Abstract
Transamination reactions of two different aminosilanes (trimethyl(tert-butylamino)silane and dimethyl(diamino)silane) with amines (diethylamine, pyrrolidine, tert-butylamine, iso-propylamine, and n-propylamine) are studied. For trimethyl(organoamino)silanes, the reaction of trimethyl(iso-propylamino)- or trimethyl(diethylamino)silane as aminosilanes and aniline or pyrrolidine are good starting materials to produce trimethyl(anilino)- and trimethyl(pyrrolidino)silane. The investigated equilibrium constants of the reactions of monoaminosilanes show a good correlation with quantum chemically calculated values. The reaction of two different diaminosilanes Me2Si(NR1R2)2 and Me2Si(NR3R4)2 containing different amino moieties leads to the formation of mixed diaminosilanes Me2Si(NR1R2)(NR3R4) in most cases.
1 Introduction
Aminosilanes are of great importance in research and industry. For example, they can be used for the synthesis of urea derivatives (Xu et al., 2019) and are investigated to use CO2 as oxygen source of polysiloxanes (Kraushaar et al., 2012; Wiltzsch et al., 2011) and isocyanates (Gründler et al., 2021). Another approach is the insertion of isocyanates in the Si–N bonds to give various new materials, which are interesting, especially as polymers or precursors for ceramics (Herbig et al., 2018a,b; Kraushaar et al., 2017).
For the synthesis of aminosilanes, chlorosilanes are usually reacted with the corresponding amines, whereby the following reaction (Scheme 1) takes place. It can be seen that only half of the amine used reacts to give the aminosilane, and the other half precipitates as hydrochloride.

Synthesis of aminosilanes starting with chlorosilanes and amines.
Another approach deals with the transamination reaction of aminosilanes. In the case of primary amines, this reaction proceeds according to Scheme 2. In Scheme 1, R1 is usually an alkyl moiety, and R2 and R3 can be alkyl or aryl groups. In addition, reactions of aminosilanes with secondary amines and heterocycles are also possible. The advantage of this transamination reaction over the conventional method for the preparation of aminosilanes is that no hydrogen chloride is released during transamination and, therefore, no amine hydrochloride is formed. Separation and purification steps like filtration can be omitted. Furthermore, only one equivalent of the amine needs to be used. This is particularly important if the amine used is expensive or difficult to access. In such cases, a chlorosilane can first be reacted according to Scheme 1 with an easily accessible amine and the resulting aminosilane can then be reacted with the amine that is difficult to access (Larsson and Mjörne, 1949). It is significant to determine favorable combinations of aminosilane and amine that will allow this reaction to be used in a meaningful way. Therefore, the equilibrium and reaction rate constants of the reactions need to be considered. Previous investigations have already shown an increase in the yield using acid catalysts like (NH4)2SO4 and NH4Cl. Additionally, in certain cases, a higher boiling point of the used solvent increases the yield (Fessenden and Crowe, 1961).
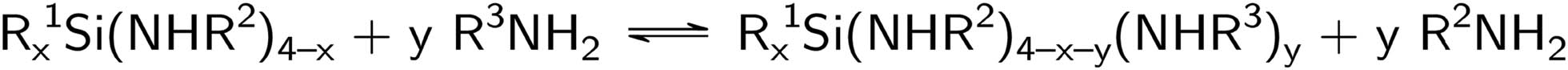
Alternative synthesis route to aminosilanes using a transamination reaction.
A well-known transamination reaction is the reaction of hexamethyldisilazane (HMDS) with amines. In the case of secondary amines, no reaction takes place if the amine has an α-branch (Fessenden and Crowe, 1961; Langer et al., 1958). Reactions of heterocycles with an excess of HMDS gave yields from 42% to 80% (Birkofer et al., 1960). The reaction of hexaphenylcyclotrisilazane with aniline resulted in diphenylbis(anilino)silane in a yield of 23% (Larsson and Bjellerup, 1953). In these reactions with HMDS, ammonia is formed as a joint product which can be easily removed from the reaction mixture.
According to Le Chatelier’s principle, the transamination reaction can be used as a synthesis route if the equilibrium can be shifted to the product side by removing one of the products. Aminosilanes derived from organic amines release new amines in the equilibrium. Therefore, it is not surprising that initial studies indicated that the equilibrium position is very much dependent on the amine used as a nucleophile and the amine that is released. If the released amine can be removed effortlessly, the equilibrium position can be shifted almost completely to the product side. With this technique, many primary aminosilanes could be synthesized in good yields (Larsson et al., 1950, 1951). Using amines with a low sterical hindrance, the formation of disilazanes R1N(SiR2 3)2 was observed, especially in the presence of (NH4)2SO4 as a proton donor. The use of amines with pronounced sterical demand leads to the corresponding monoaminosilanes in good yields (Hils et al., 1966).
For the study of reactions of diaminosilanes with heterocycles, dimethylbis(diethylamino)silane was reacted with γ-butyrolactam and δ-valerolactam. The reaction with γ-butyrolactam gave good results, while the reaction with δ-valerolactam gave an undefinable product mixture (Herbig et al., 2019).
Reactions of amines with sterically demanding residues, low basicity, or high melting points with diaminosilanes were not successful (Yoder and Zuckerman, 1965, 1966).
Furthermore, the reaction of dimethylbis(diethylamino)silane with tetra- and hexamethylenediamine was investigated. The reaction with tetramethylenediamine initially yielded a cyclic compound, which is unstable and tends to polymerize. Hexamethylenediamine directly gives a polymer. It is observed that polymerization with dimethylbis(diethylamino)silane produces polymers with about twice the molar mass of the products obtained by the currently used method with dimethyldichlorosilane (Henglein and Lienhard, 1959).
The reaction of triaminosilanes with primary amines is divided into three steps in which the amine residues are exchanged one after the other. Thus, a mixture of four components can be formed. The proportions of the different substances depend on the ratio of the equilibrium constants. An exchange reaction of two different triaminosilanes was also investigated (Tansjö et al., 1959).
Further, studies dealt with the reactions of secondary amines with triaminosilanes. When a molar ratio of silane/secondary amine of 1:2 was used, complete conversion was always obtained. This illustrates that the reaction was hardly dependent on the amines employed (Radhamani and Padma, 1993).
Triaminosilanes can also be reacted with lactams. These reactions gave several products that only partially could be characterized (Herbig et al., 2019).
Tetrakis(iso-propylamino)silane was also reacted with γ-butyrolactam. Initially, only the disubstituted bis(y-butyrolactam )bis(iso-propylamino)silane was obtained. The reaction in THF gave the highest yield. Another trisubstituted product, tris(γ-butyrolactamo)(iso-propylamino)silane, could only be detected besides the disubstituted product after a reaction time of 5 days (Herbig et al., 2019).
Except for these examples, no systematic investigations on the influence of the amine on the success of transamination reactions have been reported so far. Here, we present studies on the thermodynamics of the transamination of trimethylsilylamines and dimethyldiaminosilanes.
2 Results
To investigate the influence of the amines used in transamination reactions, mixtures of trimethylsilylated amines, dimethyldiaminosilanes, and unsilylated amines were prepared and analyzed using NMR spectroscopy. For reactions of trimethylsilylated amines with unsilylated amines, kinetic aspects were also investigated.
2.1 Monoaminosilanes
The thermodynamic stability of the products is primarily important for the equilibrium constants of the isodesmic reaction between aminosilanes and amines. For reactions of trimethyl(tert-butylamino)silane (M2) with various amines (A1, A3–A6, Table 1) the equilibrium constants were calculated from experimental values.
Substances under investigation
| R = | RH | Me3SiR | Me2SiR2 |
|---|---|---|---|
| i PrNH | A1 | M1 | D11 |
| t BuNH | A2 | M2 | D22 |
| n PrNH | A3 | M3 | D33 |
| C4H8N (pyrrolidyl) | A4 | M4 | D44 |
| PhNH | A5 | M5 | D55 |
| Et2N | A6 | M6 | D66 |
-
All amino moieties are represented with a number (1–6); amines are abbreviated as A, (mono)aminotrimethylsilanes as M and diaminodimethylsilanes as D. In D, two numbers were used to distinguish between both amino moieties.
Additionally, these values were calculated using quantum chemical methods (see Supporting Information) with the conductor-like polarizable continuum model (CPCM) (Barone and Cossi, 1998) for a chloroform solution and without any solvent model (in the gas phase). With the CPCM, the solvent was considered as an approximation, even if the solutions are almost equimolar mixtures of chloroform, aminosilane, and amine. A comparison of the quantum chemically calculated values with the experimentally determined values is shown in Table 2. The calculated values are similar to those determined experimentally. It should be noted that large deviations already occur with a small difference in the theoretical and experimental Gibbs free energy ΔΔG of the reaction (Table 2).
Calculated and experimental equilibrium constants K and the difference of the theoretical and experimental Gibbs free energies of the reactions of M2 with amines A1, A3–A6
| Amine | K exp | K calc | ΔΔG (kJ·mol−1) | ||
|---|---|---|---|---|---|
| Without solvent model | Including solvent model (chloroform) | Without solvent model | Including solvent model (chloroform) | ||
| A1 | 0.67 | 2.70 | 3.23 | 1.58 | 3.33 |
| A3 | 49.36 | 21.99 | 20.78 | 1.96 | 3.32 |
| A4 | 33.28 | 166.86 | 114.60 | 4.02 | 7.98 |
| A5 | 127.32 | 192.03 | 410.37 | 1.05 | 9.69 |
| A6 | 0.12 | 0.28 | 0.45 | 2.04 | 0.32 |
Despite all the differences between the experimental setup and the used quantum chemical models, the match of calculated and experimentally determined values is good enough to confirm that quantum chemical calculations using this protocol can be used to estimate the equilibrium position of the transamination reaction. Keeping Occram’s razor in mind, it can be concluded that no solvent model has to be used in the calculations to get suitable estimations before performing transamination experiments.
Since the experimental data were generated by the analysis of the reaction of M2 with A1 and A3–A6, but the quantum chemical calculations were done for all combinations, an experimental equilibrium constant of the reaction of, for example, M3 with A1, has to be calculated for a full comparison.
From two experiments with the same aminosilane and different amines, the equilibrium constant of other aminosilane–amine combinations can be calculated. For example, from the equilibrium constant of the reaction of M2 with A1 and the reaction of M2 with A3, the equilibrium constant of the reaction of M3 with A1 can be calculated, which is shown in Eqs. 1–3. So, it was possible to calculate the equilibrium constants for all possible combinations of amine and aminosilanes based on the experimentally determined values (Table 3):
Equilibrium constants K derived from experimental data of all possible variations of the starting materials
| Amine | A1 | A2 | A3 | A4 | A5 | A6 |
|---|---|---|---|---|---|---|
| Aminosilane | ||||||
| M1 | — | 1.495 | 73.786 | 49.752 | 190.318 | 0.181 |
| M2 | 0.669 | — | 49.363 | 33.284 | 127.323 | 0.121 |
| M3 | 0.014 | 0.020 | — | 0.674 | 2.579 | 0.002 |
| M4 | 0.020 | 0.030 | 1.483 | — | 3.825 | 0.004 |
| M5 | 0.005 | 0.008 | 0.388 | 0.261 | — | 0.001 |
| M6 | 5.529 | 8.264 | 407.959 | 275.074 | 1,052.256 | — |
Table 3 can be used to identify favorable combinations for the synthesis of aminosilanes using transamination reactions. It should be noted that the aminosilane used must first be prepared via the previously used salt elimination, so the amine used for this synthesis should be available at a low cost.
When using trimethyl(diethylamino)silane, the equilibrium constants are greater than one. Diethylamine is inexpensive and has a low boiling point of 56.3°C (Wächter, 2012). The use of trimethyl(iso-propylamino)silane also causes most equilibrium constants to be greater than 1. Its use has the advantage that iso-propylamine is less expensive and has a lower boiling point than diethylamine at 32.4°C (Wächter, 2012). Low costs and low boiling points are the reasons why these two amines are well suited for the salt elimination route to synthesize aminosilanes that are needed as starting material for the transamination. Due to low costs, it is possible to use two equivalents of the amine in the salt elimination, and the low boiling point simplifies the distillation of the amine during transamination.
If aniline is used as a nucleophile, equilibrium constants significantly greater than 1 are observed, meaning that it displaces all other amines from the silane. Pyrrolidine also has equilibrium constants greater than 1 with trimethyl(diethylamino)- and -(iso-propylamino)silane. Aniline and pyrrolidine are costly and have a high boiling point of 184°C (Wächter, 2012) and 89°C (Schwetlick, 2015), respectively. Thus, the silylation of aniline and pyrrolidine is well suited for the use of the transamination route because only one equivalent of the amine is needed.
After preparing the mixtures of trimethylsilylated amines and unsilylated amines, 29Si NMR spectra were recorded continuously. The reactions take around 1 day to reach the equilibrium. With the amount of substance of the used chemicals, for each spectrum, the concentrations (resp. mass) of all silylated species were calculated.
From the determined data (Table 4), it can be seen that the reaction with pyrrolidine is the fastest, followed by iso- and n-propylamine and aniline. The reaction with diethylamine leads to the smallest rate and therefore the slowest reaction. Here, only the rate constants k 1 are discussed, since k −1 results from the rate constant k 1 and the equilibrium constant K and was not determined directly.
Reaction rate constants k 1 of the investigated reactions of amines A1, A3–A6 with M2
| Amine | Reaction rate constant k 1 (mL·mol−1·s−1) |
|---|---|
| A1 | 35.169 |
| A3 | 49.469 |
| A4 | 82.437 |
| A5 | 8.291 |
| A6 | 2.085 |
The value of the reaction rate constants with starting material variation can be explained primarily by sterical effects. Another possible explanation is the nucleophilicity of the used amine, which plays only a minor role. If the amine is sterically hindered or if the free electron pair is delocalized, the reaction proceeds more slowly. A stronger nucleophilicity, on the other hand, favors the reaction and accelerates it. The electron lone pair of aniline is delocalized among the aromatic ring; therefore, the electron density on the nitrogen is very low. This hinders the nucleophilic attack on the silicon and slows down the reaction. The electron density and thus the nucleophilicity of the aliphatic amines are similar compared to other substance classes, but still different compared to each other (Kanzian et al., 2009), and therefore the sterical hindrance is more decisive in comparison to aniline. Pyrrolidine is the most rigid amine studied, has the highest nucleophilicity (Kanzian et al., 2009), and therefore has the highest reaction rate. The sterical hindrance of the two propylamines are very similar, and the reaction constants are very similar, too. The nucleophilicity of n-propylamine is slightly higher than that of iso-propylamine (Kanzian et al., 2009) and therefore the reaction constant of n-propylamine is slightly higher. Diethylamine has the highest sterical hindrance because of the two free-moving ethyl moieties.
The thermodynamically favorable combinations of amine and aminosilane considered before can now also be evaluated kinetically. The reaction with pyrrolidine has a large rate constant due to the low sterical hindrance and large nucleophilicity. Thus, the reaction times turn out to be small. With aniline, the reaction rate is low due to the delocalization of the free electron pair. Therefore, it takes much longer to reach the equilibrium. These facts have to be considered in planning transamination experiments.
The influence of temperature and pressure on the equilibrium constant and rate constant was studied, monitoring the reaction of M2 with A1 at various temperatures and pressures. The reaction parameters are given in the Supporting Information. The calculated values of the rate constants and the equilibrium constants are given in Table 5. It can be seen that the reaction favors the formation of M1 at lower temperatures, and the reaction rate increases with increasing temperature. Because of the influence of the equilibrium constant on the calculation of the rate constant, a bigger error has to be assumed.
Temperature dependency of the rate constants and equilibrium constants of the reaction of A1 with M2
| Temperature (K) | Rate constant k 1 (mL·mol−1·s−1) | Equilibrium constant |
|---|---|---|
| 280.3 | 1.99 | 8.29 ± 0.07 |
| 289.3 | 3.44 | 3.25 ± 0.02 |
| 296.0 | 35.17 | 0.66 ± 0.08 |
The investigation of the activation volume gave no results, presumably due to the pressure differences which were possible inside the used NMR tubes in the various measurements being too small.
2.2 Diaminosilanes
For the investigation of the transamination of diaminosilanes of the type Me2Si(NR1R2)2 (with R1 = H, alkyl, aryl and R2 = alkyl, aryl), only equilibrium constants were determined. Using a diaminosilane with an amine, two equilibrium constants are possible, as seen in Scheme 3. They are calculated from the molar ratios determined by 29Si NMR and the amount of amine used, as shown in Eqs. 4 and 5. The reaction of two different diaminosilanes is also possible (Scheme 4) and leads to only one equilibrium constant (Eq. 6):
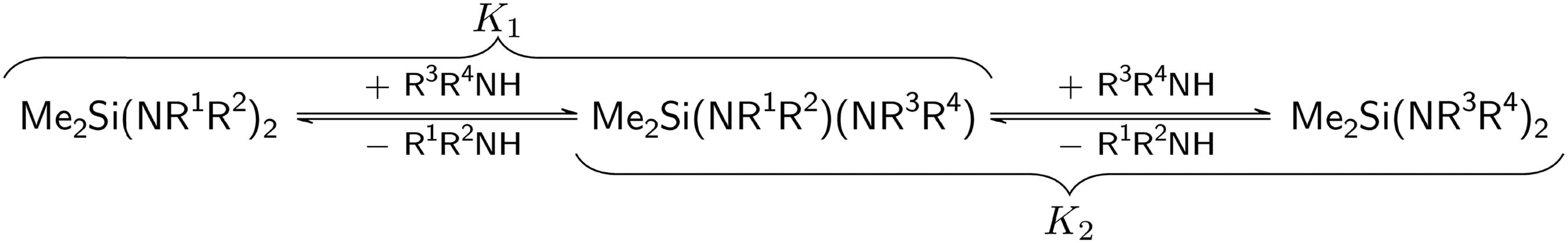
Transamination reaction of dimethyldiaminosilanes with amines, divided into two coupled equilibria with equilibrium constants K 1 and K 2.
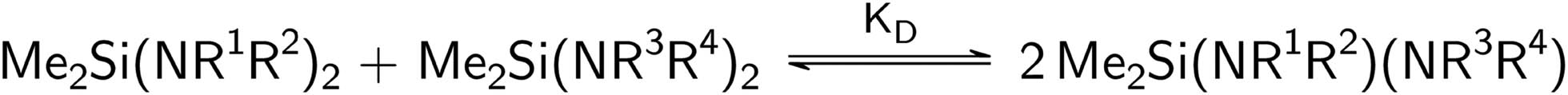
Transamination equilibrium of two dimethyldiaminosilanes.
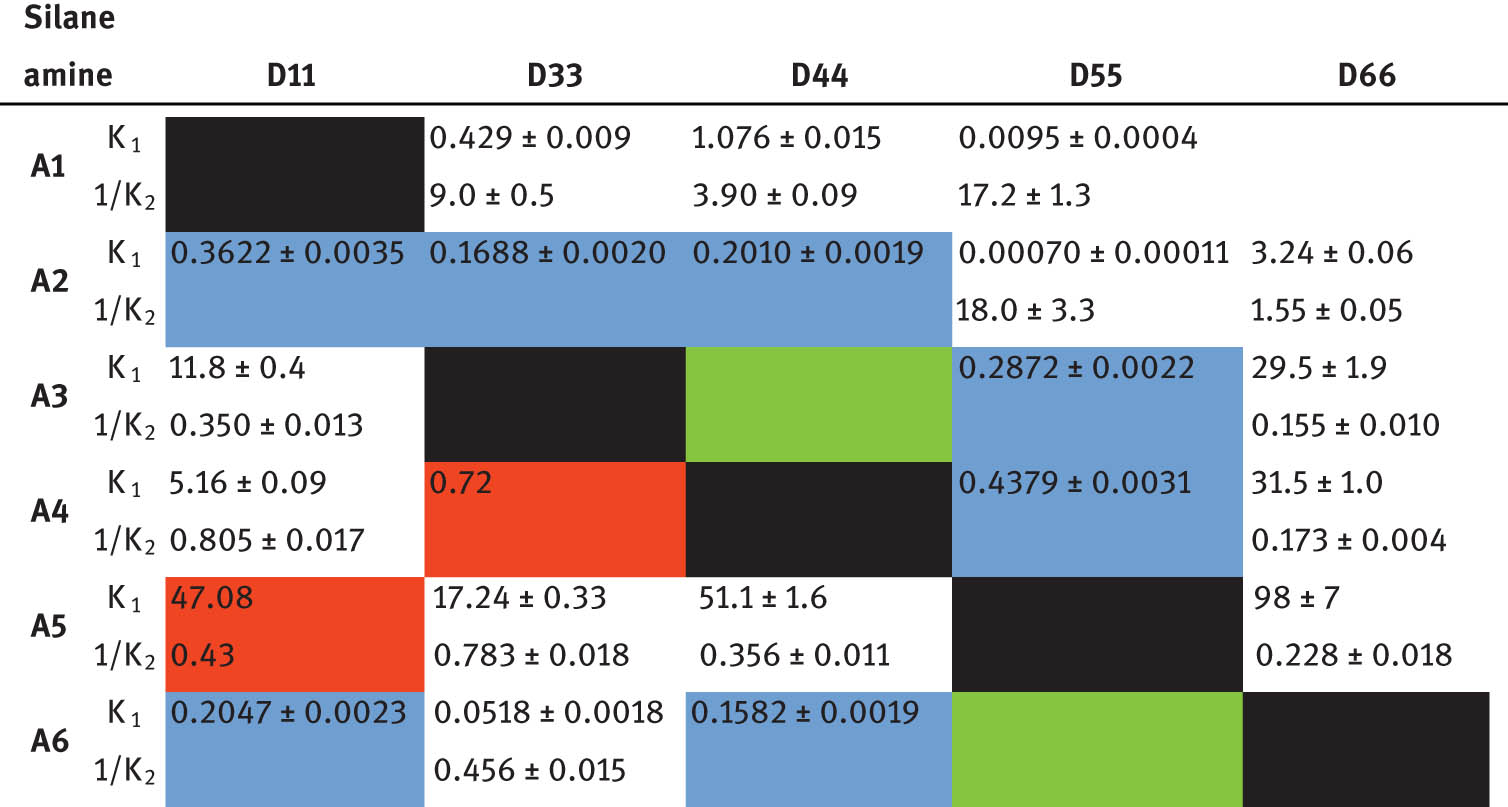
The equilibrium constants for the combination of diaminosilanes and amines were determined via 29Si NMR spectroscopy and are shown in Table 6 and the combination of two aminosilanes in Table 7.
Equilibrium constants of the reaction of a diaminosilane with an amine
 |
-
Green: just starting material; red: no resolved signal (deconvolution is used, if possible); blue: just two signals (no second reaction).
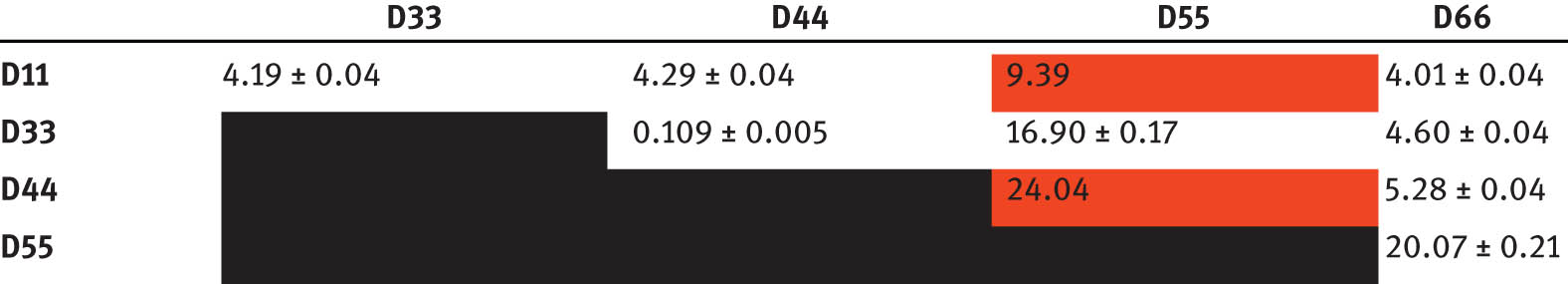
Equilibrium constants K D of the reaction of two diaminosilanes
 |
-
Red: no resolved signal (deconvolution was used, if possible).
In Table 6, the calculated equilibrium constants of the second reaction (K 2) are shown as reciprocal to simplify the comparison to K 1. It can be seen that the values of K 1 of one reaction and 1/K 2 of the reaction of the opposite direction are similar to each other.
A correlation between the reactions of diaminosilanes and monoaminosilanes can be seen. If the value of the equilibrium constant of the reaction of a monoaminosilane with an amine is small, the value of the reaction of the corresponding diaminosilane with the same amine is also small, and vice versa. For example, the reaction of the silane with aniline substituents with diethylamine has, in both investigated systems, the largest equilibrium constant (1,052 for diaminosilanes, 98 for monoaminosilanes).
In Table 7, it can be seen that most combinations of two diaminosilanes have an equilibrium constant K D larger than 1. This shows that mixed aminosilanes are formed preferably. Only the combination of dimethylbis(pyrrolidino)silane and dimethylbis(n-propylamino)silane shows an equilibrium constant smaller than 1, which indicates that only a small amount of the mixed aminosilane was formed.
In the NMR spectra of some reactions, additional signals between 0 and −4 ppm could be observed. These probably originate from the decomposition of CDCl3 and the subsequent reaction with the diaminosilanes. To demonstrate that this decomposition does not affect the calculated equilibrium constants, other solvents were tested. Therefore, the reaction of dimethylbis(n-propylamino)silane with dimethylbis(diethylamino)silane was performed in deuterated benzene and acetone. In acetone, only one NMR spectra could be measured. Afterward, the mixture decomposes into products, which were not further investigated. The resulting equilibrium constant is within the error range of the reaction in CDCl3. During these investigations, it was noticed that the reaction rate significantly depends on the solvent. In CDCl3 and deuterated acetone, the equilibrium was reached after about 1–2 weeks, and in deuterated benzene after several months. Thus, an investigation of the influence of the solvent could be of interest for further studies.
3 Discussion
The calculation of the equilibrium and reaction rate constants of the transamination of monoaminosilanes with amines shows that trimethyl(diethylamino)silane (M6) and trimethyl(iso-propylamino)silane (M1) are very well suited as starting materials for the transamination reactions. This makes them particularly suitable for the production of the aniline (A5) and pyrrolidine (A4) derivatives since these are much more cost-intensive to produce by the conventional route.
The transamination reaction of two diaminosilanes with different amine moieties is suitable for preparing mixed amine diaminosilanes which are otherwise hard to synthesize.
4 Conclusions
In this work, thermodynamic and kinetic aspects of transamination reactions were investigated. By varying the reactants and determining the rate and equilibrium constants, it was possible to draw conclusions about the usefulness of transamination reactions for the synthesis of selected aminosilanes.
Trimethyl(diethylamino)- and trimethyl(iso-propylamino)silane are very well-suited as starting materials for the synthesis of trimethylsilylamines. They are inexpensive to produce or purchase, the boiling points of the resulting amines are very low, and the equilibria are very much on the product side. This makes them particularly suitable for the production of aniline and pyrrolidine derivatives since these amines are much more cost-intensive. In addition, their boiling points are significantly higher than those of the amines released. Pyrrolidine also has the advantage that the rate constant is much larger than that of aniline due to the lower sterical hindrance and higher nucleophilicity. The influence of catalysts or other solvents was not further investigated in this work.
To find suitable starting materials for transamination reactions, quantum chemical calculations without a solvent model are useful to estimate the equilibrium constants for similar systems, while having low computational costs.
Investigation of the temperature dependence of the reaction of trimethyl(tert-butylamino)silane with iso-propylamine revealed a high influence of the temperature on the rate constant and the equilibrium constant.
For the reactions of diaminosilanes Me2Si(NR1R2)2, only the equilibrium constants were investigated. For reactions with amines, it was found that these behave analogous to those of monoaminosilanes. This means that if the equilibrium constant of the reaction of a monoaminosilane with an amine is high, the equilibrium constant of the reaction of the analogous diaminosilane with the same amine is also high.
For the reaction of two diaminosilanes, it was found that the equilibrium is on the side of the mixed diaminosilanes Me2Si(NR1R2)(NR3R4). Therefore, this reaction can be used to synthesize these asymmetric diaminosilanes.
Experimental
Methods
The source and purification of the used chemicals can be found in the Supporting Information.
NMR spectra were measured with a BRUKER AVANCE III 500 MHz (frequencies: 1H: 500.13 MHz; 13C: 125.76 MHz; 29Si: 99.36 MHz) spectrometer in CDCl3 with TMS as an internal standard if not stated otherwise. Kinetic experiments were performed with a saturated solution of Cr(acac)3 in CDCl3 as a solvent to reduce D1 time. To exclude any influence of Cr(acac)3 in NMR solvents, the mixtures of M2 with hexamethyldisiloxane (HMDSO, (Me3Si)2O) in CDCl3 (with or without Cr(acac)3) were prepared and analyzed. The ratio of the molar fraction of both silicon compounds and the fraction of integral values of the signals considering the amount of silicon atoms in the compounds are the same, taking the estimated experimental error into account. To exclude errors introduced by the NMR measurement parameters, a mixture of two aminosilanes was analyzed and the values of the used molar ratio of the aminosilanes and the ratio of the integral values were compared. Both fractions are in good agreement with each other considering the experimental error (weight). These values and spectra can be found in the Supporting Information.
Determination of the equilibrium constants
The equilibrium constants were determined via 29Si NMR spectroscopy. All components (aminosilanes, amines, and CDCl3) were mixed directly in the NMR tubes. All amounts are given in the Supporting Information. The sealed tubes were stored at room temperature for at least 1 week, and then the 29Si NMR spectrum was measured. The signals were integrated with the Software NMRium from Zakodium Sàrl. After storing the tubes for one additional week at room temperature, a second 29Si NMR spectrum was measured, and the signals were integrated. If the integrals gave nearly the same values, no further measurements were taken, otherwise, the procedure was repeated until two consecutive measurements gave the same or at least very similar values for the integrals. Afterwards the amount of the substances contained in the reaction solution was calculated. The calculation of the equilibrium constants, including uncertainties, was done with a Python script, which can be found in the Supporting Information. The range for the integration was determined manually. For the determination of the standard deviation, a small range without any signal was used (from −250 to −190 ppm). The integration of the signals was done using the trapz function of the numpy Python package, and the integrated values were assumed to be equivalent to the concentration of the aminosilanes. From these values, the equilibrium constants were calculated.
Determination of the reaction rate constants
For the determination of the reaction rate constants, samples were prepared, like for the determination of equilibrium constants described before. 29Si NMR spectra were measured at an interval of about 40 min. The amounts of the starting material are given in the Supporting Information. As a solvent, a 1 M solution of Cr(acac)3 in CDCl3 was used. A first integration of the individual spectra was done with the Software MestReNova from Mestrelab Research. With the determination of the equilibrium constants under the same conditions (NMR parameters, including temperature), these values were used for the rate constant determination. A detailed description of the mathematical procedure can be found in the Supporting Information. The calculation of the reaction rate constants was performed with the same Python script as for the equilibrium constants.
Materials
The 1H, 13C, and 29Si NMR spectra of the used aminosilanes can be found in the Supporting Information. As all aminosilanes are found in the literature, only the synthesis and 29Si NMR shifts are given here.
The trimethyl(organoamino)silanes were purchased as stated in the Supporting Information.
Dimethylbis(iso-propylamino)silane (D11)
Dimethyldichlorosilane (7.816 g, 60.6 mmol) was added to an ice-cooled solution of iso-propylamine (15.173 g, 256.7 mmol) in about 80 mL of dry diethylether. The resulting white suspension was stirred for 60 min under reflux and then filtered (G4-fritt). All volatiles were removed in vacuo. About 6 mL of DBU was added, the solution was left overnight, and then filtered (G4-fritt). The mixture was distilled in vacuo yielding 8.76 g (50.3 mmol, 83%) of pure dimethylbis(iso-propylamino)silane (colorless liquid).
δSi (99 MHz; CDCl3; Me4Si) −11.8 ppm (−11.4 ppm, Wiltzsch et al., 2011).
Dimethylbis(n-propylamino)silane (D33)
Dimethyldichlorosilane (4.136 g, 32.0 mmol) was added to an ice-cooled solution of n-propylamine (7.516 g, 127.2 mmol) in about 60 mL of dry diethylether. The resulting white suspension was stirred for 30 min under reflux and then filtered (G4-fritt). All volatiles were removed in vacuo yielding 4.494 g (25.8 mmol, 80%) of pure dimethylbis(n-propylamino)silane (colorless liquid).
δSi (99 MHz; CDCl3; Me4Si) −8.1 ppm (−7.9 ppm, Kraushaar et al., 2012; −9.1 ppm, Kraushaar et al., 2017).
Dimethylbis(pyrrolidino)silane (D44)
Dimethyldichlorosilane (4.011 g, 31.1 mmol) was added to an ice-cooled solution of pyrrolidine (10.398 g, 146.2 mmol) in about 30 mL of dry n-pentane. The resulting white suspension was stirred for 30 min under reflux and then filtered (G4-fritt). All volatiles were removed in vacuo yielding 5.44 g (27.4 mmol, 88%) of pure dimethylbis(pyrrolidino)silane (colorless liquid).
δSi (99 MHz; CDCl3; Me4Si) −8.2 ppm (−8.1 ppm, Herbig et al., 2019).
Dimethylbis(anilino)silane (D55)
Dimethyldichlorosilane (4.365 g, 33.8 mmol) was added to an ice-cooled solution of aniline (14.136 g, 151.8 mmol) in about 35 mL of dry diethylether. The resulting white suspension was stirred for 30 min under reflux and then filtered (G4-fritt). All volatiles were removed in vacuo yielding 7.13 g (29.4 mmol, 87%) of pure dimethylbis(anilino)silane (white solid).
δSi (99 MHz; CDCl3; Me4Si) −10.4 ppm (−10.6 ppm, Kraushaar et al., 2017; −10.4 ppm, Gründler et al., 2021).
Acknowledgement
The authors thank TU Bergakademie Freiberg (Freiberg, Germany) for financial support. B. Kutzner and E. Brendler (Institute for Analytical Chemistry, TU Bergakademie Freiberg) are acknowledged for help with the NMR measurements. Part of this work was performed within the research group “Chemical utilization of carbon dioxide with aminosilanes (CO2-Sil)” that was financially supported by the European Union (European Social Fund), the Ministry of Science and Art of Saxony, and the Sächsische Aufbaubank. The authors thank the reviewer for the helpful comments, which were used to improve the manuscript.
-
Funding information: This research was funded by European Social Fund/Sächsische Aufbaubank/Ministry of Science and Art of Saxony, Deutsche Forschungsgemeinschaft.
-
Author contributions: Steven Knerr: data curation, formal analyses, investigation, methodology, software, visualization, writing – original draft; Marcus Herbig: conceptualization, data curation, funding acquisition, methodology, software, writing – original draft, writing – review and editing, project administration; Edwin Kroke: funding acquisition, resources, supervision, writing – review and editing.
-
Conflict of interest: The authors state no conflict of interest.
-
Data availability statement: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Barone V., Cossi M., Quantum calculation of molecular energies and energy gradients in solution by a conductor solvent model. J. Phys. Chem. A, 1998, 102, (11), 1995–2001. 10.1021/jp9716997.Search in Google Scholar
Birkofer L., Richter P., Ritter A., Aktivierung N-haltiger Heterocyclen durch Silylierung. Chem. Ber., 1960, 93, 2804–2809. 10.1002/cber.19600931207.Search in Google Scholar
Fessenden R., Crowe D.F., An extension of and the reversibility of the silylamine-amine exchange reaction. J. Org. Chem., 1961, 26, 4638–4641. 10.1021/jo01069a106.Search in Google Scholar
Gründler F., Scholz H., Herbig M., Schwarzer S., Wagler J., Kroke E., Formation of Aromatic O-Silylcarbamates from Aminosilanes and their subsequent thermal decomposition with formation of isocyanates. Eur. J. Inorg. Chem., 2021, 2021, 2211–2224. 10.1002/ejic.202100118.Search in Google Scholar
Henglein V.F.A., Lienhard K., Über die Darstellung und Eigenschaften von monomeren, polymeren und cyclischen Methylsilyldiaminen. Makromol. Chem., 1959, 32, 218–225. 10.1002/macp.1959.020320121.Search in Google Scholar
Herbig M., Böhme U., Kroke E., Insertion of CO2 and related heteroallenes into the Si-N-bond of methyl(N-morpholino)silanes. Inorg. Chim. Acta., 2018a, 473, 20–28. 10.1016/j.ica.2017.12.020.Search in Google Scholar
Herbig M., Böhme U., Kroke E., Lactamomethylsilanes – Synthesis, structures, and reactivity towards CO2 and phenylisocyanate. Z. Anorg. Allg. Chem., 2019, 645, 377–387. 10.1002/zaac.201800424.Search in Google Scholar
Herbig M., Böhme U., Schwarzer A., Kroke E., Formation of 1-aza-2-silacyclopentanes and unexpected products from the insertion of phenylisocyanate into 2,2-dimethyl-1-(trimethylsilyl)-1-aza-2-silacyclopentane. Main. Group. Met. Chem., 2018b. 10.1515/mgmc-2018-0005.Search in Google Scholar
Hils J., Hagen V., Ludwig H., Rühlmann K., Über die Si-N-Bindung, XXII. Die Darstellung von N,N-Bis-trimethylsilyl-aminen. Chem. Ber., 1966, 776–779. 10.1002/cber.19660990308.Search in Google Scholar
Kanzian T., Nigst T.A., Maier A., Pichl S., Mayr H., Nucleophilic reactivities of primary and secondary amines in acetonitrile. Eur. J. Org. Chem., 2009, 2009, 6379–6385. 10.1002/ejoc.200900925.Search in Google Scholar
Kraushaar K., Herbig M., Schmidt D., Wagler J., Böhme U., Kroke E., Insertion of phenyl isocyanate into mono- and diaminosilanes. Z. Naturforsch. B, 2017, 72, 909–921. 10.1515/znb-2017-0149.Search in Google Scholar
Kraushaar K., Wiltzsch C., Wagler J., Böhme U., Schwarzer A., Roewer G., et al. From CO2 to Polysiloxanes: Di(carbamoyloxy)silanes Me2Si[(OCO)NRR]2 as Precursors for PDMS. Organometallics, 2012, 31, 4779–4785. 10.1021/om300313f.Search in Google Scholar
Langer S.H., Connell S., Wender I., Preparation and properties of trimethylsilyl ethers and related compounds. J. Org. Chem., 1958, 23, 50–58. 10.1021/jo01095a017.Search in Google Scholar
Larsson E., Bjellerup L., The reaction of diphenyldichlorosilane with ammonia and amines. J. Am. Chem. Soc., 1953, 75, 995–997. 10.1021/ja01100a512.Search in Google Scholar
Larsson E., Carlsson C.G., Webb M., Rottenberg M., Über die Reaktion zwischen Triäthyl-amino-silan und einigen primären Aminen. Acta Chem. Scand., 1950, 4, 45–48. 10.3891/acta.chem.scand.04-0045.Search in Google Scholar
Larsson E., Mårin R., Haug C.M., Stene J., Sörensen N.A., Über Tri-n-propyl-amino-silan und einige verwandte Verbindungen. Acta Chem. Scand., 1951, 5, 1173–1178. 10.3891/acta.chem.scand.05-1173.Search in Google Scholar
Larsson E., Mjörne O., Über eine Reaktion zwischen Triäthyl-N-äthylamino-silan und einigen primären Aminen. Svensk. Kem. Tid., 1949, 59–61.10.3891/acta.chem.scand.04-0045Search in Google Scholar
Radhamani K.N., Padma D.K., A convenient high yield room temperature synthesis of mixed tri(amino)silanes by transamination of tris(dicyclohexylamino)silane and their characterization. Phosphorus, Sulfur. Silicon Relat. Elem., 1993, 79, 65–68. 10.1080/10426509308034398.Search in Google Scholar
Schwetlick K. Organikum. Wiley-VCH GmbH, Weinheim, 2015.Search in Google Scholar
Tansjö L., Strømme K.O., Stenhagen E., Andersson G., Stenhagen E., Palmstierna H., N-Substituted Alkyltriaminosilanes. II. On the reaction of N-Substituted n-Propyltriaminosilanes with Amines. Acta Chem. Scand., 1959, 13, 29–34. 10.3891/acta.chem.scand.13-0029.Search in Google Scholar
Wächter M. Tabellenbuch der Chemie. Wiley-VCH GmbH, Weinheim, 2012.Search in Google Scholar
Wiltzsch C., Kraushaar K., Schwarzer A., Kroke E., CO2 as an oxygen source for polysiloxanes - preparation, crystal structure and thermal decomposition of two novel silylcarbamates. Z. Naturforsch. B, 2011, 66, 917–922. 10.1515/znb-2011-0908.Search in Google Scholar
Xu M., Jupp A.R., Ong M.S.E., Burton K.I., Chitnis S.S., Stephan D.W., Synthesis of urea derivatives from CO2 and silylamines. Angew. Chem. Int. Ed., 2019, 58, 5707–5711. 10.1002/anie.201900058.Search in Google Scholar PubMed
Yoder C.H., Zuckerman J.J., Silicon imidazolidines. Inorg. Chem., 1965, 4, 116–118. 10.1021/ic50023a029.Search in Google Scholar
Yoder C.H., Zuckerman J.J., Amination and transamination as routes to fourth group diamines. J. Am. Chem. Soc., 1966, 88, 4831–4839. 10.1021/ja00973a015.Search in Google Scholar
© 2023 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Two new zinc(ii) coordination complexes constructed by phenanthroline derivate: Synthesis and structure
- Lithium fluoroarylsilylamides and their structural features
- On computation of neighbourhood degree sum-based topological indices for zinc-based metal–organic frameworks
- Two novel lead(ii) coordination complexes incorporating phenanthroline derivate: Synthesis and characterization
- Thermodynamics of transamination reactions with aminotrimethylsilanes and diaminodimethylsilanes
- From synthesis to biological impact of palladium bis(benzimidazol-2-ylidene) complexes: Preparation, characterization, and antimicrobial and scavenging activity
- Novel ruthenium(ii) N-heterocyclic carbene complexes: Synthesis, characterization, and evaluation of their biological activities
- Entropy measures of the metal–organic network via topological descriptors
- Rapid Communication
- Synthesis and crystal structure of an ionic phenyltin(iv) complex of N-salicylidene-valine
- Special Issue: Theoretical and computational aspects of graph-theoretic methods in modern-day chemistry (Guest Editors: Muhammad Imran and Muhammad Javaid)
- Topological indices for random spider trees
- On distance-based indices of regular dendrimers using automorphism group action
- Retraction
- Retraction to “Aluminium(iii), Fe(ii) Complexes and Dyeing Properties of Apigenin(5,7,4′-trihydroxy flavone)”
Articles in the same Issue
- Research Articles
- Two new zinc(ii) coordination complexes constructed by phenanthroline derivate: Synthesis and structure
- Lithium fluoroarylsilylamides and their structural features
- On computation of neighbourhood degree sum-based topological indices for zinc-based metal–organic frameworks
- Two novel lead(ii) coordination complexes incorporating phenanthroline derivate: Synthesis and characterization
- Thermodynamics of transamination reactions with aminotrimethylsilanes and diaminodimethylsilanes
- From synthesis to biological impact of palladium bis(benzimidazol-2-ylidene) complexes: Preparation, characterization, and antimicrobial and scavenging activity
- Novel ruthenium(ii) N-heterocyclic carbene complexes: Synthesis, characterization, and evaluation of their biological activities
- Entropy measures of the metal–organic network via topological descriptors
- Rapid Communication
- Synthesis and crystal structure of an ionic phenyltin(iv) complex of N-salicylidene-valine
- Special Issue: Theoretical and computational aspects of graph-theoretic methods in modern-day chemistry (Guest Editors: Muhammad Imran and Muhammad Javaid)
- Topological indices for random spider trees
- On distance-based indices of regular dendrimers using automorphism group action
- Retraction
- Retraction to “Aluminium(iii), Fe(ii) Complexes and Dyeing Properties of Apigenin(5,7,4′-trihydroxy flavone)”

