Abstract
C21H19NO3, monoclinic, P21/n (no. 14), a = 10.137(2) Å, b = 12.3205(18) Å, c = 14.700(3) Å, β = 106.432(1)°, V = 1760.9(6) Å3, Z = 4, Rgt(F) = 0.0511, wRref(F2) = 0.1808, T = 293 K.
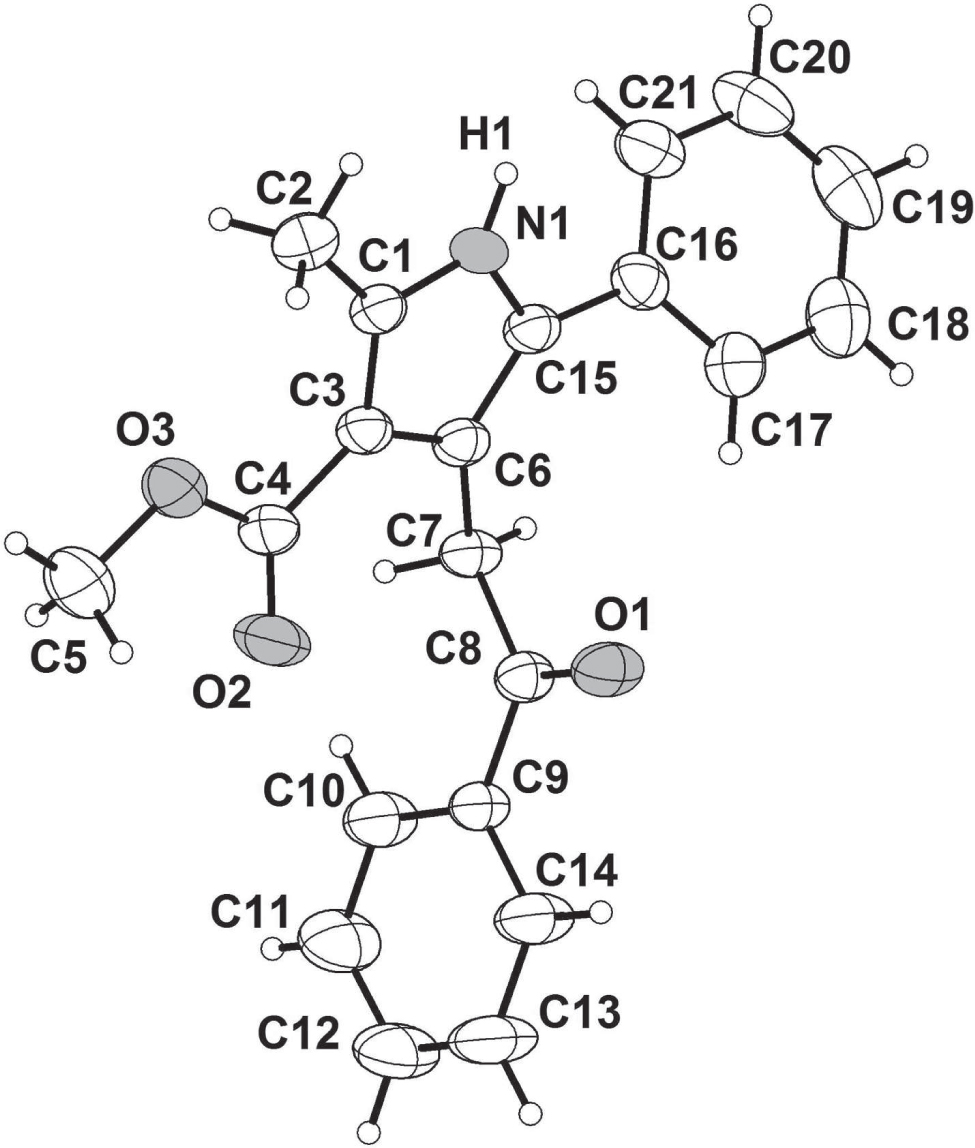
The asymmetric unit of the title crystal structure is shown in the figure. Tables 1 and 2 contain details on crystal structure and measurement conditions and a list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless prism |
| Size: | 0.20 × 0.20 × 0.20 mm |
| Wavelength: | Mo Kα radiation (0.71075 Å) |
| μ: | 0.8 cm−1 |
| Diffractometer, scan mode: | Rigaku CCD, φ and ω |
| 2θmax, completeness: | 55°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 9945, 4030, 0.037 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 2909 |
| N(param)refined: | 226 |
| Programs: | Sir92 [26], Crystal Structure [27], SHELX [28] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| O1 | 0.27793(16) | 0.25321(10) | 1.14220(9) | 0.0550(4) |
| O2 | −0.07988(17) | 0.27786(11) | 1.08119(12) | 0.0637(5) |
| O3 | −0.19384(16) | 0.37749(11) | 1.16108(11) | 0.0575(4) |
| N1 | 0.14082(16) | 0.58495(11) | 1.21518(10) | 0.0401(4) |
| C1 | 0.02765(19) | 0.52695(13) | 1.21638(12) | 0.0388(4) |
| C2 | −0.0593(3) | 0.56050(17) | 1.27746(15) | 0.0535(5) |
| C3 | 0.01822(18) | 0.44248(13) | 1.15224(12) | 0.0368(4) |
| C4 | −0.0863(2) | 0.35798(14) | 1.12689(13) | 0.0427(5) |
| C5 | −0.2987(3) | 0.2949(2) | 1.1419(3) | 0.0741(8) |
| C6 | 0.13031(18) | 0.45162(13) | 1.11203(11) | 0.0364(4) |
| C7 | 0.1487(2) | 0.38174(13) | 1.03292(12) | 0.0391(4) |
| C8 | 0.19889(19) | 0.26766(13) | 1.06336(12) | 0.0380(4) |
| C9 | 0.15498(19) | 0.17520(13) | 0.99565(12) | 0.0393(4) |
| C10 | 0.0592(3) | 0.18690(17) | 0.90722(14) | 0.0571(6) |
| C11 | 0.0190(3) | 0.09870(19) | 0.84799(16) | 0.0722(8) |
| C12 | 0.0732(3) | −0.00235(19) | 0.87543(17) | 0.0706(8) |
| C13 | 0.1666(4) | −0.01561(17) | 0.96217(18) | 0.0751(8) |
| C14 | 0.2075(3) | 0.07227(16) | 1.02163(16) | 0.0630(7) |
| C15 | 0.20615(19) | 0.54096(13) | 1.15281(12) | 0.0371(4) |
| C16 | 0.32817(19) | 0.59416(15) | 1.13870(12) | 0.0415(4) |
| C17 | 0.4330(2) | 0.53439(17) | 1.11884(13) | 0.0492(5) |
| C18 | 0.5490(3) | 0.5857(3) | 1.10801(16) | 0.0635(6) |
| C19 | 0.5626(3) | 0.6963(3) | 1.11660(18) | 0.0709(7) |
| C20 | 0.4596(3) | 0.7563(2) | 1.13637(19) | 0.0705(7) |
| C21 | 0.3427(3) | 0.70704(17) | 1.14685(16) | 0.0573(6) |
| H1 | 0.1685 | 0.6420 | 1.2490 | 0.0481* |
| H2A | −0.1456 | 0.5878 | 1.2385 | 0.0643* |
| H2B | −0.0131 | 0.6163 | 1.3203 | 0.0643* |
| H2C | −0.0756 | 0.4991 | 1.3131 | 0.0643* |
| H5A | −0.3361 | 0.2864 | 1.0746 | 0.0889* |
| H5B | −0.3706 | 0.3158 | 1.1690 | 0.0889* |
| H5C | −0.2593 | 0.2274 | 1.1692 | 0.0889* |
| H7A | 0.2140 | 0.4165 | 1.0052 | 0.0469* |
| H7B | 0.0616 | 0.3770 | 0.9841 | 0.0469* |
| H10 | 0.0218 | 0.2549 | 0.8879 | 0.0685* |
| H11 | −0.0451 | 0.1077 | 0.7891 | 0.0866* |
| H12 | 0.0464 | −0.0615 | 0.8351 | 0.0848* |
| H13 | 0.2027 | −0.0840 | 0.9812 | 0.0901* |
| H14 | 0.2717 | 0.0623 | 1.0803 | 0.0756* |
| H17 | 0.4251 | 0.4593 | 1.1128 | 0.0591* |
| H18 | 0.6184 | 0.5449 | 1.0947 | 0.0762* |
| H19 | 0.6408 | 0.7304 | 1.1091 | 0.0851* |
| H20 | 0.4690 | 0.8313 | 1.1428 | 0.0846* |
| H21 | 0.2734 | 0.7488 | 1.1594 | 0.0687* |
Source of material
Anhydrous InCl3 (22 mg, 10 mmol) and NH4OAc (85 mg, 1.1 mmol) were added to a mixture of 1,2-dibenzoyl ethylene (1 mmol) and a methyl acetoacetate compound (1 mmol) in dry THF (15 mL). The reaction mixture was stirred at room temperature. After complete disappearance of the starting material (monitored by TLC using methanol/chloroform, 1:9), the solvent was evaporated in a rotary evaporator. The reaction mixture was diluted with water (10 mL) and extracted with CHCl3 (25 mL). The organic layer was separated, washed with brine, and then dried over anhydrous Na2SO4. Removal of the solvent resulted in the crude product, which was chromatographed over silica gel using petroleum ether and increasing proportions of chloroform as eluent to get the title compound. The light yellowish crystals of the title compound were obtained by slow evaporation of a solvent solution at room temperature. 319 mg (96%); m.p. 158–160 °C, Rf 0.2 (chloroform/petroleum ether, 3:1). IR (KBr)/cm-1 3249 (N—H), 1702 (C—O), 1658 (C—O), 1446, 1344, 1260, 1220, 1140, 1094. 1H-NMR (400 MHz, CDCl3) δ: 8.40 (br s, NH), 8.06 (d, J = 7.2, 2H, ArH), 7.56 (t, J = 7.6, 1H, ArH), 7.46 (t, J = 8.0, 2H, ArH), 7.31, −7.21 (m, 7H, ArH), 4.42 (s, 2H, −C(O)CH2−), 3.54 (s, 3H, −OCH3), 2.51 (s, 3H, pyrrole ring 2-CH3). 13C-NMR (125 MHz, CDCl3) δ: 199.2, 165.9, 137.5, 136.4, 132.8, 132.0, 129.8, 128.8, 128.5, 128.2, 127.3, 127.2, 115.0, 111.6, 50.4, 36.4, 14.0. Anal. Calc. for C21H19NO3: C 75.66, H 5.74, N 4.20. Found: C 75.61, H 5.71, N 4.19%.
Experimental details
The structure was solved by direct method [26] and expanded using Fourier techniques. Carbon-bound hydrogen atoms were placed in calculated positions (C—H = 0.95 Å for aromatic carbon atoms and C—H = 0.99 Å for methylene groups) and were included in the refinement in the riding model approximation, with Uiso(H) set to 1.2Ueq(C).
Discussion
Tetra-substituted pyrroles were prepared from 1,2-dibenzoyl ethylene and methyl acetoacetate employing NH4OAc as a nitrogen source, through a combination of Michael addition and Paal–Knorr methods [1; 2; 3]. The pyrrole [4; 5; 6; 7] ring is one of the most common skeletal features found in heterocycles and natural products [8; 9; 10]. Generally, pyrroles possess a broad spectrum of biological activities such as antimicrobial [11], telomerase inhibitory [12], antifungal [13], cardiotonic [14], pheromonal [15], and phytotoxic effects [16]. The classical approaches for synthesizing pyrroles are Knorr [17; 18], Hantzsch [19; 20; 21], and Paal-Knorr [1; 2; 3] methods. A whole new library of pyrrole derivatives can thus be prepared from commercially available starting materials. Typical examples of such derivatives reported being; Ethyl 2,5-di-tert-butyl-5-ethoxy-4-oxo-4,5-dihydro-1H-pyrrole-3-carboxylate [22], Ethyl 1,4-bis(4-chlorophenyl)-2-methyl-1H-pyrrole-3-carboxylate [23], Methyl 1-benzyl-5-methyl-2,4-diphenyl-1H-pyrrole-3-carboxylate [24] and 4-(3,3,4,4,5,5-hexafluoro-2-(5-(4-methoxyphenyl)-2-methylthiophene-3-yl)cyclopent-1-enyl)-1,5-dimethyl-1H-pyrrole-2-carbonitrile [25]. All bond lengths and angle in the title molecule (cf. the figure) are in the normal ranges.
Acknowledgements
The authors gratefully acknowledge financial assistance from the University Research Council of the University of Johannesburg.
References
1 Bharadwaj, A. R.; Scheidt, K. A.: Catalytic multicomponent synthesis of highly substituted pyrroles utilizing a one-pot Sila-Stetter/Paal-Knorr strategy. Org. Lett. 6 (2004) 2465–2468.10.1021/ol049044tSearch in Google Scholar PubMed
2 Minetto, G.; Raveglia, L. F.; Taddei, M.: Microwave-assisted Paal-Knorr reaction. A rapid approach to substituted pyrroles and furans. Org. Lett. 6 (2004) 389–392.10.1021/ol0362820Search in Google Scholar PubMed
3 Banik, B. K.; Samajdar, S.; Banik, I. J.: Simple synthesis of substituted pyrroles. J. Org. Chem. 69 (2004) 213–216.10.1021/jo035200iSearch in Google Scholar PubMed
4 Toube, T. P.: Acylpyrroles. In: Chemistry of heterocyclic compounds: pyrroles, part 2: The synthesis, reactivity, and physical properties of substituted pyrroles, Volume 48 (Ed. R. A. Jones), p. 1–129, Wiley, New York, NY, 1992.10.1002/9780470187340.ch1Search in Google Scholar
5 Sundberg, R. J.: In Comprehensive Heterocyclic Chemistry; Katritzky, A. R.; Rees, C. W.; Scriven, E. F. V.; Eds.; Pergamon: Oxford, vol-2 (1996) 119.Search in Google Scholar
6 Gilchrist, T. L.: Heterocyclic Chemistry, 3rd edition Addison Wesley Longman, Essex, 1997.Search in Google Scholar
7 Joule, J. A.; Mills, K.: Heterocyclic Chemistry, Blackwell Science: Oxford, 2000; Chapter 13.Search in Google Scholar
8 Fan, H.; Peng, J.; Hamann, M. T.; Hu, J. F.: Lamellarins and related pyrrole-derived alkaloids from marine organisms. Chem. Rev. 108 (2008) 264–287.10.1021/cr078199mSearch in Google Scholar PubMed PubMed Central
9 Gribble, G. W.: In Comprehensive Heterocyclic Chemistry; Katritzky, A. R.; Rees, C. W.; Scriven, E. F. V.: Eds.; Pergamon: Oxford. Vol. 2, 207–257, 1996.Search in Google Scholar
10 Fürstner, A.: Chemistry and biology of roseophilin and the prodigiosin alkaloids: A survey of the last 2500 years. Angew. Chem. Int. Ed. 42 (2003) 3582–3603.10.1002/anie.200300582Search in Google Scholar PubMed
11 Raimondi, M. V.; Cascioferro, S.; Schillaci, D.; Petruso, S.: Synthesis and antimicrobial activity of new bromine-rich pyrrole derivatives related to monodeoxypyoluteorin. Eur. J. Med. Chem. 41 (2006) 1439–1445.10.1016/j.ejmech.2006.07.009Search in Google Scholar PubMed
12 Shi, D. F.; Wheelhouse, R. T.; Sun, D. Y.; Hurley, L. H.: Quadruplex-interactive agents as telomerase inhibitors: Synthesis of porphyrins and structure activity relationship for the inhibition of telomerase. J. Med. Chem. 44 (2001) 4509–4523.10.1021/jm010246uSearch in Google Scholar PubMed
13 Onnis, V.; De Logu, A.; Cocco, M. T.; Fadda, R.; Meleddu, R.; Congiu, C.: 2-Acylhydrazino-5-arylpyrrole derivatives: synthesis and antifungal activity evaluation. Eur. J. Med. Chem. 44 (2009) 1288–1295.10.1016/j.ejmech.2008.08.003Search in Google Scholar PubMed
14 Obame, F. N.; Plin-Mercier, C.; Assaly, R.; Zini, R.; DuboisRande, J. L.; Berdeaux, A.; Morin, D.: Cardioprotective effect of morphine and a blocker of glycogen synthase kinase 3 beta SB216763 [3-(2,4-dichlorophenyl)-4(1-methyl-1H-indol-3-yl)-1H-pyrrole-2,5-dione], via inhibition of the mitochondrial permeability transition pore. J. Pharmacol. Exp. Ther. 326 (2008) 252–258.10.1124/jpet.108.138008Search in Google Scholar PubMed
15 Caputo, J. F.; Caputo, R. E.; Brand, J. M.: Significance of the pyrrolic nitrogen atom in receptor recognition of Atta texana (Buckley) (Hymenoptera:Formicidae) trail pheromone and parapheromones. J. Chem. Ecol. 5 (1979) 273–278.10.1007/BF00988241Search in Google Scholar
16 Zhao, Y.; Li, Y.; Ou, X.; Zhang, P.; Huang, Z.; Bi, F.; Huang, R.; Wang, Q.: Synthesis, insecticidal, and acaricidal activities of novel 2-aryl-pyrrole derivatives containing ester groups. J. Agric. Food Chem. 56 (2008) 10176–10182.10.1021/jf802464dSearch in Google Scholar
17 Alberola, A.; Ortega, A. G.; Sadaba, M. L.; Sanudo, C.: Versatility of Weinreb amides in the Knorr pyrrole synthesis. Tetrahedron. 55 (1999) 6555–6566.10.1016/S0040-4020(99)00289-6Search in Google Scholar
18 Castro, A. J.; Giannini, D. D.; Greenlee, W. F.: Synthesis of a 2,3′-bipyrrole. Denitrosation in the Knorr pyrrole synthesis. J. Org. Chem. 35 (1970) 2815–2816.10.1021/jo00833a080Search in Google Scholar
19 Hong, D.; Zhu, Y.; Li, Y.; Lin, X.; Lu, P.; Wang, Y.: Three-component synthesis of polysubstituted pyrroles from a-diazoketones, nitroalkenes, and amines. Org. Lett. 13 (2011) 4668–4671.10.1021/ol201891rSearch in Google Scholar
20 Matiychuk, V. S.; Martyak, R. L.; Obushak, N. D.; Ostapiuk, Y. V.; Pidlypnyi, N. I.: 3-Aryl-2-Chloropropanals in Hantzsch synthesis of pyrroles. Chem. Heterocycl. Comp. 40 (2004) 1218–1219.10.1023/B:COHC.0000048299.17625.7fSearch in Google Scholar
21 Palacios, F.; Aparico, D.; de los Santos, J. M.; Vicario, J.: Regioselective alkylation reactions of hydrazones derived from phosphine oxides and phosphonates. Synthesis of phosphorus substituted 1-amino-pyrrolones, pyridinones and pyrroles. Tetrahedron 57 (2001) 1961–1972.10.1016/S0040-4020(01)00033-3Search in Google Scholar
22 Rosen, G. M.; Muralidharan, S.; Zavalij, P. Y.; Fletcher, S.; Kao, J. P.: Ethyl 2,5-di-tert-butyl-5-ethoxy-4-oxo-4,5-dihydro-1H-pyrrole-3-carboxylate. Acta Crystallogr. E69 (2013) o878.10.1107/S1600536813012282Search in Google Scholar PubMed PubMed Central
23 Nandeesh, K. N.; Chandra, Mahendra, M.; Palani, K.; Mantelingu, K.: Ethyl 1,4-bis(4-chlorophenyl)-2-methyl-1H-pyrrole-3-carboxylate. Acta Crystallogr. E69 (2013) o1269.10.1107/S1600536813019247Search in Google Scholar PubMed PubMed Central
24 Lopchuk, J. M.; Gribble, G. W.; Jasinski, J. P.: Methyl 1-benzyl-5-methyl-2,4-diphenyl-1H-pyrrole-3-carboxylate. Acta Crystallogr. E70 (2014) o338–o33910.1107/S1600536814003316Search in Google Scholar PubMed PubMed Central
25 Pu, S.; Liu, G.; Shen, L.; Xu, J.: Efficient synthesis and properties of isomeric photochromic diarylethenes having a pyrrole unit. Org. Lett. 9 (2007) 2139–2142.10.1021/ol070622qSearch in Google Scholar PubMed
26 SIR92: Altomare, A.; Cascarano, G.; Giacovazzo, C.; Guagliardi, A.; Burla, M.; Polidori, G.; Camalli, M.: J. Appl. Cryst. 27 (1994) 435.10.1107/S0021889894000221Search in Google Scholar
27 Crystal Structure 4.0: Crystal Structure Analysis Package, Rigaku Corporation. Tokyo 196-8666, Japan, 2000–2010.Search in Google Scholar
28 Sheldrick, G. M.: A short history of SHELX. Acta Crystallogr. A64 (2008) 112–122.10.1107/S0108767307043930Search in Google Scholar PubMed
©2016 Gadada Naganagowda et al., published by De Gruyter.
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 3.0 License.
Articles in the same Issue
- Cover and Frontmatter
- Editorial
- Twenty years of crystal structure publication and the road ahead
- Crystal Structures
- Crystal structure of poly-[triaqua-(μ4-5′-carboxy-[1,1′-biphenyl]-2,3,3′-tricarboxylate-κ6O1,O2:O3,O4:O5:O6)praseodymium(III), C16H13O11Pr
- Crystal structure of (R)-1-(2,3-dihydro-1H-pyrrolizin-5-yl)-2,3-dihydroxypropan-1-one, C10H13NO3
- Crystal structure of (E)-4-nitro-2-((2-phenoxyphenylimino)methyl)phenol, C19H14N2O4
- Crystal structure of 3,3′-di(furan-2-yl)-5,5′-bi-1,2,4-triazine
- Crystal structure of 11-(p-coumaroyloxy)-tremetone, C22H20O5
- The crystal structure of 1,3-bis(2,6-diiso-propylphenyl)imidazol-2-ylidene)-dibromido-(1-methyl-1H-imidazole-κ1N)palladium(II) – ethyl acetate – water (1/1/1), C31H42Br2N4Pd
- Crystal structure of 2-((3-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)-1-phenyl-1H-pyrazol-4-yl)methylene)-1H-indene-1,3(2H)-dione, C28H19N5O2
- Crystal structure of 2-(5-(4-fluorophenyl)-3-p-tolyl-4,5-dihydro-1H-pyrazol-1-yl)-4-(5-methyl-1-p-tolyl-1H-1,2,3-triazol-4-yl)thiazole, C29H25FN6S
- Crystal structure of poly-[aqua-(μ7-benzene-1,3,5-tricarboxylato)-(μ3-1,2,4-triazol-1-ido)dicobalt(II)], C11H7Co2N3O7
- Crystal constructure of 16(S)-methyl-6α-carboxy-1, 15-dioxo-6, 7-seco-ent-kaur-2-en-7, 20-olide, C20H24O6
- Crystal structure of 1-(benzo[d]thiazol-2-yl)-3-phenylthiourea, C14H11N3S2
- Crystal structure of 3-(2-bromophenyl)-1,1-dimethylthiourea, C9H11BrN2S
- Crystal structure of 1-(adamantan-1-yl)-3-(3-chlorophenyl)thiourea, C17H21ClN2S
- Crystal structure of 3-(adamantan-1-yl)-1-(4-bromophenyl)urea, C17H21BrN2O
- Crystal structure of (Z)-Ethyl 2-cyano-2-(3-phenylthiazolidin-2-ylidene) acetate, C14H14N2O2S
- Crystal structure of methyl 2b-ethyl-1a,2a,2b,2b1,3,5,10,11-octahydro-1H-oxireno[2′,3′:6,7]indolizino[8,1-cd]carbazole-4-carboxylate, C21H24N2O3
- Crystal structure of 2-amino-5-oxo-4-(3,4,5-trimethoxy-phenyl)-4,5,6,7-tetrahydro-cyclopenta[b]pyran-3-carbonitrile, C18H18N2O5
- Crystal structure of 1,2,3-trimethyl-2,3-dihydro-1H-perimidine, C14H16N2
- Crystal structure of bis(2,6-dihydroxymethyl)pyridine-κ3N,O,O′)-bis(μ2-6-chloropyridin-2-olato-κ3N,O:O)-bis(6-chloropyridin-2-olato-κO)-bis(nitrato-κ2O,O′)digadolinium(III), C34H30Cl4Gd2N8O14
- Crystal structure of 8-isopropyl-8-aza-bicyclo[3.2.1]octan-3-yl 3-hydroxy-2-phenylpropanoate, C19H27NO3
- Crystal structure of 1-methyl-3-[((naphthalen-2-ylsulfonyl)oxy)imino]indolin-2-one, C19H14N2O4S
- Crystal structure (7,8-bis(diisopropylphosphino)-7,8-dicarba-nido-undecaborane-κ2P,P′)-(benzoato-κ2O,O′)nickel(II), C21H42B9NiO2P2
- Crystal structure of methyl-2-methyl-4-(2-oxo-2-phenylethyl)-5-phenyl-1H-pyrrole-3-carboxylate, C21H19NO3
- Crystal structure of 2-[(2-oxo-thiazolidine-3-carbonyl)sulfamoyl]-methy-benzoic acid methyl ester, C13H14N2O6S2
- Crystal structure of N′-(2-phenylacetyl)thiophene-2-carbohydrazide monohydrate, C13H14N2O3S
- Crystal structure of 1,1′-(hexane-1,6-diyl)bis(3-methyl-1H-imidazol-3-ium) bis(hexafluoro phosphate), C14H24F12N4P2
- Crystal structure of di-μ-chlorido-bis[1,2-bis(dicyclohexylphosphino)-1,2-dicarba-closo-dodecaborane-κ2P,P′]zinc(II), C52H108B20Cl2P4Zn2
- Crystal structure of dibromido-bis[μ-1-[(2-methyl-1H-benzoimidazol-1-yl)methyl]-1H-benzotriazole-κN]mercury(II), C30H26Br2HgN10
- Crystal structure of bis(μ-nitrato-κ2O:O)-bis[1,2-bis(diphenylphosphino)-1,2-dicarba-closo-dodecaborane-κ2P,P′]disilver(I) dicloromethane monosolvate, C54H64B20Cl4O6P4Ag2
- Crystal structure of dinuclear dichloridobis(dimethylformamide-kO)bis[μ2-3-(2-oxyphenyl)-5-(pyrazin-2-yl)-1,2,4-triazol-1ido-κ4-O,N:N′,N′′(2−)]diiron(III) − dimethylformamide (1/1), C36H42Cl2Fe2N14O6
- Crystal structure of diaqua-dinitrato-κO-bis(4-(1H-pyrazol-3-yl)pyridine-κN)manganese(II), C16H18MnN8O8
- Crystal structure of (Z)-6-methoxy-2-(2,2,2-trifluoro-1-hydroxyethylidene)-2,3-dihydro-1H-inden-1-one, C12H6F6O3
- Crystal Structure of 4-(2-chloroacetamido)pyridinium chloride monohydrate, C7H10Cl2N2O2
- Crystal structure of 2-amino-4-(4-chloro-phenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile, C16H13ClN2O2
- Crystal structure of (E)-1-(2-(thiophen-2-ylmethylene)hydrazinyl)phthalazine hydrochloride–ethanol (1/1), C15H17ClN4OS
- Crystal structure of N,N-diethyl-5-bromo-3,4-dihydro-2,4-dioxopyrimidine-1(2H)-carboxamide, C9H12BrN3O3
- Crystal structure of 3-(2-(4-chlorophenyl)-3-hydroxy-3,3-diphenylpropyl)-1,1-dimethylurea, C24H25ClN2O2
- Crystal structure of 3-(4-chlorophenyl)-1,1-dimethylthiourea, C9H11ClN2S
- Crystal structure of 2-amino-4-(4-bromo-phenyl)-7-methyl-5-oxo-4H,5H-pyrano[4,3-b]pyran-3-carbonitrile, C16H11BrN2O3
- Crystal structure of 4-(3,4-dimethyl-phenyl)-2-methyl-5-oxo-1,4,5,6,7,8-hexahydro-quinoline-3-carboxylic acid ethyl ester, C21H25NO3
- Crystal structure of (E)-2-({4-hydroxy-5-methoxy-3-[(4-methyl-1-piperazinyl)methyl]phenyl} methylidene)-1-indanone, C23H26N2O3
- Crystal structure of tripropylammonium 2′-carboxy-[1,1′-biphenyl]-2-carboxylate – [1,1′-biphenyl]-2,2′-dicarboxylic acid (2/1), C60H72N2O12
- Crystal structure of catena-poly-{aqua-[μ2-1,2-bis((1H-imidazol-1-yl)methyl)benzene-κ2N:N′]-[μ2-4,4′-(dimethylsilanediyl)dibenzato-κ3O,O′:O′]nickel(II)}, C30H30N4NiO5Si
- The crystal structure of 1-(4-bromophenyl)-2-(4-(4-fluorophenyl)piperazin-1-yl)ethanol, C18H20BrFN2O1
- Crystal structure of trimethylammonium 4-((4-carboxyphenyl)sulfonyl)benzoate, C17H19NO6S
- Crystal structure of syn-2,4-di-o-tolylpentane-2,4-diol, C19H24O2
- Crystal structure of 2-[3,5-bis(trifluoromethyl)benzylsulfanyl]-5-(5-bromothiophen-2-yl)-1,3,4-oxadiazole, C15H7BrF6N2OS2
- Crystal structure of (E)-3-((naphthalen-1-ylimino)methyl)-4-nitrophenol, C17H12N2O3
- Crystal structure of 2-dichloromethyl-2-p-nitrophenyl-1,3-dioxolane, C10H9Cl2NO4
- Crystal structure of (1,4,8,11-tetraazacyclotetradecane)palladium(II) tetracyanopalladate(II), C14H24N8Pd2
- Crystal structure of 2-(4-oxo-2-thioxothiazolidin-3-yl)acetic acid monohydrate, C5H7NO4S2
- Crystal structure of a P4-bridged (η5-pentamethyl-cyclopentadienyl)(η5-adamantylcyclopentadienyl) titanium(III)complex, C50H66P4Ti2
- Crystal structure of cis-bis(2,2′-bipyrimidine-κ2N,N′)bis(thiocyanato-κN)nickel(II), C18H12N10NiS2
- Crystal structure of cis-bis(2,2′-bipyridine-κ2N,N′)dibromidomanganese(II), C20H16Br2MnN4
- Crystal structure of cis-bis(2,2′-bipyridine-κ2N,N′)bis(thiocyanato-κN)nickel(II), C22H16N6NiS2
- Crystal structure of trans-dibromido(1,4,8,11-tetraazacyclotetradecane)nickel(II), C10H24Br2N4Ni
- Crystal structure of cis-tetrabromidobis(pyridine-κN)platinum(IV), C10H10Br4N2Pt
- Crystal structure of (E)-5-((4-chlorophenyl)diazenyl)-2-(5-(4-fluorophenyl)-3-(thiophen-2-yl)-4,5-dihydro-1H-pyrazol-1-yl)-4-methylthiazole, C23H17ClFN5S2
- The crystal structure of 3-((1R,2S)-1-methylpyrrolidin-1-ium-2-yl)pyridin-1-ium tetrachloridocobaltate(II) monohydrate, C10H18Cl4CoN2O
Articles in the same Issue
- Cover and Frontmatter
- Editorial
- Twenty years of crystal structure publication and the road ahead
- Crystal Structures
- Crystal structure of poly-[triaqua-(μ4-5′-carboxy-[1,1′-biphenyl]-2,3,3′-tricarboxylate-κ6O1,O2:O3,O4:O5:O6)praseodymium(III), C16H13O11Pr
- Crystal structure of (R)-1-(2,3-dihydro-1H-pyrrolizin-5-yl)-2,3-dihydroxypropan-1-one, C10H13NO3
- Crystal structure of (E)-4-nitro-2-((2-phenoxyphenylimino)methyl)phenol, C19H14N2O4
- Crystal structure of 3,3′-di(furan-2-yl)-5,5′-bi-1,2,4-triazine
- Crystal structure of 11-(p-coumaroyloxy)-tremetone, C22H20O5
- The crystal structure of 1,3-bis(2,6-diiso-propylphenyl)imidazol-2-ylidene)-dibromido-(1-methyl-1H-imidazole-κ1N)palladium(II) – ethyl acetate – water (1/1/1), C31H42Br2N4Pd
- Crystal structure of 2-((3-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)-1-phenyl-1H-pyrazol-4-yl)methylene)-1H-indene-1,3(2H)-dione, C28H19N5O2
- Crystal structure of 2-(5-(4-fluorophenyl)-3-p-tolyl-4,5-dihydro-1H-pyrazol-1-yl)-4-(5-methyl-1-p-tolyl-1H-1,2,3-triazol-4-yl)thiazole, C29H25FN6S
- Crystal structure of poly-[aqua-(μ7-benzene-1,3,5-tricarboxylato)-(μ3-1,2,4-triazol-1-ido)dicobalt(II)], C11H7Co2N3O7
- Crystal constructure of 16(S)-methyl-6α-carboxy-1, 15-dioxo-6, 7-seco-ent-kaur-2-en-7, 20-olide, C20H24O6
- Crystal structure of 1-(benzo[d]thiazol-2-yl)-3-phenylthiourea, C14H11N3S2
- Crystal structure of 3-(2-bromophenyl)-1,1-dimethylthiourea, C9H11BrN2S
- Crystal structure of 1-(adamantan-1-yl)-3-(3-chlorophenyl)thiourea, C17H21ClN2S
- Crystal structure of 3-(adamantan-1-yl)-1-(4-bromophenyl)urea, C17H21BrN2O
- Crystal structure of (Z)-Ethyl 2-cyano-2-(3-phenylthiazolidin-2-ylidene) acetate, C14H14N2O2S
- Crystal structure of methyl 2b-ethyl-1a,2a,2b,2b1,3,5,10,11-octahydro-1H-oxireno[2′,3′:6,7]indolizino[8,1-cd]carbazole-4-carboxylate, C21H24N2O3
- Crystal structure of 2-amino-5-oxo-4-(3,4,5-trimethoxy-phenyl)-4,5,6,7-tetrahydro-cyclopenta[b]pyran-3-carbonitrile, C18H18N2O5
- Crystal structure of 1,2,3-trimethyl-2,3-dihydro-1H-perimidine, C14H16N2
- Crystal structure of bis(2,6-dihydroxymethyl)pyridine-κ3N,O,O′)-bis(μ2-6-chloropyridin-2-olato-κ3N,O:O)-bis(6-chloropyridin-2-olato-κO)-bis(nitrato-κ2O,O′)digadolinium(III), C34H30Cl4Gd2N8O14
- Crystal structure of 8-isopropyl-8-aza-bicyclo[3.2.1]octan-3-yl 3-hydroxy-2-phenylpropanoate, C19H27NO3
- Crystal structure of 1-methyl-3-[((naphthalen-2-ylsulfonyl)oxy)imino]indolin-2-one, C19H14N2O4S
- Crystal structure (7,8-bis(diisopropylphosphino)-7,8-dicarba-nido-undecaborane-κ2P,P′)-(benzoato-κ2O,O′)nickel(II), C21H42B9NiO2P2
- Crystal structure of methyl-2-methyl-4-(2-oxo-2-phenylethyl)-5-phenyl-1H-pyrrole-3-carboxylate, C21H19NO3
- Crystal structure of 2-[(2-oxo-thiazolidine-3-carbonyl)sulfamoyl]-methy-benzoic acid methyl ester, C13H14N2O6S2
- Crystal structure of N′-(2-phenylacetyl)thiophene-2-carbohydrazide monohydrate, C13H14N2O3S
- Crystal structure of 1,1′-(hexane-1,6-diyl)bis(3-methyl-1H-imidazol-3-ium) bis(hexafluoro phosphate), C14H24F12N4P2
- Crystal structure of di-μ-chlorido-bis[1,2-bis(dicyclohexylphosphino)-1,2-dicarba-closo-dodecaborane-κ2P,P′]zinc(II), C52H108B20Cl2P4Zn2
- Crystal structure of dibromido-bis[μ-1-[(2-methyl-1H-benzoimidazol-1-yl)methyl]-1H-benzotriazole-κN]mercury(II), C30H26Br2HgN10
- Crystal structure of bis(μ-nitrato-κ2O:O)-bis[1,2-bis(diphenylphosphino)-1,2-dicarba-closo-dodecaborane-κ2P,P′]disilver(I) dicloromethane monosolvate, C54H64B20Cl4O6P4Ag2
- Crystal structure of dinuclear dichloridobis(dimethylformamide-kO)bis[μ2-3-(2-oxyphenyl)-5-(pyrazin-2-yl)-1,2,4-triazol-1ido-κ4-O,N:N′,N′′(2−)]diiron(III) − dimethylformamide (1/1), C36H42Cl2Fe2N14O6
- Crystal structure of diaqua-dinitrato-κO-bis(4-(1H-pyrazol-3-yl)pyridine-κN)manganese(II), C16H18MnN8O8
- Crystal structure of (Z)-6-methoxy-2-(2,2,2-trifluoro-1-hydroxyethylidene)-2,3-dihydro-1H-inden-1-one, C12H6F6O3
- Crystal Structure of 4-(2-chloroacetamido)pyridinium chloride monohydrate, C7H10Cl2N2O2
- Crystal structure of 2-amino-4-(4-chloro-phenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile, C16H13ClN2O2
- Crystal structure of (E)-1-(2-(thiophen-2-ylmethylene)hydrazinyl)phthalazine hydrochloride–ethanol (1/1), C15H17ClN4OS
- Crystal structure of N,N-diethyl-5-bromo-3,4-dihydro-2,4-dioxopyrimidine-1(2H)-carboxamide, C9H12BrN3O3
- Crystal structure of 3-(2-(4-chlorophenyl)-3-hydroxy-3,3-diphenylpropyl)-1,1-dimethylurea, C24H25ClN2O2
- Crystal structure of 3-(4-chlorophenyl)-1,1-dimethylthiourea, C9H11ClN2S
- Crystal structure of 2-amino-4-(4-bromo-phenyl)-7-methyl-5-oxo-4H,5H-pyrano[4,3-b]pyran-3-carbonitrile, C16H11BrN2O3
- Crystal structure of 4-(3,4-dimethyl-phenyl)-2-methyl-5-oxo-1,4,5,6,7,8-hexahydro-quinoline-3-carboxylic acid ethyl ester, C21H25NO3
- Crystal structure of (E)-2-({4-hydroxy-5-methoxy-3-[(4-methyl-1-piperazinyl)methyl]phenyl} methylidene)-1-indanone, C23H26N2O3
- Crystal structure of tripropylammonium 2′-carboxy-[1,1′-biphenyl]-2-carboxylate – [1,1′-biphenyl]-2,2′-dicarboxylic acid (2/1), C60H72N2O12
- Crystal structure of catena-poly-{aqua-[μ2-1,2-bis((1H-imidazol-1-yl)methyl)benzene-κ2N:N′]-[μ2-4,4′-(dimethylsilanediyl)dibenzato-κ3O,O′:O′]nickel(II)}, C30H30N4NiO5Si
- The crystal structure of 1-(4-bromophenyl)-2-(4-(4-fluorophenyl)piperazin-1-yl)ethanol, C18H20BrFN2O1
- Crystal structure of trimethylammonium 4-((4-carboxyphenyl)sulfonyl)benzoate, C17H19NO6S
- Crystal structure of syn-2,4-di-o-tolylpentane-2,4-diol, C19H24O2
- Crystal structure of 2-[3,5-bis(trifluoromethyl)benzylsulfanyl]-5-(5-bromothiophen-2-yl)-1,3,4-oxadiazole, C15H7BrF6N2OS2
- Crystal structure of (E)-3-((naphthalen-1-ylimino)methyl)-4-nitrophenol, C17H12N2O3
- Crystal structure of 2-dichloromethyl-2-p-nitrophenyl-1,3-dioxolane, C10H9Cl2NO4
- Crystal structure of (1,4,8,11-tetraazacyclotetradecane)palladium(II) tetracyanopalladate(II), C14H24N8Pd2
- Crystal structure of 2-(4-oxo-2-thioxothiazolidin-3-yl)acetic acid monohydrate, C5H7NO4S2
- Crystal structure of a P4-bridged (η5-pentamethyl-cyclopentadienyl)(η5-adamantylcyclopentadienyl) titanium(III)complex, C50H66P4Ti2
- Crystal structure of cis-bis(2,2′-bipyrimidine-κ2N,N′)bis(thiocyanato-κN)nickel(II), C18H12N10NiS2
- Crystal structure of cis-bis(2,2′-bipyridine-κ2N,N′)dibromidomanganese(II), C20H16Br2MnN4
- Crystal structure of cis-bis(2,2′-bipyridine-κ2N,N′)bis(thiocyanato-κN)nickel(II), C22H16N6NiS2
- Crystal structure of trans-dibromido(1,4,8,11-tetraazacyclotetradecane)nickel(II), C10H24Br2N4Ni
- Crystal structure of cis-tetrabromidobis(pyridine-κN)platinum(IV), C10H10Br4N2Pt
- Crystal structure of (E)-5-((4-chlorophenyl)diazenyl)-2-(5-(4-fluorophenyl)-3-(thiophen-2-yl)-4,5-dihydro-1H-pyrazol-1-yl)-4-methylthiazole, C23H17ClFN5S2
- The crystal structure of 3-((1R,2S)-1-methylpyrrolidin-1-ium-2-yl)pyridin-1-ium tetrachloridocobaltate(II) monohydrate, C10H18Cl4CoN2O


