Abstract
C16H18Cl2N4O2, monoclinic, P21/c (no. 14), a = 8.8369(3) Å, b = 11.4750(4) Å, c = 8.8258(3) Å, β = 95.958(2)°, V = 890.1 Å3, Z = 2, Rgt(F) = 0.0316, wRref(F2) = 0.0941, T = 200 K.
Source of material
The compound was prepared upon reacting the two-fold symmeytric benzimidazole derived from 2,3-dimercaptotartaric acid with ReOCl3(PPh3)2 in acetonitrile. Crystals suitable for the diffraction study were obtained upon free evaporation of the solvent at ambient conditions.
Experimental details
Carbon-bound H atoms were placed in calculated positions (C–H 0.95 Å) and were included in the refinement in the riding model approximation, with Uiso(H) set to 1.2Ueq(C). Both nitrogen-bound H atoms as well as the H atoms of the water molecule were located on a difference Fourier map and refined freely applying DFIX and DANG instructions for the water molecule.
Data collection and handling.
| Crystal: | Brown platelets, |
| size 0.136×0.26×0.597 mm | |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 3.81 cm−1 |
| Diffractometer, scan mode: | Bruker APEX-II CCD, φ and ω |
| 2θmax: | 56.64° |
| N(hkl)measured, N(hkl)unique: | 15586, 2221 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 1977 |
| N(param)refined: | 125 |
| Programs: | SHELX, WinGX, MERCURY, |
| PLATON [8–11] |
Atomic coordinates and displacement parameters (in Å2).
| Atom | Site | x | y | z | Uiso |
|---|---|---|---|---|---|
| H(2A) | 4e | 0.0843 | 0.4155 | 0.0925 | 0.049 |
| H(13) | 4e | 0.4848 | 0.6821 | 0.4443 | 0.045 |
| H(14) | 4e | 0.5256 | 0.8843 | 0.4551 | 0.053 |
| H(15) | 4e | 0.3779 | 1.0113 | 0.2984 | 0.053 |
| H(16) | 4e | 0.1843 | 0.9434 | 0.1185 | 0.044 |
| H(1) | 4e | 0.059(2) | 0.730(2) | 0.021(2) | 0.044(5) |
| H(2) | 4e | 0.275(2) | 0.525(2) | 0.282(2) | 0.044(5) |
| H(1A) | 4e | 0.245(2) | 0.362(2) | 0.443(2) | 0.060(6) |
| H(1B) | 4e | 0.267(2) | 0.318(1) | 0.306(2) | 0.064(7) |
Discussion
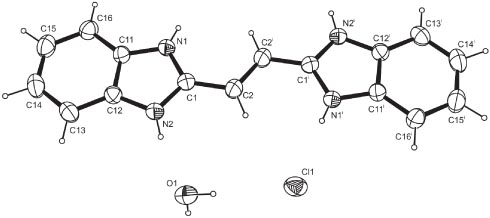
Next to cardiovascular diseases, cancer has become one of the main fatal diseases in industrialized countries. Apart from classical surgery, chemo- and radiotherapeutic treatments have entered the arsenal of possible cures for certain types of cancer. All methods, however, suffer from their own set of problematic side-effects and, as a consequence, the development of radiopharmaceuticals – combining the advantages of chemotherapy as well as radiation methods while at the same time avoiding their unique respective undesired side-effects – has been a topic of research [1, 2]. Tailoring and fine-tuning of the envisioned radiopharmaceuticals’ properties such as lipophilicity and, in particular, inertness is of paramount importance with respect to possible future in vivo applications in contemporary medicine and requires sound knowledge about structural parameters of the ligands applied if a more heuristic approach in the synthesis is to triumph over pure trial-and-error as it is encountered in this specific field of coordination chemistry up to the present day. In addition, the spatial requirements of pharmaceuticals are of importance as this factor influences on their interaction with enzymatic systems and, as a consequence, the pathway and rate of conjugation, functionalization and secretion from the body. In continuation of our ongoing research on the field of rhenium coordination compounds, the coordination of a multidentate ligand towards a rhenium(V) starting material was attempted. A structural analysis of the crystalline reaction product showed the formation of an unexpected compound. The crystal structures of the hydrochloride and hydrobromide of a simpler derivative of benzimidazole have been reported earlier [3]. The title compound is the hydrochloride salt of a symmetric derivative of ethylene bearing a benzimidazole substituent on each carbon atom. The asymmetric unit contains half a molecule of the organic cation, a chloride counterion as well as a molecule of water. The central C=C double bond is (E)-configured. Protonation occurred on the – formally – sp2-hybridized nitrogen atoms in each substituent. The molecule is essentially planar (r.m.s of all fitted non-hydrogen atoms in the asymmetric unit = 0.0122 Å) with one of the two nitrogen atoms deviating most from the least-squares plane by 0.0273(10) Å. The central C=C bond is measured at 1.378(3) Å which is in good agreement with the values for other ethylene derivatives whose metrical parameters have been deposited with the Cambridge Structural Database [4]. In the crystal, classical hydrogen bonds of the N–H⋯Cl and the O–H⋯Cl type are observed next to C–H⋯O and C–H⋯Cl contacts whose range invariably falls below the sum of van-der-Waals radii of the atoms participating in them [5]. While both hydrogen bonds established by the water molecule are aimed towards the chloride anion as acceptor, the N–H supported hydrogen bonds apply both – the chloride anion as well as the oxygen atom of the water molecule – as acceptors. The C–H⋯Cl contacts are established by the hydrogen atoms of the central ethylene scaffold and, therefore, see the chloride anion chelated by an N–H as well as a C–H contact. A second C–H⋯Cl interaction stems from one of the hydrogen atoms in ortho position to the annealed heterocyclic moiety on the phenyl group while the other such hydrogen atom gives rise to C–H⋯O contacts. In total, the cations are connected to sheets parallel [101] that are extended to a three dimensional network by means of further chloride- and water-supported contacts. In terms of graph-set analysis [6, 7], the descriptor for the classical hydrogen bonds is DDDD on the unary level while the C–H⋯X contacts necessitate a DD descriptor on the same level. The shortest intercentroid distance in between two centers of gravity was measured at 3.7863(8) Å and is apparent in between the two different aromatic moieties in neighbouring molecules.
Atomic coordinates and displacement parameters (in Å2).
| Atom | Site | x | y | z | U11 | U22 | U33 | U12 | U13 | U23 |
|---|---|---|---|---|---|---|---|---|---|---|
| Cl(1) | 4e | 0.12164(4) | 0.18436(3) | 0.14845(4) | 0.0478(2) | 0.0346(2) | 0.0368(2) | 0.0111(1) | 0.0006(1) | 0.0026(1) |
| O(1) | 4e | 0.2931(1) | 0.37220(9) | 0.3675(1) | 0.0505(6) | 0.0385(5) | 0.0375(6) | 0.0001(5) | 0.0019(5) | 0.0079(4) |
| N(1) | 4e | 0.1222(1) | 0.70366(9) | 0.0830(1) | 0.0331(5) | 0.0270(5) | 0.0303(5) | −0.0002(4) | −0.0034(4) | 0.0025(4) |
| N(2) | 4e | 0.2549(1) | 0.5839(1) | 0.2354(1) | 0.0361(6) | 0.0274(5) | 0.0322(5) | −0.0001(4) | −0.0038(4) | 0.0039(4) |
| C(1) | 4e | 0.1425(2) | 0.5921(1) | 0.1225(1) | 0.0344(6) | 0.0281(6) | 0.0323(6) | −0.0013(5) | −0.0011(5) | 0.0030(5) |
| C(2) | 4e | 0.0585(2) | 0.4918(1) | 0.0569(2) | 0.0452(8) | 0.0289(6) | 0.0446(8) | −0.0051(5) | −0.0098(6) | 0.0034(5) |
| C(11) | 4e | 0.2231(1) | 0.7711(1) | 0.1759(1) | 0.0307(6) | 0.0288(6) | 0.0286(6) | −0.0012(5) | 0.0028(5) | −0.0012(5) |
| C(12) | 4e | 0.3091(1) | 0.6946(1) | 0.2721(1) | 0.0309(6) | 0.0311(6) | 0.0288(6) | −0.0012(5) | 0.0030(5) | −0.0008(5) |
| C(13) | 4e | 0.4249(2) | 0.7340(1) | 0.3791(2) | 0.0318(6) | 0.0477(8) | 0.0320(6) | −0.0013(6) | −0.0017(5) | −0.0011(6) |
| C(14) | 4e | 0.4474(2) | 0.8534(2) | 0.3844(2) | 0.0398(7) | 0.0512(8) | 0.0396(7) | −0.0118(6) | 0.0003(6) | −0.0120(6) |
| C(15) | 4e | 0.3589(2) | 0.9300(1) | 0.2894(2) | 0.0510(8) | 0.0354(7) | 0.0467(8) | −0.0101(6) | 0.0064(6) | −0.0089(6) |
| C(16) | 4e | 0.2446(2) | 0.8913(1) | 0.1830(2) | 0.0435(7) | 0.0287(6) | 0.0389(7) | −0.0013(5) | 0.0047(6) | −0.0014(5) |
Acknowledgments:
The authors thank Mr. David Rogers for helpful discussions.
References
1. Yumata, N. C.; Habarurema, G.; Mukiza, J.; Gerber, T. I. A.; Hosten, E.; Taherkhani, F.; Nahali, M.: Rhenium complexes of di-2-pyridyl ketone, 2-benzoylpyridine and 2-hydroxybenzophenone: A structural and theoretical study. Polyhedron 62 (2013) 89–103.Search in Google Scholar
2. Potgieter, K. C.; Gerber, T. I. A.; Betz, R.; Rhyman, L.; Ramasami, P.: Structural and DFT/TD-DFT investigation of tris(bidentate) complexes of rhenium(III) synthesized from the cis-[ReO2]+ core and benzenethiol derivatives. Polyhedron 59 (2013) 91–100.Search in Google Scholar
3. Prabakaran, P.; Murugesan, S.; Robert, J. J.; Panneerselvam, P.; Muthiah, P. T.; Bocelli, G.; Righi, L.: A Novel Hydrogen-Bonded Duplex Made up of Water Molecules and Halide Ions in the Sandwich Inclusion Structures of (C10H8N3S)+·X·2H2O [X = Cl−, Br−]. Chem. Lett. 29 (2000) 1080–1081.Search in Google Scholar
4. Allen, F. H.: The Cambridge Structural Database: a quarter of a million crystal structures and rising. Acta Crystallogr. B58 (2002) 380–388.Search in Google Scholar
5. Bondi, A.: van der Waals Volumes and Radii. J. Phys. Chem. 68 (1964) 441–451.Search in Google Scholar
6. Bernstein, J.; Davis, R. E.; Shimoni, L.; Chang, N.-L.: Patterns in Hydrogen Bonding: Functionality and Graph Set Analysis in Crystals. Angew. Chem. Int. Ed. Engl. 34 (1995) 1555–1573.Search in Google Scholar
7. Etter, M. C.; MacDonald, J. C.; Bernstein, J.: Graph-set analysis of hydrogen-bond patterns in organic crystals. Acta Crystallogr. B46 (1990) 256–262.Search in Google Scholar
8. Sheldrick, G. M.: A short history of SHELX. Acta Crystallogr. A64 (2008) 112–122.10.1107/S0108767307043930Search in Google Scholar PubMed
9. Farrugia, L. J.: WinGX and ORTEP for Windows: an update. J. Appl. Crystallogr. 45 (2012) 849–854.Search in Google Scholar
10. Macrae, C. F.; Bruno, I. J.; Chisholm, J. A.; Edgington, P. R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; van de Streek, J.; Wood, P. A.: Mercury CSD 2.0 – new features for the visualization and investigation of crystal structures. J. Appl. Crystallogr. 41 (2008) 466–470.Search in Google Scholar
11. Spek, A. L.: Structure validation in chemical crystallography. Acta Crystallogr. D65 (2009) 148–155.10.1107/S090744490804362XSearch in Google Scholar PubMed PubMed Central
©2015 Xandri Schoultz et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 3.0 License.
Articles in the same Issue
- Frontmatter
- Crystal structure of bis(ethyltriphenylphos-phonium) tetrabromidozincate(II), C40H40Br4P2Zn
- Crystal structure of chlorido-(2-ethoxy-6-(((quinolin-8-yl)methyl)-phenolato-κ3N,N′,O)-copper(II), C18H15ClCuN2O2
- Crystal structure of bis(ethyltriphenylphos-phonium) tetrabromidocobaltate(II), C40H40Br4CoP2
- Crystal structure of di(pyrrolidin-1-yl)methane-thione, C9H16N2S
- Crystal structure of ammonium phenylglyoxylate, C8H9NO3
- Crystal structure of 2-hydroxy-2-methyl-2-phenyl acetic acid hemihydrate, C9H11O3.50
- Crystal structure of 2-((E)-2-(1H-benzo[d]imidazol-2-yl)vinyl)-1H-benzo[d]imidazolium dichloride dihydrate, C16H18Cl2N4O2
- Crystal structure of 1,2-dimethylimidazolium iodide, C5H9IN2
- Crystal structure of catena-poly[(μ2-4,4′-bipyridine-κ2N:N′)-tetraqua-cobalt(II)] – (3-(carboxylatomethyl)benzoic acid) – water (1/2/2), C28H34CoN2O14
- Crystal structure of bis(2,2′-bipyridine-κ2N,N′)-bis(thiophene-3,4-dicarboxylato-κ2O,O′)-cadmium(II), C32H22CdN4O8S2
- Crystal structure of tetraaqua-(4,4′-diamino-1,1′-biphenyl-2,2′-disulfonato-κN)(4,4′-bipyridyl-κN)zinc(II) trihydrate, C22H32N4O13S2Zn
- Crystal structure of poly[[[(1,3-dimethyl-2-imidazolidinone-κ1O11) zinc(II)]-μ-1,4-benzenedi-carboxylato-κ4O1,O2:O3,O4]—1,3-dimethyl-2-imidazolidinone (1:1)], [Zn(C5H10N2O)(C8H4O4)]· C5H10N2O, C18H24N4O6Zn
- Crystal structure of poly[[(1,3-dimethyl-2-imidazolidinone-κ1O) zinc(II)]-μ-furan-2,5-dicarboxylato-κ4O,O′:O′′,O′′′], [Zn(C5H10N2O)(C6H2O5)], C22H24N4O12Zn2
- Crystal structure of catena-poly[(μ2-4,4′-bipyridine-κ2N:N′)-tetraquamanganese(II)] – (3-(carboxylatomethyl)benzoic acid) – water (1/2/2), C28H34MnN2O14
- Crystal structure of chlorido-tricarbonyl-bis(2-pyridylmethanone-N,N′)-rhenium(I), C14H8ClN2O4Re
- Crystal structure of (2-(benzoyl)phenolato-κ2O,O′)-trans-dichlorido -cis- bis(methoxido) niobium(V), C15H15Cl2NbO4
- Crystal structure of potassium diaqua dihydroxy(methylenediphosphonato-κ2-O,O′)cobaltate(III), CH10CoKO10P2
- Crystal structure of hexakis(4-(dimethylamino)pyridin-1-ium) decavanadate-water (1:16), C42H98N12O44V10
- Crystal structure of 4-diethylaminobenzaldehyde isonicotinoylhydrazone monohydrate, C17H20N4O·H2O, C17H22N4O2
- Crystal structure of bis[μ-1-[(2-ethyl-1H-imidazol-1-yl)methyl]-1H-benzotriazole-κ2N:N′]bis[dibromido]dimercury(II), C24H26Br4Hg2N10
- Crystal structure of poly{bis-[(4,4′-biphenyl-dicarboxylate-κ2O:O′)-μ-1,1′-oxy-bis-(ethane-2,1-diyl)-bis-(1H-imidazole)-κ2N:N′] zinc(II)}dihydrate, C48H48N8O12Zn2
- Crystal structure of sodium poly[(μ4-2,2′,2′′-nitrilotriacetato-κ4O,O′,O′′,N)magnesium(II)] monohydrate
- The crystal structure of 1-(2-(2-chloroethoxy)phenyl)ethanone
- The crystal structure of tris(μ2-1,3-bis(4,4,4-Trifluoro-3-oxido-1-(oxo)but-2-en-1-yl)phenyl-/κ4O,O′:O′′,O′′′)-bis(1,2-dimethoxyethane-O,O′)-diterbium(III)
- Crystal structure of (S)-2-(2,2′-diethoxy-[1,1′-binaphthalen]-6-yl)-4,4,5,5-tetra-methyl-2-imidazoline-1-oxyle, C31H33N2O3
- Crystal structure of cis-tetrachloridobis-(pyridine-κN)platinum(IV), C10H10Cl4N2Pt
- Crystal structure of catena-poly[aqua-(μ2-5-norbornene-2,3-dicarboxylato-κ3O,O′:O′′)-(1,10-phenanthroline-κ2N,N′)zinc(II)], [Zn(C9H8O4)(C12H8N2)(H2O)]n
Articles in the same Issue
- Frontmatter
- Crystal structure of bis(ethyltriphenylphos-phonium) tetrabromidozincate(II), C40H40Br4P2Zn
- Crystal structure of chlorido-(2-ethoxy-6-(((quinolin-8-yl)methyl)-phenolato-κ3N,N′,O)-copper(II), C18H15ClCuN2O2
- Crystal structure of bis(ethyltriphenylphos-phonium) tetrabromidocobaltate(II), C40H40Br4CoP2
- Crystal structure of di(pyrrolidin-1-yl)methane-thione, C9H16N2S
- Crystal structure of ammonium phenylglyoxylate, C8H9NO3
- Crystal structure of 2-hydroxy-2-methyl-2-phenyl acetic acid hemihydrate, C9H11O3.50
- Crystal structure of 2-((E)-2-(1H-benzo[d]imidazol-2-yl)vinyl)-1H-benzo[d]imidazolium dichloride dihydrate, C16H18Cl2N4O2
- Crystal structure of 1,2-dimethylimidazolium iodide, C5H9IN2
- Crystal structure of catena-poly[(μ2-4,4′-bipyridine-κ2N:N′)-tetraqua-cobalt(II)] – (3-(carboxylatomethyl)benzoic acid) – water (1/2/2), C28H34CoN2O14
- Crystal structure of bis(2,2′-bipyridine-κ2N,N′)-bis(thiophene-3,4-dicarboxylato-κ2O,O′)-cadmium(II), C32H22CdN4O8S2
- Crystal structure of tetraaqua-(4,4′-diamino-1,1′-biphenyl-2,2′-disulfonato-κN)(4,4′-bipyridyl-κN)zinc(II) trihydrate, C22H32N4O13S2Zn
- Crystal structure of poly[[[(1,3-dimethyl-2-imidazolidinone-κ1O11) zinc(II)]-μ-1,4-benzenedi-carboxylato-κ4O1,O2:O3,O4]—1,3-dimethyl-2-imidazolidinone (1:1)], [Zn(C5H10N2O)(C8H4O4)]· C5H10N2O, C18H24N4O6Zn
- Crystal structure of poly[[(1,3-dimethyl-2-imidazolidinone-κ1O) zinc(II)]-μ-furan-2,5-dicarboxylato-κ4O,O′:O′′,O′′′], [Zn(C5H10N2O)(C6H2O5)], C22H24N4O12Zn2
- Crystal structure of catena-poly[(μ2-4,4′-bipyridine-κ2N:N′)-tetraquamanganese(II)] – (3-(carboxylatomethyl)benzoic acid) – water (1/2/2), C28H34MnN2O14
- Crystal structure of chlorido-tricarbonyl-bis(2-pyridylmethanone-N,N′)-rhenium(I), C14H8ClN2O4Re
- Crystal structure of (2-(benzoyl)phenolato-κ2O,O′)-trans-dichlorido -cis- bis(methoxido) niobium(V), C15H15Cl2NbO4
- Crystal structure of potassium diaqua dihydroxy(methylenediphosphonato-κ2-O,O′)cobaltate(III), CH10CoKO10P2
- Crystal structure of hexakis(4-(dimethylamino)pyridin-1-ium) decavanadate-water (1:16), C42H98N12O44V10
- Crystal structure of 4-diethylaminobenzaldehyde isonicotinoylhydrazone monohydrate, C17H20N4O·H2O, C17H22N4O2
- Crystal structure of bis[μ-1-[(2-ethyl-1H-imidazol-1-yl)methyl]-1H-benzotriazole-κ2N:N′]bis[dibromido]dimercury(II), C24H26Br4Hg2N10
- Crystal structure of poly{bis-[(4,4′-biphenyl-dicarboxylate-κ2O:O′)-μ-1,1′-oxy-bis-(ethane-2,1-diyl)-bis-(1H-imidazole)-κ2N:N′] zinc(II)}dihydrate, C48H48N8O12Zn2
- Crystal structure of sodium poly[(μ4-2,2′,2′′-nitrilotriacetato-κ4O,O′,O′′,N)magnesium(II)] monohydrate
- The crystal structure of 1-(2-(2-chloroethoxy)phenyl)ethanone
- The crystal structure of tris(μ2-1,3-bis(4,4,4-Trifluoro-3-oxido-1-(oxo)but-2-en-1-yl)phenyl-/κ4O,O′:O′′,O′′′)-bis(1,2-dimethoxyethane-O,O′)-diterbium(III)
- Crystal structure of (S)-2-(2,2′-diethoxy-[1,1′-binaphthalen]-6-yl)-4,4,5,5-tetra-methyl-2-imidazoline-1-oxyle, C31H33N2O3
- Crystal structure of cis-tetrachloridobis-(pyridine-κN)platinum(IV), C10H10Cl4N2Pt
- Crystal structure of catena-poly[aqua-(μ2-5-norbornene-2,3-dicarboxylato-κ3O,O′:O′′)-(1,10-phenanthroline-κ2N,N′)zinc(II)], [Zn(C9H8O4)(C12H8N2)(H2O)]n

