Athanasios Loupas analyzed the data and all authors contributed to the interpretation of the findings. Maria Kalogeropoulou and Marios Athanasios Loupas drafted the first manuscript version, and all the authors commented on this version. All authors read and approved the final manuscript.
-
Conflict of interest: The authors state no conflict of interest.
-
Informed consent: Patients and legal guardians/parents were informed about the study objectives as well as the confidentiality and anonymity of the data and gave their written consent to participate in the study, with the option of data withdrawal.
-
Ethical approval: The present study was conducted in accordance with the Declaration of Helsinki and the Greek legislation (Law 2328/1995, Presidential Decree 310/1996, Law 3603/2007, Law 2472/1997, Law 3471/2006), stating that there is no need for ethics approval in telephone and internet surveys such the one presented here.
-
Data availability statement: All data generated or analyzed during this study are included in this published article (and its supplementary file).
References
[1] Friedman JM Neurofibromatosis 1. In: Adam MP, Mirzaa GM, Pagon RA, Wallace SE, Bean LJH, Gripp KW, et al. editors. Seattle, WA: GeneReviews; 1993.Suche in Google Scholar
[2] Evans DG, Huson SM, Donnai D, Neary W, Blair V, Newton V, et al. A clinical study of type 2 neurofibromatosis. Q J Med. 1992;84:603–18.Suche in Google Scholar
[3] Plotkin SR, Messiaen L, Legius E, Pancza P, Avery RA, Blakeley JO, et al. Updated diagnostic criteria and nomenclature for neurofibromatosis type 2 and schwannomatosis: An international consensus recommendation. Genet Med. 2022;24:1967–77.10.1212/WNL.98.18_supplement.3996Suche in Google Scholar
[4] National Institute on Neurological Disorders and Stroke. Neurofibromatosis; 2023. [Cited 2023 Oct 16]. https://www.ninds.nih.gov/health-information/disorders/neurofibromatosis.Suche in Google Scholar
[5] Orphanet. Neurofibromatosis type 1; 2014. [cited 2023 Oct 16], https://www.orpha.net/consor/cgi-bin/OC_Exp.php? Lng = GB&Expert = 636.Suche in Google Scholar
[6] Orphanet. Full NF2-related schwannomatosis; 2009. [cited 2023 Oct 16], https://www.orpha.net/consor/cgi-bin/OC_Exp.php? lng = EN&Expert = 637.Suche in Google Scholar
[7] Copley-Merriman C, Yang X, Juniper M, Amin S, Yoo HK, Sen SS. Natural history and disease burden of neurofibromatosis type 1 with plexiform neurofibromas: A systematic literature review. Adolesc Health Med Ther. 2021;12:55–66.10.2147/AHMT.S303456Suche in Google Scholar PubMed PubMed Central
[8] Yang X, Yoo HK, Amin S, Cheng WY, Sundaresan S, Zhang L, et al. Clinical and humanistic burden among pediatric patients with neurofibromatosis Type 1 and plexiform neurofibroma in the USA. Childs Nerv Syst. 2022;38:1513–22.10.1007/s00381-022-05513-8Suche in Google Scholar PubMed PubMed Central
[9] Wolkenstein P, Durand-Zaleski I, Moreno JC, Zeller J, Hemery F, Revuz J. Cost evaluation of the medical management of neurofibromatosis 1: a prospective study on 201 patients. Br J Dermatol. 2000;142:1166–70.10.1046/j.1365-2133.2000.03543.xSuche in Google Scholar PubMed
[10] Yang X, Desai K, Agrawal N, Mirchandani K, Chatterjee S, Sarpong E, et al. Treatment, resource use and costs among pediatric patients with neurofibromatosis Type 1 and plexiform Neurofibromas. Pediatric Health Med Ther. 2020;11:421–8.10.2147/PHMT.S265690Suche in Google Scholar PubMed PubMed Central
[11] Varni JW, Sherman SA, Burwinkle TM, Dickinson PE, Dixon P. The PedsQL family impact module: preliminary reliability and validity. Health Qual Life Outcomes. 2004;2:55.10.1186/1477-7525-2-55Suche in Google Scholar PubMed PubMed Central
[12] Hellenic Statistical Authority (ELSTAT). Population-Housing Census. 2021 [Cited 2023 Oct 16]. https://www.statistics.gr/en/2021-census-pop-hous.Suche in Google Scholar
[13] Government gazette. FEK 946/2012; 2012.Suche in Google Scholar
[14] European Medicines Agency (EMA). Koselugo, EPAR – Product Information; 2023 [Cited 2023 Oct 18]. https://www.ema.europa.eu/en/documents/product-information/koselugo-epar-product-information_en.pdf.Suche in Google Scholar
[15] European Medicines Agency (EMA). Keppra, EPAR – Product Information; 2023 [Cited 2023 Oct 18]. https://www.ema.europa.eu/en/documents/product-information/keppra-epar-product-information_en.pdf.Suche in Google Scholar
[16] European Medicines Agency (EMA). Briviact, EPAR – Product Information; 2023 [Cited 2023 Oct 18]. https://www.ema.europa.eu/en/documents/product-information/briviact-epar-product-information_en.pdf.Suche in Google Scholar
[17] European Medicines Agency (EMA). Cabometyx, EPAR – Product Information; 2023 [Cited 2023 Oct 18]. https://www.ema.europa.eu/en/documents/product-information/cabometyx-epar-product-information_en.pdf.Suche in Google Scholar
[18] Ater JL, Zhou T, Holmes E, Mazewski CM, Booth TN, Freyer DR, et al. Randomized study of two chemotherapy regimens for treatment of low-grade glioma in young children: a report from the Children’s Oncology Group. J Clin Oncol. 2012;30:2641–7.10.1200/JCO.2011.36.6054Suche in Google Scholar PubMed PubMed Central
[19] Greek Ministry of Health. Drug price bulletin, published 16. 12. 2022; [Cited 2023 Oct 16]; http://www.moh.gov.gr/articles/times-farmakwn/deltia-timwn.Suche in Google Scholar
[20] National Institute for Health and Care Excellence (NICE). Selumetinib for treating symptomatic and inoperable plexiform neurofibromas associated with type 1 neurofibromatosis in children aged 3 and over; 2022.Suche in Google Scholar
[21] Government gazette. FEK 1702/B′/2011; 2011.Suche in Google Scholar
[22] Govermnet gazette. FEK 946/B‘/2012; 2012.Suche in Google Scholar
[23] Hellenic Statistical Authority (ELSTAT). Per Capita Sizes: GDP and National Income; 2021 [Cited 2023 Oct 18]. https://www.statistics.gr/el/statistics/-/publication/SEL33/.Suche in Google Scholar
[24] Yang X, Desai K, Agrawal N, Mirchandani K, Chatterjee S, Sarpong E, et al. Characteristics, treatment patterns, healthcare resource use, and costs among pediatric patients diagnosed with neurofibromatosis type 1 and plexiform neurofibromas: a retrospective database analysis of a medicaid population. Curr Med Res Opin. 2021;37:1555–61.10.1080/03007995.2021.1940907Suche in Google Scholar PubMed
© 2023 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Research Articles
- Social factors related to depression during COVID-19
- Prevalence of diaper need and diaper dermatitis and associated risk factors among children aged 1–24 months in a referral hospital in Ghana: A cross-sectional study
- A gravity model approach to understand the spread of pandemics: Evidence from the COVID-19 outbreak
- Raising security of first responders with C-ITS?
- Recomposition of work and attitudes of family assistants within Covid-19 in Poland: A pilot study
- Allelic variants of CYP2B6 gene expression and its implication on the pathogenesis of malaria among a cohort of outpatients in North-Central Nigeria
- Impact of medically supervised fasting on the vitamin D, glycemic control, quality of life and need for medication among type 2 diabetes mellitus: Protocol for a randomized control trial (FAVIT Trial)
- A Tumblr thematic analysis of perinatal health: Where users go to seek support
- Assessing the significance of socioeconomic and demographic factors on COVID-19 cases in Turkey along with the development levels of provinces
- Economic burden and health-related quality of life in patients with neurofibromatosis type 1 in Greece
- Review Articles
- The current status of diversity among physician assistants in surgery: A systematic review
- Planting a path to kidney health: The vegetarian diet and diabetic nephropathy
- Short Communications
- World Heart Day: Clinical case to raise awareness on cardiovascular disease in women
- Preserving sight: Managing and preventing diabetic retinopathy
- The rise of anti-vaccination legislation in two Midwestern US states: Implications for politics, policy, and society
- Letter to the Editor
- Tranexamic acid and pre-hospital trauma setting: Is everything clear by now?
- Pneumocephalus was commonly evident and pneumorrhachis was very commonly evident among our peri-anesthesia patients whose peri-partum neurological symptoms had warranted radiological investigations
- Ventilator-associated pneumonia: Epidemiological changes or disregarded bundles?
- Special Issue on Public Health Resilience - Part I
- Editorial: Special issue on public health resilience
- Message framing, partisanship, and popular support for COVID-19 vaccine mandate for all adults: Evidence from a preregistered survey experiment
Artikel in diesem Heft
- Research Articles
- Social factors related to depression during COVID-19
- Prevalence of diaper need and diaper dermatitis and associated risk factors among children aged 1–24 months in a referral hospital in Ghana: A cross-sectional study
- A gravity model approach to understand the spread of pandemics: Evidence from the COVID-19 outbreak
- Raising security of first responders with C-ITS?
- Recomposition of work and attitudes of family assistants within Covid-19 in Poland: A pilot study
- Allelic variants of CYP2B6 gene expression and its implication on the pathogenesis of malaria among a cohort of outpatients in North-Central Nigeria
- Impact of medically supervised fasting on the vitamin D, glycemic control, quality of life and need for medication among type 2 diabetes mellitus: Protocol for a randomized control trial (FAVIT Trial)
- A Tumblr thematic analysis of perinatal health: Where users go to seek support
- Assessing the significance of socioeconomic and demographic factors on COVID-19 cases in Turkey along with the development levels of provinces
- Economic burden and health-related quality of life in patients with neurofibromatosis type 1 in Greece
- Review Articles
- The current status of diversity among physician assistants in surgery: A systematic review
- Planting a path to kidney health: The vegetarian diet and diabetic nephropathy
- Short Communications
- World Heart Day: Clinical case to raise awareness on cardiovascular disease in women
- Preserving sight: Managing and preventing diabetic retinopathy
- The rise of anti-vaccination legislation in two Midwestern US states: Implications for politics, policy, and society
- Letter to the Editor
- Tranexamic acid and pre-hospital trauma setting: Is everything clear by now?
- Pneumocephalus was commonly evident and pneumorrhachis was very commonly evident among our peri-anesthesia patients whose peri-partum neurological symptoms had warranted radiological investigations
- Ventilator-associated pneumonia: Epidemiological changes or disregarded bundles?
- Special Issue on Public Health Resilience - Part I
- Editorial: Special issue on public health resilience
- Message framing, partisanship, and popular support for COVID-19 vaccine mandate for all adults: Evidence from a preregistered survey experiment


