Abstract
Supramolecular chemistry has become a key area in emerging bottom-up nanoscience and nanotechnology. In particular, supramolecular systems that can produce a photonic output are increasingly important research targets and present various possibilities for practical applications. Accordingly, photonic properties of various supramolecular systems at the nanoscale are important in current nanotechnology. In this short review, nanophotonics in supramolecular chemistry will be briefly summarized by introducing recent examples of control of photonic responses of supramolecular systems. Topics are categorized according to the fundamental actions of their supramolecular systems: (i) self-assembly; (ii) recognition; (iii) manipulation.
Edited by Volker Sorger
1 Introduction
Over the past 20 years, major discoveries in science and innovation in technology have led to the development of nanoscience and nanotechnology where the fabrication of ultrasmall objects and/or control of highly precise structures are critical [1–5]. Successful techniques for nanostructure control currently rely heavily on sophisticated top down (engineering down) nanofabrications. These top-down approaches are based on suitable lithographic or ion implantation techniques and have resulted in various types of densely integrated device structures that make an immense contribution to high technology fields such as silicon-integrated chip technology. However, according to the so-called Moore’s law [6, 7], the current rate of miniaturization in silicon device technology will very soon be adversely affected by the physical limits of device dimensions imposed by ultra-violet, electron/ion beam and soft X-ray lithographic techniques.
In a breakthrough made possible by nanoscience and nanotechnology, an alternate approach based on bottom-up (engineering-up) concepts has been introduced. These approaches rely on spontaneous processes of self-assembly [8–11] and subsequent formation of nanostructure formation [12–15]. Self-assembly processes are often conducted under mild conditions unlike top-down nanofabrication procedures that often require ultrahigh vacuum and laser irradiation. Thus, bottom-up approaches are applicable for the formation of nanostructures of various soft materials including organic molecules, biomolecules and their hybrids. Therefore, supramolecular chemistry, which deals with molecule-molecule interactions, has become a key science in emerging bottom-up nanoscience and nanotechnology.
In order to convert scientific knowledge to a technical application, basic phenomena should be integrated into functional systems such as stimuli-responsive devices. Nanotechnology involving bottom-up approaches often requires supramolecular systems to produce an output implying some useful functionality including electronic, photonic, chemical and biological outputs [16–18]. Of these, systems based on photonic outputs are of increasing current interest with various possibilities for practical application. Therefore, photonic properties of nanosized forms of various supramolecular systems will be of crucial importance in nanotechnology in the near future.
In this short review, nanophotonic aspects of supramolecular chemistry are briefly summarized by introducing typical recent examples of control of photonic responses from supramolecular systems. The topics are roughly categorized according to the fundamental actions of the supramolecular systems: (i) self-assembly; (ii) recognition; (iii) manipulation. The examples introduced illustrate clearly the critical relationship between nanophotonics and supramolecular chemistry.
2 Self-assembly
Organic dye molecules when contained in supramolecular assemblies exhibit very different spectroscopic characteristics compared to those observed for their mono-dispersed states in solution [19–21]. As is known for different assembly modes such as J- and H-aggregates, assembly motifs sensitively influence the spectroscopic properties of both their absorption and emission profiles. Therefore, photonic properties of self-assembled structures of chromophore molecules are attractive research targets in supramolecular chemistry.
Ajayaghosh and coworkers have investigated the photonic characteristics of gel-type assemblies of rather small chromophore molecules [22–24]. For example, they researched thienylenevinylene-based gels (Figure 1A) that exhibit epitaxial self-assembly to form aligned supramolecular wires [25]. The hydrogen-bonded networks obtained possessed enhanced electrical properties with high charge carrier mobility. In addition, thienylenevinylene molecules with amide end groups exhibited a considerable difference in their absorption spectra when contained in supramolecular assemblies. A large blue shift for the assembled structures was attributed to a large oscillator strength and strong exciton coupling based on formation of a one-dimensional arrangement of the molecules in H-type aggregates. Reversible formation of the aggregates from individual molecules was also confirmed in variable temperature experiments. The same research group reported attogram sensing of the explosive 2,4,6-trinitrotoluene (TNT) by observing the photonic behavior of a self-assembled gel of perfluoroarene-based oligo(p-phenylenevinylene) (Figure 1B) due to their fluorescence responses [26]. When predeposited on disposable paper strips the gel could be used to detect TNT at the attogram (10-18 g) level with a detection limit of 0.23 ppq. TNT molecules fit vertically into the free spaces of the gel through electrostatic interactions with its fibers. Edge-to-face π-stacking between the electron-deficient aromatic core of trinitrotoluene and the electron-rich perfluoroarene moieties of the gel resulted in formation of a strongly bound complex. The entrapped trinitrotoluene molecules play the role of a fluorescence trap. The reported attogram detection level would be highly beneficial if used in a simple and low-cost protocol for the on-site detection of TNT on contaminated specimens.
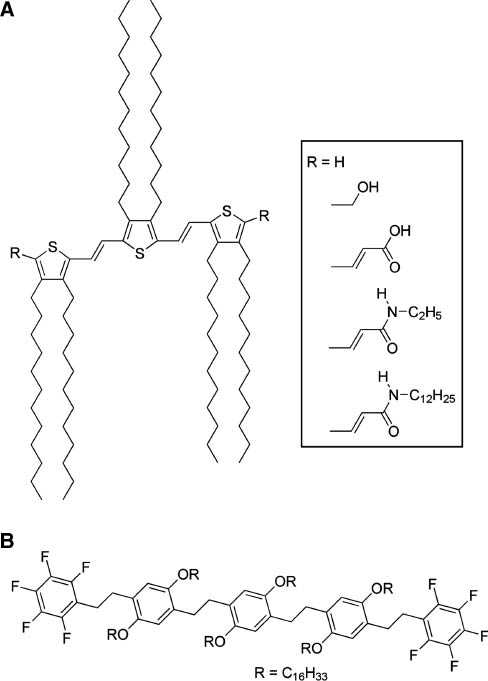
Gel-forming chromophores: (A) thienylenevinylene-based gelator; (B) perfluoroarene-based oligo(p-phenylenevinylene) gelator.
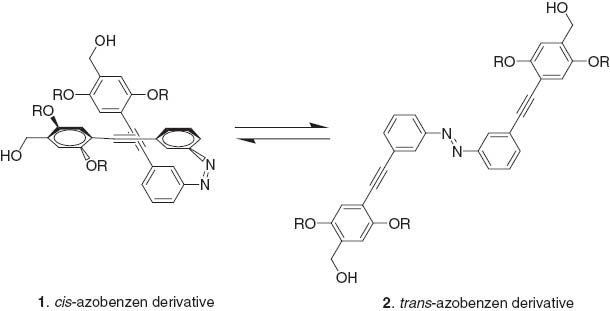
Ajayaghosh et al. have also demonstrated photo-induced reversible dot to rod variation of assembled structures of azobenzene derivatives 1, 2 [27]. Irradiation of nanodots with UV light induced partial conversion of trans-azobenzene 2 to its cis-isomer 1. Even though the conversion yield is not very large a locally high concentration of the cis-isomer 1 on the surface of the dots enhanced the surface dipole moment making the nanodot structures unstable due to an increase in ζ-potential. This situation facilitates interparticle association, resulting in formation of rod-shaped structures. They also synthesized azobenzene-linked phenyleneethynylenes that could form helical supramolecular assemblies [28]. Helicity of the assemblies formed were of a specific chirality that could be reversibly switched to the opposite helical sense through a chiral-center-controlled photoisomerization of the attached azobenzene moieties.
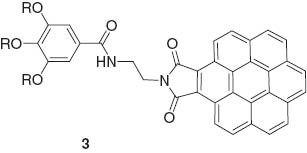
George and coworkers used an unsymmetrical coronene monoimide 3 for formation of hydrogen-bonding-mediated supramolecular gels. Gels are formed through the concept of repulsion between dipoles to maintain a head-head staggered organization [29]. Hydrogen-bonding enforcement minimizes dipolar repulsion between chromophores.
The formation of head-head assembly is highly advantageous for nonlinear optical activity. Sierra, Barluenga, and coworkers reported the aggregation-induced enhanced emission effect in self-assembled structures of 1,6-bis(4-hydroxyphenyl)-3-hexen-1,5-diyne bearing two bulky end groups derived from 3,4,5-tris(3,4,5-trihydroxybenzyloxy)benzoic acid (Figure 2A), probably due to the restriction of intramolecular rotation [30]. This property makes it possible to obtain molecular self-assemblies as liquid-crystalline phases or organogels with intense luminescence. Del Guerzo and coworkers utilized the superior characteristics of self-assembled systems for preparation of highly organized and anisotropic nanoobjects [31]. White-light-emitting self-assembled nanofibers were obtained by blending three components, 2,3-bis(decyloxy)anthracene (blue), 2,3-bis(hexadecyloxy)-5,12-diphenyltetracene (green), and 2,3-bis(hexadecyloxy)-5,6,11,12-tetraphenyltetracene (red) (Figure 2B). Liu et al. reported preparation of two-component supramolecular hydrogels based on the noncovalent self-assembly between different benzene dicarboxylic acids and histidine-based bolaamphiphiles 4 [32].
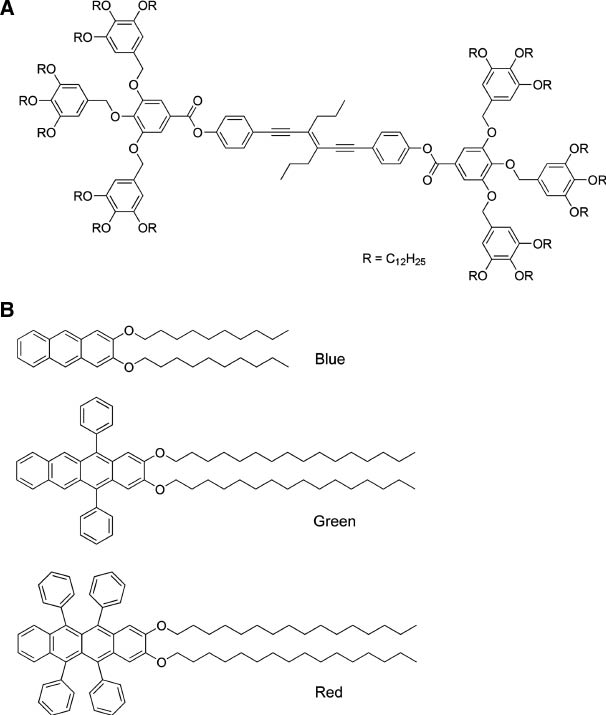
Molecules for color emission upon self-assembly: (A) 1,6-bis(4-hydroxyphenyl)-3-hexen-1,5-diyne bearing two bulky end groups derived from 3,4,5-tris(3,4,5-trihydroxybenzyloxy)benzoic acid; (B) RGB colored components of 2,3-bis(decyloxy)anthracene (blue), 2,3-bis(hexadecyloxy)-5,12-diphenyltetracene (green), and 2,3-bis(hexadecyloxy)-5,6,11,12-tetraphenyltetracene (red).
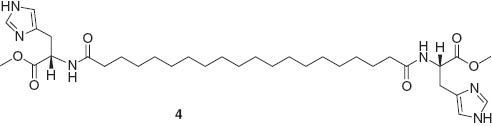
The structures of supramolecular assemblies alter depending on the relative positions of carboxylic acids at the benzene unit, leading to diverse photonic properties. Some of the assemblies exhibited a strong luminescence enhancement by doping with europium (III) chloride. This feature is difficult to achieve using normal synthetic polymers.
Kim and coworkers investigated fluorescence properties of pyrene-containing poly(arylene ether sulfone) compounds that exhibited two fluorescence switching modes in different gelation solvents [33]. In tetrahydrofuran, the assembly exhibited gelation-induced enhancement of excimer emission. In contrast, strong hydrogen bonding networks induced stacking among the pyrene moieties, resulting in fluorescence quenching in methylene chloride. Yi and coworkers synthesized phenol-substituted 1,8-naphthalimide derivatives 5 (donor) and an iridium(III) complex with orange light emission (acceptor) for tuneable emission upon mixing [34].
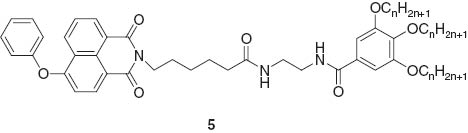
This two-component gel responded to the presence of cysteine with a visible change in luminescence. The original color of the two component gel was yellow and addition of cysteine induced color change of the gel to white. The luminescence color of the gel changed from white to blue upon addition of cysteine. The iridium complex is probably concentrated at the gel interface and reacts with cysteine, resulting in color fading of the gel.
3 Recognition
Photonic aspects of supramolecular systems can be applied for use in materials detection [17, 35–39]. Detection of particular chemical species accompanied by specific variations of fluorescence emission is a powerful method in analytical chemistry. Fluorescent probes that bind to specific ions through supramolecular recognition are widely used in analyses of living cells [40–42]. In particular, development of a fluorescent probe for Ca2+ ion detection fura-2 6 by Tsien and coworkers [43] promoted significant activity in the corresponding research field.
Fluorescent probe molecules for Na+, K+, Mg2+, and Zn2+ have also been developed [44–48] as well as probes for small molecules such as nitrogen monooxide (NO) [49, 50]. Another important milestone in biological imaging is the application of green fluorescence protein (GFP) to cell media [51–53], which lead to the Nobel prize in Chemistry being awarded to Chalfie, Tsien, and Shimomura in 2008. GFP-based probes have been used for detection of various biological substances including Ca2+ and cyclic-AMP [54, 55]. In the following sections, recent developments on fluorescence probe technology are first briefly overviewed.
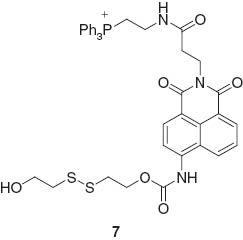
The design of fluorescent probes for organic molecules with complex structures has been an active research area where improvements of specificities in the recognition process are anticipated. Yang, Chan, and coworkers reported fluorescent probe molecules with a bis-spiropyran ligand for detection of glutathione (Figure 3) [56]. Multi-point molecular recognition leads to high affinity and specificity for glutathione and this system was used to realize in vivo detection of glutathione in living cells. Detection systems for small organic molecules accompanied by selective chemical reactions in cells have also been developed. For example, Kang, Kim, and coworkers reported an off-on fluorescent probe for mitochondrial thioredoxin [57]. The synthetic probe molecules Mito-Naph 7 react with thioredoxin to induce release of thiolate via intramolecular cyclization, resulting in fluorescent compounds.
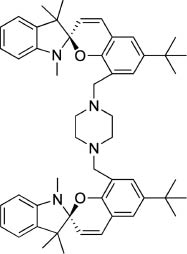
Fluorescent probe molecules with bis-spiropyran ligand for detection of glutathione.
Florescence bioimaging through catalytic activation has also been investigated. Kanai and coworkers reported fluorescent detection of acetyl CoA with the probe RH-NH28 upon activation of acetyl-CoA by an artificial reaction promoter. In addition to successful fluorescence imaging, regulation processes of biomolecular activation were also demonstrated [58].
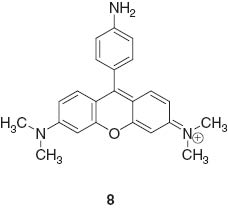
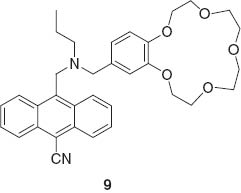
Fluorescent detection of multiple targets becomes possible when a fluorescent sensor molecule possesses multiple binding sites with different photonic responses. This concept was introduced by de Silva et al. in one example of a molecular logic gate 9 [59].
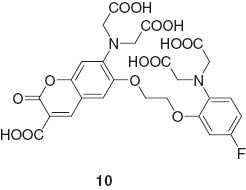
The logic gate actions of supramolecular host are based on different sensitivities to Na+ and H+ based on an internal charge transfer mechanism. Suzuki and coworkers reported multi-detection probe molecules KCM-1 10 for Ca2+ and Mg2+ [60].
This system realized concurrent detection of both the important ion species in living cells. Lin et al. developed a fluorescent probe 11 responding to H2O2, NO, and H2O2/NO with three different sets of photonic signals. Intracellular imaging of these species was also demonstrated [61].
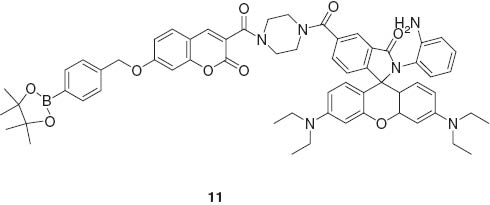
Because of low background emission and high transparency, in vivo imaging with probes responding to irradiation of near infrared light have been paid attention as seen in the pioneering example by Weissleder et al. [62]. Combination of chemiluminescence with near infrared systems was reported by Nagano, Urano, and coworkers [63]. They realized in vivo near-infrared bioimaging using an infrared-active dye probe as an acceptor for intramolecular bioluminescence resonance energy transfer of luciferin (Figure 4).
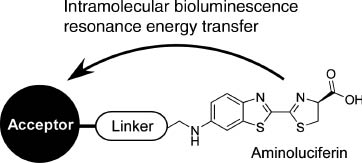
A probe design for in vivo near infrared bioimaging using intramolecular bioluminescence resonance energy transfer of luciferin.
Fluorescence detection of ions is also important in environmental science. After nuclear power station disasters, detection of caesium becomes an important matter of public safety. In the vicinity of radiation leaks such as those which occurred at Chernobyl and more recently Fukushima, radiocaesium species including the two longer-lived isotopes of caesium, Cs-134 and Cs-137 are a major source of environmental contamination. During reactor exposure, caesium was released in a particulate form that requires detection amongst large volumes of contaminated ground soils in the solid state. Therefore, it is necessary to develop sensors for particles that contain caesium cations (Cs+) that could be used to screen large areas of contaminated land especially in the vicinities of radiation leaks. Host-guest interactions accompanying detectable signals such as fluorescence emission provide greater spatial resolution.
The host molecule used for this purpose was 1-(4′-hydroxy phenyl)-4-phenylbenzene connected to a 4-nitrophenyl ether group through a tetraethylene glycol linker separating the two aromatic moieties (Figure 5) [64]. Caesium cation-containing particles can be visualized after spraying with this host molecule in methanol, which is especially suitable for detecting caesium salts in particulate form and promotes the ability to detect them with the naked eye in the solid state. Of the cations tested, fluorescence was absent in the presence of Mg2+ and Ca2+. Green fluorescence due to the presence of Cs+ on dry soil was observed after spraying of the host solution, while cations such as Na+ and K+ lead to rather weak blue emission (Figure 6). Because the wavelength of the fluorescence emission maximum is dependent on the concentration of Cs+ in the mixed solid with K+, construction of a calibration curve becomes possible. The calibration curve suggests that concentrations of Cs+ far lower than 1 ppm could be detected. Based on density functional theory, geometries of the resulting complexes were estimated. A weak hydrogen bond between the 4-nitro group of the 4-nitrophenyl ether moiety and one methine of the terphenol is predicted, which apparently stabilizes a quasi-macrocyclic conformation of the host molecule. This simple optical method has a higher spatial resolution for Cs-137 than existing radioscopes and gamma ray cameras. Only contaminated particles are detected by eye and can be easily removed. Therefore, the volume of radioactively contaminated material for extraction is greatly reduced.
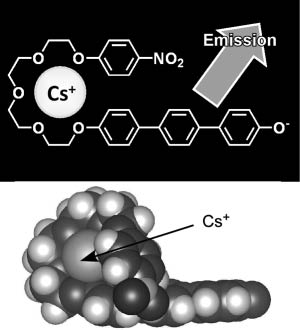
A fluorescence probe for caesium detection: structure and models for caesium inclusion.
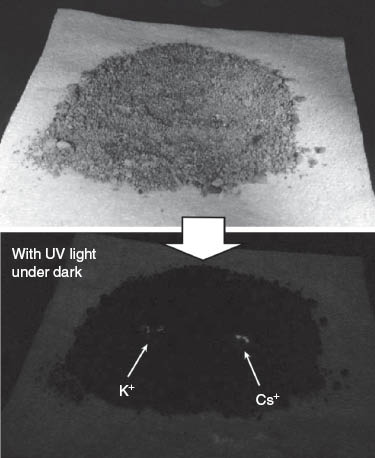
Fluorescence detection of caesium ions with potasium ion as a control.
4 Manipulation
As previously mentioned, photonic properties of supramolecular systems vary dynamically accompanying changes in assembly structures and molecular association/dissociation. Photonic responses from supramolecular systems can be also controlled through manipulation of supramolecular structures by external mechanical forces [65–67]. Mechanical deformation of supramolecular materials is sometimes accompanied by alteration of photonic properties of supramolecular structures. For example, Jia and coworkers demonstrated mechanochromic luminescent behavior of assemblies of a pyrene-appended dipeptide compound (Figure 7) [68]. In the as-prepared powder, hydrogen bonding inhibits molecular mobility and confines the orientation of pyrene groups to a partially overlapped architecture in the excited state. Application of shearing force to the sample weakens the intermolecular interactions, leading to the disruption of the ordered structure. Increased motional freedom increases the possibility of excimer-type emission formation of stacked pairs of pyrene moieties. Thus, force-induced turn-on fluorescence is realized. Baumgartner and coworkers prepared highly fluorescent organogels from a phosphole-lipid system [69]. A significant emission shift from blue to orange can be induced by mechanical grinding and thermal annealing due to control of fluorescence resonance energy transfer in the donor-acceptor system.
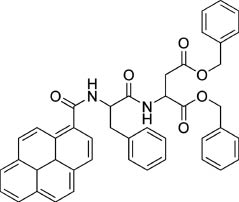
Pyrene-bearing dipeptide compound for mechanochromic luminescence.
Liquid crystals are good candidates for stimuli-responsive photonic materials because their internally ordered structures can be easily altered by external forces. For example, Sagara and Kato reported luminescent liquid crystals containing the luminophore 9,10-bis(phenylethynyl)anthracene, which presents fluorescence emission of three colors (reddish-orange, yellow, and green) with switching by mechanical and thermal stimuli [70]. Reddish-orange, yellow, and green colors correspond to cubic phase, mesomorphic phase, and columnar phase, respectively.
Gel films are also stimuli-responsive materials. Mizoshita et al. demonstrated mechanical reversible fluorescence switching of a perylene bisimide dye 12 [71].
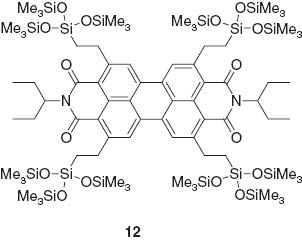
The dye used cannot form hydrogen bonds and undergoes strong face-to-face π-π stacking of the core. Intermolecular interactions respond to external stimuli in the solid state. Variations in fluorescent emission caused by isothermal mechanical shearing in its films could be quickly erased by treatment of the films with solvent vapors.
Jia and coworkers prepared coassemblies of a dipeptide containing a pyrene group and a dipeptide containing lactam rhodamine B (Figure 8) that exhibited multi-luminescent color switching from blue to green and a reddish color by applying a mechanical stimulus [72]. These color transitions included an emission wavelength shift from blue to green, and an unusual switching of emission intensity at long wavelength (non-luminescent to reddish luminescence). Thomas, Fraser, and coworkers demonstrated a visible fluorescence color change from blue-green to yellow-green for difluoroboron dibenzoylmethane-polylactide in powder and film forms by applying a shearing force [73]. A mechanically induced conversion from crystalline to amorphous induces a molecular rearrangement which permits greater rotational freedom to form excimeric or intermolecular charge-transfer species. Dong, Tang, and coworkers reported unusual aggregation-induced emission for diphenyldibenzofulvene molecules 13 [74].
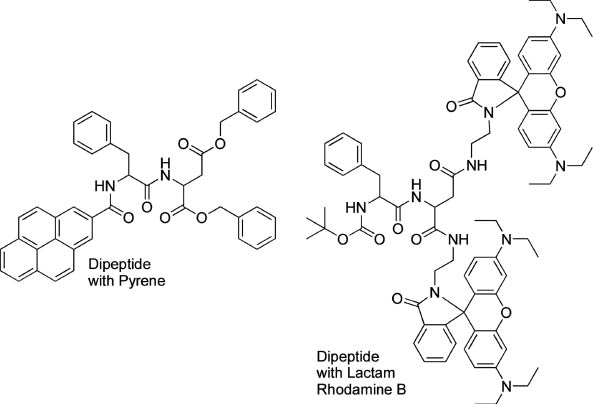
Dipeptides containing a pyrene group and dipeptide containing lactam rhodamine B for multi-luminescent color switching by application of a mechanical stimulus.
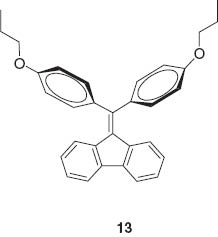
Fluorescence of their crystals could be turned off and on respectively upon grinding and annealing, which is attributed to amorphization and crystallization. Fraser and coworkers demonstrated reversible mechanochromic luminescence of a difluoroboron avobenzone solid 14 [75].
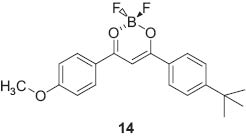
Only a small mechanical perturbation such as a slight touch with the tip of a cotton swab changed the green-blue film emission to yellow. This mechanochromic fluorescence was recovered spontaneously at room temperature or more quickly with heating. Kanbara and coworkers reported two-step luminescence changes of a Pt(II) complex bearing hexanoylamide moieties due to mechanical and vapochromic effects [76]. Luminescence color was strongly affected by the Pt-Pt distance in molecular packing, which depends in turn upon the hydrogen-bonding network structure.
A Langmuir monolayer at the air-water interface provides a unique medium where lateral mechanical forces can be confined within a monomolecular plane. Molecular aggregation and variations in their packing are easily controlled so that monolayers at the air-water interface become model systems for investigation of molecular aggregation and any accompanying photonic properties [77, 78]. We have pioneered mechanical operation of molecular machines at the air-water interface [79–82]. As shown below, reversible capture and release of fluorescence guest molecules by a mechanically operated molecular machine induced modulation of fluorescence emission.
We used a steroid cyclophane as a molecular machine that contains a cyclic core of a 1,6,20,25-tetraaza[6.1.6.1]paracyclophane connected to four cholic acid moieties through a flexible l-lysine spacer (Figure 9) [83, 84]. Cholic acid moieties are rigid and possess hydrophobic and hydrophilic faces which are thought to predispose it to lie flat on the water surface. Therefore, the steroid cyclophane molecules are in an open conformation when lying on water in an uncompressed state and coat the water surface at a molecular area of ca. 7 nm2. Upon compression of its monolayer the steroid cyclophane molecule takes up a cavity conformation in the condensed phase with an empty space remaining between the steroidal walls. Binding behavior of an aqueous naphthalene-type fluorescent guest molecule to these monolayers was investigated at the water’s surface by using in situ fluorescence spectroscopy. The fluorescence intensity of the probe molecule is known to be significantly suppressed in a polar medium, and a large fluorescence can be observed only when it is inserted into the hydrophobic core of the host cyclophane. Formation of the three-dimensional cavity of the steroid cyclophane induced an increase in the surface fluorescence probably because of inclusion of the fluorescent guest into the cavity at the surface. This piezoluminescent response could also be demonstrated upon repeated compression and expansion cycles of the steroid cyclophane monolayers in the presence of the fluorescent guest molecule. A periodic change in the fluorescence intensity was confirmed according to this compression-expansion cycle, indicating that capture and release of the fluorescent guest occurs by the dynamic cavity formation of the steroid cyclophane (Figure 10).
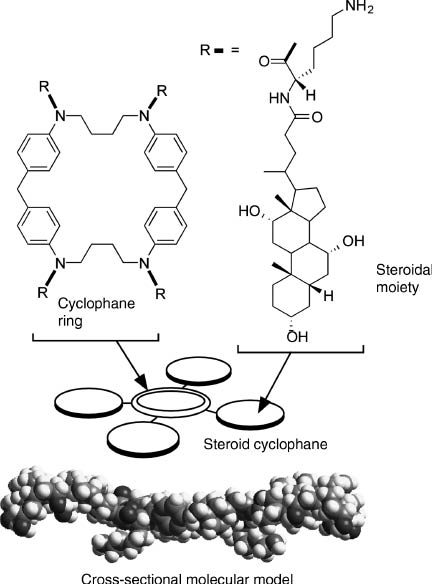
Steroid cyclophane as a molecular machine.
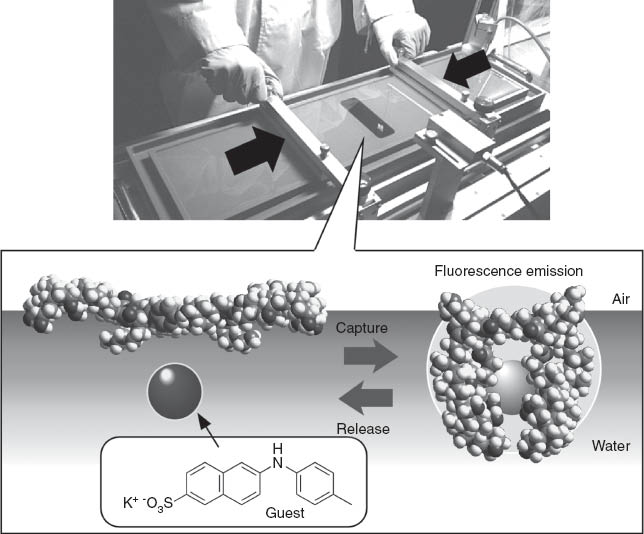
Fluorescence response based on capture and release of guest molecules by mechanical force.
Binding curve analyses suggest that the guest molecule is included in the steroid cyclophane cavity with a 1:1 stoichiometry at the air-water interface. Judging from other experimental evidence, including fluorescence data for the transferred monolayer and binding constants, the increased fluorescence intensity probably does not originate from the increase in the quantity of bound fluorescent guest but it reflects an increase in the intrinsic fluorescence intensity of the bound guest. The fluorescence quantum yield of the fluorescent guest decreases with the formation of a non-emissive twisted intramolecular charge transfer state, which is generally known to be destabilized in nonpolar media. The three-dimensional cavity of the steroid cyclophane provides a nonpolar environment for binding of fluorescent guest. In addition, the restricted motion of fluorescent guest at high surface pressure might suppress the formation of the twisted intramolecular charge transfer state, resulting in enhancement of the fluorescence.
In another example of mechanically modulated photonic detection of guest molecules, we have recently developed the novel concept of mechanically-controlled indicator displacement assay where the well-designed monolayer acts as a mechanically-controllable signal-emission unit [85]. An amphiphilic dilysine peptide host having three important motifs, a phenylboronic acid, a cholesterol moiety, and a carboxyfluorescein indicator at the N-terminus of the peptide, was used as a receptor component at the air-water interface (Figure 11). Carboxyfluorescein fluorescent probe is known to serve as a fluorescence resonance energy transfer acceptor for coumarin-based indicators, such as 4-methylesculetin. We prepared a mixed monolayer of the receptor molecule and a matrix lipid, methyl stearate, where the indicators were also mixed at a surface pressure of 10 mN m-1, excitation at 373 nm produced a blue fluorescence emission around 450 nm arising from the excited 4-methylesculetin chromophore. The same 373 nm excitation produced a new green fluorescence around 530 nm as the surface pressure increased to 20 mN m-1. The presence of this peak signaled an energy transfer between excited 4-methylesculetin and ground state fluorescein. Figure 2B shows the dependence of the fluorescence of the monolayer as a function of ML concentration, with a constant surface pressure of 20 mN m-1. Fluorescence resonance energy transfer efficiency depends on both the distance and conformation of the fluorophores, as well as overlap efficiency of donors and acceptors.
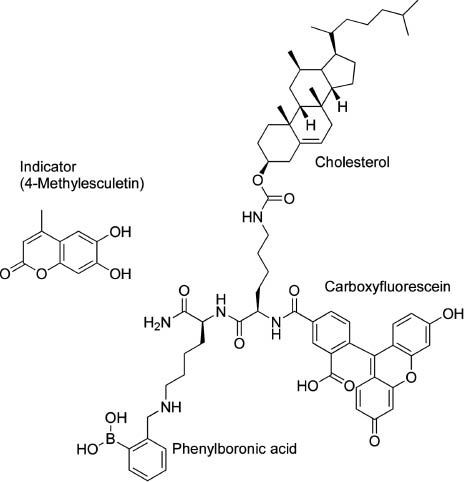
Molecular design for a mechanically-controlled indicator displacement assay.
d-glucose, as a model for the displacement guest, was added to the water subphase beneath the receptor monolayer, resulting in a decrease in the magnitude of the green fluorescence observed for the monolayer with little change in the 4-methylesculetin peak (Figure 12). Resonance energy transfer from the excited 4-methylesculetin to the fluorescein moiety was turned off when the glucose concentration was increased in the subphase. Analyses of the ratio of fluorescence peaks (R=F525/F450) indicates a 1:1 stoichiometric binding where very strong binding of the guest to the host monolayer saturated in the presence of one equivalent of guest. The present method, using host-indicator complexes at a specifically defined interfacial region, might be used for detection at stressed regions by external mechanical forces. This strategy may be further applicable to biological and environmental analyses.
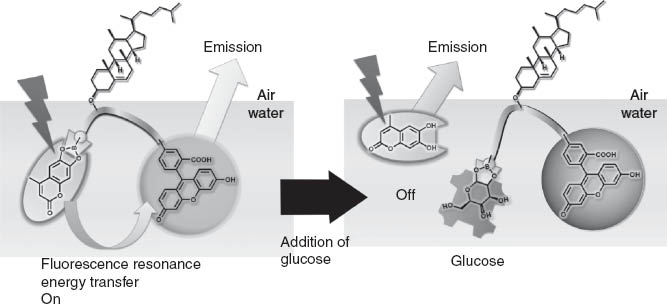
Detection of glucose based on regulation of fluorescence resonance energy transfer.
5 Perspectives
This review briefly overviews trends and possibilities of the potential applications of supramolecular chemistry compiled from recent research examples. Supramolecular chemistry is one field of chemistry while photonics is generally considered to be a branch of physics. Despite this, the potential for overlap between these fields is surprisingly large especially in a nanoscience/nanotechnology context. In nanoscience, chemistry and physics will be agglomerated to reveal new concepts and methodologies. Supramolecular chemistry plays a crucial role in emerging bottom-up nanotechnology where photonic responses are important with connotations for practical applications due to various properties. Therefore, the fusion of the two scientific categories, supramolecular chemistry and nanophotonics, to create a new scientific paradigm, supramolecular nanophotonics, should be highlighted as an important feature of current nanoscience and nanotechnology.
This work was partly supported by World Premier International Research Center Initiative (WPI Initiative), MEXT, Japan and Core Research for Evolutional Science and Technology (CREST) program of Japan Science and Technology Agency (JST), Japan.
References
[1] Hino T, Hasegawa T, Terabe K, Tsuruoka T, Nayak A, Ohno T, Aono M. Atomic switches: atomic-movement-controlled nanodevices for new types of computing. Sci Technol Adv Mater 2011;12:013003.10.1088/1468-6996/12/1/013003Search in Google Scholar
[2] Hasegawa T, Terabe K, Tsuruoka T, Aono M. Atomic switch: atom/ion movement controlled devices for beyond von-neumann computers. Adv Mater 2012;24:252–67.10.1002/adma.201102597Search in Google Scholar
[3] Qu X, Brame J, Li Q, Alvarez PJJ. Nanotechnology for a safe and sustainable water supply: enabling integrated water treatment and reuse. Acc Chem Res 2013;46:834–43.10.1021/ar300029vSearch in Google Scholar
[4] Han A, Hou H, Li L, Kim HS, de Figueiredo P. Microfabricated devices in microbial bioenergy sciences. Trend Biotechnol 2013;31:225–32.10.1016/j.tibtech.2012.12.002Search in Google Scholar
[5] Yu H-Z, Li Y, Ou LM-L. Reading disc-based bioassays with standard computer drives. Acc Chem Res 2013;46:258–68.10.1021/ar300104bSearch in Google Scholar
[6] Kish LB. End of Moore’s law: thermal (noise) death of integration in micro and nano electronics. Phys Lett A 2002;305:144–9.10.1016/S0375-9601(02)01365-8Search in Google Scholar
[7] Thompson SE, Parthasarathy S. Moore’s law: the future of Si microelectronics. Mater Today 2006;9:20–5.10.1016/S1369-7021(06)71539-5Search in Google Scholar
[8] Ariga K, Hill JP, Lee, MV, Vinu A, Charvet R, Acharya S. Challenges and breakthroughs in recent research on self-assembly. Sci Technol Adv Mater 2008;9:014109.10.1088/1468-6996/9/1/014109Search in Google Scholar PubMed PubMed Central
[9] Sakakibara K, Hill JP, Ariga K. Thin-film-based nanoarchitectures for soft matter: controlled assemblies into two-dimensional worlds. Small 2011;7:1288–308.10.1002/smll.201002350Search in Google Scholar PubMed
[10] Li M, Ishihara S, Ji Q, Akada M, Hill JP, Ariga K. Paradigm shift from self-assembly to commanded assembly of functional materials: recent examples in porphyrin/fullerene supramolecular systems. Sci Technol Adv Mater 2012;13:053001.10.1088/1468-6996/13/5/053001Search in Google Scholar PubMed PubMed Central
[11] Ward MD, Raithby PR. Functional behaviour from controlled self-assembly challenges and prospects. Chem Soc Rev 2013;42:1619–36.10.1039/C2CS35123DSearch in Google Scholar
[12] Ariga K, Li M, Richards GJ, Hill JP. Nanoarchitectonics: a conceptual paradigm for design and synthesis of dimension-controlled functional nanomaterials. J Nanosci Nanotechnol 2011;11:1–13.10.1166/jnn.2011.3839Search in Google Scholar PubMed
[13] Ariga K, Vinu A, Yamauchi Y, Ji Q, Hill JP. Nanoarchitectonics for mesoporous materials. Bull Chem Soc Jpn 2012;85:1–32.10.1246/bcsj.20110162Search in Google Scholar
[14] Ariga K, Ji Q, Hill JP, Bando Y, Aono M. Forming nanomaterials as layered functional structures towards materials nanoarchitectonics. NPG Asia Mater 2012;4:e17.10.1038/am.2012.30Search in Google Scholar
[15] Cook TR, Zheng Y-R, Stang PJ. Metal-organic frameworks and self-assembled supramolecular coordination complexes: comparing and contrasting the design, synthesis, and functionality of metal-organic materials. Chem Rev 2013;113:734–77.10.1021/cr3002824Search in Google Scholar PubMed PubMed Central
[16] Stuart MAC, Huck WTS, Genzer J, Mueller M, Ober C, Stamm M, Sukhorukov GB, Szleifer I, Tsukruk VV, Urban M, Winnik F, Zauscher S, Luzinov I, Minko S. Emerging applications of stimuli-responsive polymer materials. Nat Mater 2010;9: 101–13.10.1038/nmat2614Search in Google Scholar PubMed
[17] Pinalli R, Dalcanale E. Supramolecular sensing with phosphonate cavitands. Acc Chem Res 2013;46:399–411.10.1021/ar300178mSearch in Google Scholar PubMed
[18] Jackson AW, Fulton DA. Making polymeric nanoparticles stimuli-responsive with dynamic covalent bonds. Polym Chem 2013;4:31–45.10.1039/C2PY20727CSearch in Google Scholar
[19] Wuerthner F, Kaiser TE, Saha-Moeller CR. J-Aggregates: from serendipitous discovery to supramolecular engineering of functional dye materials. Angew Chem Int Ed 2011;50: 3376–410.10.1002/anie.201002307Search in Google Scholar PubMed
[20] Samanta SK, Bhattacharya S. Wide-range light-harvesting donor-acceptor assemblies through specific intergelator interactions via self-assembly. Chem Eur J 2012;18: 15875–85.10.1002/chem.201103855Search in Google Scholar PubMed
[21] Frischmann PD, Mahata K, Wuerthner F. Powering the future of molecular artificial photosynthesis with light-harvesting metallosupramolecular dye assemblies. Chem Soc Rev 2013;42:1847–70.10.1039/C2CS35223KSearch in Google Scholar
[22] Ajayaghosh A, Praveen VK. π-Organogels of self-assembled p-phenylenevinylenes: soft materials with distinct size, shape, and functions. Acc Chem Res 2007;40:644–56.10.1021/ar7000364Search in Google Scholar PubMed
[23] Ajayaghosh A, Praveen VK, Vijayakumar C. Organogels as scaffolds for excitation energy transfer and light harvesting. Chem Soc Rev 2008;37:109–22.10.1039/B704456ASearch in Google Scholar
[24] Babu SS, Prasanthkumar S, Ajayaghosh A. Self-assembled gelators for organic electronics. Angew Chem Int Ed 2012;51:1766–76.10.1002/anie.201106767Search in Google Scholar PubMed
[25] Prasanthkumar S, Saeki A, Seki S, Ajayaghosh A. Solution phase epitaxial self-assembly and high charge-carrier mobility nanofibers of semiconducting molecular gelators. J Am Chem Soc 2010;132:8866–710.1021/ja103685jSearch in Google Scholar PubMed
[26] Kartha KK, Babu SS, Srinivasan S, Ajayaghosh A. Attogram sensing of trinitrotoluene with a self-assembled molecular gelator. J Am Chem Soc 2012;134:4834–41.10.1021/ja210728cSearch in Google Scholar PubMed
[27] Mahesh S, Gopal A, Thirumalai R, Ajayaghosh A. Light-induced Ostwald ripening of organic nanodots to rods. J Am Chem Soc 2012;134:7227–30.10.1021/ja301002gSearch in Google Scholar PubMed
[28] Gopal A, Hifsudheen M, Furumi S, Takeuchi M, Ajayaghosh A. Thermally assisted photonic inversion of supramolecular handedness. Angew Chem Int Ed 2012;51:10505–9.10.1002/anie.201205332Search in Google Scholar PubMed
[29] Jain A, Rao KV, Kulkarni C, George A, George SJ. Fluorescent coronene monoimide gels via H-bonding induced frustrated dipolar assembly. Chem Commun 2012;48:1467–9.10.1039/C1CC14990CSearch in Google Scholar PubMed
[30] Pérez A, Serrano JL, Sierra T, Ballesteros A, de Saá D, Barluenga J. Control of self-assembly of a 3-hexen-1,5-diyne derivative: toward soft materials with an aggregation-induced enhancement in emission. J Am Chem Soc 2011;133:8110–3.10.1021/ja2018898Search in Google Scholar PubMed
[31] Giansante C, Raffy G, Schäfer C, Rahma H, Kao M-T, Olive AGL, Del Guerzo A. White-light-emitting self-assembled nanofibers and their evidence by microspectroscopy of individual objects. J Am Chem Soc 2011;133:316–25.10.1021/ja106807uSearch in Google Scholar PubMed
[32] Liu Y, Wang T, Liu M. Supramolecular polymer hydrogels from bolaamphiphilic L-histidine and benzene dicarboxylic acids: thixotropy and significant enhancement of EuIII fluorescence. Chem Eur J 2012;18:14650–9.10.1002/chem.201202637Search in Google Scholar PubMed
[33] Park J, Kim J, Seo M, Lee J, Kim SY. Dual-mode fluorescence switching induced by self-assembly of well-defined poly(arylene ether sulfone)s containing pyrene and amide moieties. Chem Commun 2012;48:10556–8.10.1039/c2cc35804bSearch in Google Scholar PubMed
[34] Cao X, Wu Y, Liu K, Yu X, Wu B, Wu H, Gong Z, Yi T. Iridium complex triggered white-light-emitting gel and its response to cysteine. J Mater Chem 2012;22:2650–7.10.1039/C2JM13826CSearch in Google Scholar
[35] Wu J, Liu W, Ge J, Zhang H, Wang P. New sensing mechanisms for design of fluorescent chemosensors emerging in recent years. Chem Soc Rev 2011;40:3483–95.10.1039/c0cs00224kSearch in Google Scholar PubMed
[36] Karuppannan S, Chambron J-C. Supramolecular chemical sensors based on pyrene monomer-excimer dual luminescence. Chem Asia J 2011;6:964–84.10.1002/asia.201000724Search in Google Scholar PubMed
[37] Hargrove AE, Nieto S, Zhang T, Sessler JL, Anslyn EV. Artificial receptors for the recognition of phosphorylated molecules. Chem Rev 2011;111:6603–782.10.1021/cr100242sSearch in Google Scholar PubMed PubMed Central
[38] Sahoo SK, Sharma D, Bera RK, Crisponi G, Callan JF. Iron(III) selective molecular and supramolecular fluorescent probes. Chem Soc Rev 2012;41:7195–227.10.1039/c2cs35152hSearch in Google Scholar PubMed
[39] Leung D, Kang SO, Anslyn EV. Rapid determination of enantiomeric excess: a focus on optical approaches. Chem Soc Rev 2012;41:448–79.10.1039/C1CS15135ESearch in Google Scholar PubMed
[40] Kobayashi H, Ogawa M, Alford R, Choyke PL, Urano Y. New strategies for fluorescent probe design in medical diagnostic imaging. Chem Rev 2010;110:2620–40.10.1021/cr900263jSearch in Google Scholar PubMed PubMed Central
[41] Chen X, Pradhan T, Wang F, Kim JS, Yoon J. Fluorescent chemosensors based on spiroring-opening of xanthenes and related derivatives. Chem Rev 2012;112:1910–56.10.1021/cr200201zSearch in Google Scholar
[42] Vummidi BR, Alzeer J, Luedtke NW. Fluorescent probes for G-quadruplex structures. ChemBioChem 2013;14:540–58.10.1002/cbic.201200612Search in Google Scholar
[43] Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 1985;260:3440–50.10.1016/S0021-9258(19)83641-4Search in Google Scholar
[44] Minta A, Tsien RY. Fluorescent indicators for cytosolic sodium. J Biol Chem 1989;264:19449–57.10.1016/S0021-9258(19)47321-3Search in Google Scholar
[45] He H, Mortellaro MA, Leiner MJ, Fraatz RJ, Tusa JK. A fluorescent sensor with high selectivity and sensitivity for potassium in water. J Am Chem Soc 2003;125:1468–69.10.1021/ja0284761Search in Google Scholar
[46] Komatsu H, Iwasawa N, Citterio D, Suzuki Y, Kubota T, Tokuno K, Kitamura Y, Oka K, Suzuki K. Design and synthesis of highly sensitive and selective fluorescein-derived magnesium fluorescent probes and application to intracellular 3D Mg2+ imaging. J Am Chem Soc 2004;126:16353–60.10.1021/ja049624lSearch in Google Scholar
[47] Hirano T, Kikuchi K, Urano Y, Higuchi T, Nagano T. Novel zinc fluorescent probes excitable with visible light for biological applications. Angew Chem Int Ed 2000;39:1052–4.10.1002/(SICI)1521-3773(20000317)39:6<1052::AID-ANIE1052>3.0.CO;2-5Search in Google Scholar
[48] Walkup GK, Burdette SC, Lippard SJ, Tsien RY. New cell-permeable. Fluorescent probe for Zn2+. J Am Chem Soc 2000;122:5644–5.10.1021/ja000868pSearch in Google Scholar
[49] Pluth MD, Tomat E, Lippard SJ. Biochemistry of mobile zinc and nitric oxide revealed by fluorescent sensors. Ann Rev Biochem 2011;80:333–55.10.1146/annurev-biochem-061009-091643Search in Google Scholar
[50] Fry NL, Mascharak PK. Photoactive ruthenium nitrosyls as NO donors: how to sensitize them toward visible light. Acc Chem Res 2011;44:289–98.10.1021/ar100155tSearch in Google Scholar
[51] Shimomura O. Discovery of green fluorescent protein (GFP) (Novel Lecture). Angew Chem Int Ed 2009;48:5590–602.10.1002/anie.200902240Search in Google Scholar PubMed
[52] Chalfie M. GFP: lighting up life (Nobel Lecture). Angew Chem Int Ed 2009;48:5603–11.10.1002/anie.200902040Search in Google Scholar PubMed
[53] Tsien RY. Constructing and exploiting the fluorescent protein paintbox (Nobel Lecture). Angew Chem Int Ed 2009;48:5612–26.10.1002/anie.200901916Search in Google Scholar PubMed
[54] Miyawaki A, Llopis J, Heim R, McCaffery JM, Adams JA, Ikura M, Tsien RY. Fluorescent indicators for Ca2+ based on green fluorescent proteins and calmodulin. Nature 1997;388:882–7.10.1038/42264Search in Google Scholar PubMed
[55] Zaccolo M, De Giorgi F, Cho CY, Feng LX, Knapp T, Negulescu PA, Taylor SS, Tsien RY, Pozzan T. A genetically encoded, fluorescent indicator for cyclic AMP in living cells. Nature Cell Biology 2000;2:25–9.10.1038/71345Search in Google Scholar PubMed
[56] Shao N, Jin J, Wang H, Zheng J, Yang R, Chan W, Abliz Z. Design of bis-spiropyran ligands as dipolar molecule receptors and application to in vivo glutathione fluorescent probes. J Am Chem Soc 2010;132:725–36.10.1021/ja908215tSearch in Google Scholar PubMed
[57] Lee MH, Han JH, Lee JH, Choi HG, Kang C, Kim JS. Mitochondrial thioredoxin-responding off-on fluorescent probe. J Am Chem Soc 2012;134:17314–9.10.1021/ja308446ySearch in Google Scholar PubMed
[58] Komatsu H, Shindo Y, Kawashima SA, Yamatsugu K, Oka K, Kanai M. Intracellular activation of acetyl-CoA by an artificial reaction promoter and its fluorescent detection. Chem Commun 2013;49:2876–8.10.1039/c3cc40616dSearch in Google Scholar PubMed
[59] De Silva AP, Gunaratne HQN, McCoy CP. A molecular photoionic and gate based on fluorescent signalling. Nature 1993;364:42–4.10.1038/364042a0Search in Google Scholar
[60] Komatsu H, Miki M, Citterio D, Kubota T, Shindo Y, Kitamura Y, Oka K, Suzuki K. Single molecular multianalyte (Ca2+, Mg2+) fluorescent probe and applications to bioimaging. J Am Chem Soc 2005;127:10798–9.10.1021/ja0528228Search in Google Scholar PubMed
[61] Yuan L, Lin W, Xie Y, Chen B, Zhu, S. Single fluorescent probe responds to H2O2, NO, and H2O2/NO with three different sets of fluorescence signals. J Am Chem Soc 2012;134:1305–15.10.1021/ja2100577Search in Google Scholar PubMed
[62] Weissleder R, Tung CH, Mahmood U, Bogdanov A. In vivo imaging of tumors with protease-activated near-infrared fluorescent probes. Nat Biotechnol 1999;17:375–8.10.1038/7933Search in Google Scholar PubMed
[63] Kojima R, Takakura H, Ozawa T, Tada Y, Nagano T, Urano Y. Design and development of near-infrared-emitting firefly luciferins available in vivo. Angew Chem Int Ed 2013;52: 1175–9.10.1002/anie.201205151Search in Google Scholar PubMed
[64] Mori T, Akamatsu M, Okamoto K, Sumita M, Tateyama Y, Sakai H, Hill JP, Abe M, Ariga K. Micrometer-level naked-eye detection of caesium particulates in the solid state. Sci Technol Adv Mater 2013;14:015002.10.1088/1468-6996/14/1/015002Search in Google Scholar PubMed PubMed Central
[65] Ito K. Slide-ring materials using topological supramolecular architecture. Curr Opin Solid State Mater Sci 2010;14:28–34.10.1016/j.cossms.2009.08.005Search in Google Scholar
[66] Boyle MM, Smaldone RA, Whalley AC, Ambrogio, MW, Botros YY, Stoddart JF. Mechanised materials. Chem Sci 2011;2:204–10.10.1039/C0SC00453GSearch in Google Scholar
[67] Ariga K, Mori T, Hill JP, Mechanical control of nanomaterials and nanosystems. Adv Mater 2012;24:158–76.10.1002/adma.201102617Search in Google Scholar PubMed
[68] Teng MJ, Jia X-R, Yang S, Chen X-F, Wei Y. Reversible tuning luminescent color and emission intensity: a dipeptide-based light-emitting material. Adv Mater 2012;24:1255–61.10.1002/adma.201104592Search in Google Scholar PubMed
[69] Ren Y, Kan WH, Thangadurai V, Baumgartner T. Bio-inspired phosphole-lipids: from highly fluorescent organogels to mechanically responsive FRET. Angew Chem Int Ed 2012;51:3964–8.10.1002/anie.201109205Search in Google Scholar PubMed
[70] Sagara Y, Kato T. Brightly tricolored mechanochromic luminescence from a single-luminophore liquid crystal: reversible writing and erasing of images. Angew Chem Int Ed 2011;50:9128–32.10.1002/anie.201100914Search in Google Scholar PubMed
[71] Mizoshita N, Tani T, Inagaki S. Isothermally reversible fluorescence switching of a mechanochromic perylene bisimide dye. Adv Mater 2012;24:3350–5.10.1002/adma.201201064Search in Google Scholar PubMed
[72] Teng M-J, Jia X-R, Chen X-F, Wei Y. A dipeptide-based multicolored-switching luminescent solid material: when molecular assemblies meet mechanochemical reaction. Angew Chem Int Ed 2012;51:6398–401.10.1002/anie.201200320Search in Google Scholar PubMed
[73] Zhang G, Singer JP, Kooi SE, Evans RE, Thomas EL, Fraser CL. Reversible solid-state mechanochromic fluorescence from a boron lipid dye. J Mater Chem 2011;21:8295–9.10.1039/c0jm03871gSearch in Google Scholar
[74] Luo X, Li J, Li C, Heng L, Dong YQ, Liu Z, Bo Z, Tang BZ. Reversible switching of the emission of diphenyldibenzofulvenes by thermal and mechanical stimuli. Adv Mater 2011;23:3261–5.10.1002/adma.201101059Search in Google Scholar PubMed
[75] Zhang G, Lu J, Sabat ML. Fraser CL. Polymorphism and reversible mechanochromic luminescence for solid-state difluoroboron avobenzone. J Am Chem Soc 2010;132: 2160–2.10.1021/ja9097719Search in Google Scholar PubMed
[76] Choi SJ, Kuwabara J, Nishimura Y, Arai T, Takaki Kanbara T. Two-step changes in luminescence color of Pt(II) complex bearing an amide moiety by mechano- and vapochromism. Chem Lett 2012;41:65–7.10.1246/cl.2012.65Search in Google Scholar
[77] Mobius D. Scheibe aggregates. Adv Mater 1995;7:437–44.10.1002/adma.19950070503Search in Google Scholar
[78] Liu X, Wang T, Liu M. Interfacial assembly of a series of cinnamoyl-containing bolaamphiphiles: spacer-controlled packing, photochemistry, and odd-even effect. Langmuir 2012;28:3474–82.10.1021/la204653bSearch in Google Scholar PubMed
[79] Ariga K, Mori T, Hill JP. Control of nano/molecular systems by application of macroscopic mechanical stimuli. Chem Sci 2011;2:195–203.10.1039/C0SC00300JSearch in Google Scholar
[80] Ariga K, Ishihara S, Izawa H, Xia H, Hill JP. Operation of micro and molecular machines: a new concept with its origins in interface science. Phys Chem Chem Phys 2011;13:4802–11.10.1039/c0cp02040kSearch in Google Scholar PubMed
[81] Ariga K, Mori T, Hill JP. Evolution of molecular machines: from solution to soft matter interface. Soft Matter 2012;8:15–20.10.1039/C1SM06832FSearch in Google Scholar
[82] Ariga K, Ito H, Hill JP, Tsukube H. Molecular recognition: from solution science to nano/materials technology. Chem Soc Rev 2012;41:5800–35.10.1039/c2cs35162eSearch in Google Scholar PubMed
[83] Ariga K, Terasaka Y, Sakai D, Tsuji H, Kikuchi J. Piezoluminescence based on molecular recognition by dynamic cavity array of steroid cyclophanes at the air-water interface. J Am Chem Soc 2000;122:7835–6.10.1021/ja000924mSearch in Google Scholar
[84] Ariga K, Nakanishi T, Terasaka Y, Tsuji H, Sakai D, Kikuchi J. Piezoluminescence at the air-water interface through dynamic molecular recognition driven by lateral pressure application. Langmuir 2005;21:976–81.10.1021/la0477845Search in Google Scholar PubMed
[85] Sakakibara K, Joyce LA, Mori T, Fujisawa T, Shabbir SH, Hill JP, Anslyn EV, Ariga K. A mechanically-controlled indicator displacement assay. Angew Chem Int Ed 2012;51:9643–6.10.1002/anie.201203402Search in Google Scholar PubMed
©2013 by Science Wise Publishing & De Gruyter Berlin Boston
Articles in the same Issue
- Masthead
- Masthead
- Graphical abstracts
- In this issue
- Letter
- Comparison of electron energy-loss and quantitative optical spectroscopy on individual optical gold antennas
- Review articles
- Fano-resonant metamaterials and their applications
- Nanophotonics and supramolecular chemistry
- Towards low energy consumption integrated photonic circuits based on Ge/SiGe quantum wells
- Light manipulation principles in biological photonic systems
Articles in the same Issue
- Masthead
- Masthead
- Graphical abstracts
- In this issue
- Letter
- Comparison of electron energy-loss and quantitative optical spectroscopy on individual optical gold antennas
- Review articles
- Fano-resonant metamaterials and their applications
- Nanophotonics and supramolecular chemistry
- Towards low energy consumption integrated photonic circuits based on Ge/SiGe quantum wells
- Light manipulation principles in biological photonic systems

