Abstract
Three tricyclohexyltin salicylates, 3-X-5-Y-2-HOC6H2COOSn(C6H11-c)3 (where X, Y=H, H 1; H, NO22; NO2, NO23), have been synthesized and characterized by means of elemental analysis, IR, and 1H NMR spectra. The crystal structures of compounds 1–3 are determined by X-ray single crystal diffraction. In these compounds, the carboxylate is monodentate, and the tin atom adopts distorted tetrahedral coordination geometry. In 2 and 3, the neighboring molecules are connected into a one-dimensional supramolecular chain by the intermolecular Sn···O weak interactions between tin and oxygen of nitro group. Bioassay results have shown that the compounds have good in vitro antibacterial activity against Escherichiacoli.
Introduction
Organotin carboxylates have received considerable attention due to their structural interest and various applications in the last few decades (Tiekink, 1991, 1994; Davies et al., 2008). Some organotin carboxylates possess potent activities against tumors, fungi, bacteria, and other microorganisms (Davies et al., 2008; Hadjikakou and Hadjiliadis, 2009; Tian et al., 2013; Amir et al., 2014; Wang et al., 2014; Mao et al., 2015). The study of structure-activity relationships has shown that organotin moiety and carboxylates appear to play an important role in determining their biological activity (Davies et al., 2008; Hadjikakou and Hadjiliadis, 2009; Amir et al., 2014; Tian et al., 2014). Thus, to synthesize new organotin carboxylates by the combination of organotin moiety with carboxylic acid with biological activity will be a good selection. Salicylic acid is widely used as a plant growth regulator and preservative in food products. It has antiseptic and antifungal properties and is widely used in organic synthesis. Several groups have reported the syntheses, structures, and biological activities of some organotin salicylates such as trimethyltin salicylate (Smith et al., 1986), triphenyltin salicylate (Vollano et al., 1984), triphenyltin 5-((E)-2-phenyl-1-diazenyl)salicylate (Basu Baul et al., 2001), dimethyltin bissalicylate (Basu Baul et al., 1996), di-n-butyltin bissalicylate (Narula et al., 1992), and di-n-butyltin bis(5-chlorosalicylate) (Gielen et al., 1994). However, less attention has been paid to tricyclohexytin salicylates. In order to continue to expand the structural chemistry and therapeutic potential of organotin salicylates, we synthesized three tricyclohexyltin salicylates, 3-X-5-Y-2-HOC6H2COOSn(C6H11-c)3 (where X, Y=H, H 1; H, NO22; NO2, NO23), and determined their crystal structures and in vitro antibacterial activity (Scheme 1).
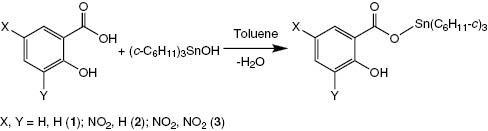
Synthesis of compounds 1–3.
Results and discussion
Synthesis
Compounds 1–3 are prepared by the reaction of tricyclohexyltin hydroxide with salicylic acid in equal mole ratio in anhydrous toluene with the yield of 85%–91% (Scheme 1).
These compounds are white or yellow crystals, air stable, and soluble in common polar organic solvents such as methanol, chloroform, acetone, and N,N-dimethylformamide, but insoluble in water and saturated hydrocarbons such as n-hexane and petroleum ether.
Spectroscopic characterization
In the IR spectra of compounds 1–3, the ν(O-H) band of phenolic hydroxyl appears at ~3200 cm-1, whereas the broad band of 3300–2600 cm-1 assigned to ν(O-H) of COOH of free salicylic acid does not appear, which indicates the deprotonation of the carboxyl of salicylic acid on complexation with the tin atom (Deacon and Phillips, 1980; Tian et al., 2015a,b). The strong bands at ~1635 and ~1315 cm-1 are assigned to the asymmetrical stretching vibration, νas(COO-), and symmetrical stretching vibration, νs(COO-), of the salicylate, respectively. The difference between the νas(COO-) and νs(COO-) bands, Δν(COO-), is 326 cm-1 for 1, 318 cm-1 for 2, and 320 cm-1 for 3, which is obviously larger than that observed in the sodium salt of free acid (227 cm-1 for sodium salicylate, 249 cm-1 for sodium 5-nitrosalicylate, and 240 cm-1 for 3,5-dinitrosalicylate), indicating that the carboxylate group is coordinated to tin in monodentate mode in the solid state (Deacon and Phillips, 1980; Szorcsik et al., 2004; Li et al., 2010).
In the 1H NMR spectra of 1–3, the single resonances of phenolic OH appear at ~12 ppm, and the resonances of COOH of the free ligands are not observed at ~15 ppm, which further confirms the replacement of the carboxylic acid protons by the tricyclohexyltin moiety on complex formation (Tian et al., 2015a,b; Zhang et al., 2015). Complexes 1–3 show the multiplets in the range of 1.25–2.09 ppm due to the cyclohexyl protons. The proton resonances of the benzene ring of 1–3 appear in the range of 6.86–9.02 ppm.
Crystal structure of 1–3
The structures of complexes 1–3 are shown in Figures 1–3, and the selected geometric parameters are given in Table 1. Compound 1 crystallizes in the monoclinic space group P21/c. The tin atom is four-coordinated, and the coordination geometry is a distorted tetrahedron shaped by three carbon atoms of cyclohexyl groups and one carboxyl O(1) from the carboxylate ligand. The bond angles around the tin atom are in the range of 94.74(7)°–117.80(8)°. The separation between the carbonyl O(2) atom of the carboxylate ligand and the Sn atom is Sn(1)···O(2) 2.971(4) Å. This distance is shorter than the sum of the Van der Waals radii of tin and oxygen (3.73 Å) and much longer than the sum of the covalent radii of tin and oxygen (2.14 Å) (Hu et al., 2003). Distortions from the ideal geometry may be rationalized partly by the weak Sn(1)···O(2) interaction. The monodentate mode of coordination of carboxylate is also reflected in two disparate C-O bond lengths (C(19)-O(1) 1.293(3) Å, C(19)-O(2) 1.235(3) Å) of the carboxylate ligand (Table 1). The four bond lengths around Sn are similar to those found in other reported tricyclohexyltin carboxylates, such as tricyclohexyltin indole-3-acetate (Molloy et al., 1986), 2-(4-chlorophenyl)-3-methylbutyrate (Song et al., 2003), ferrocenecarboxylate (Dong et al., 2014), and phenoxyacetate (Zhang et al., 2015).
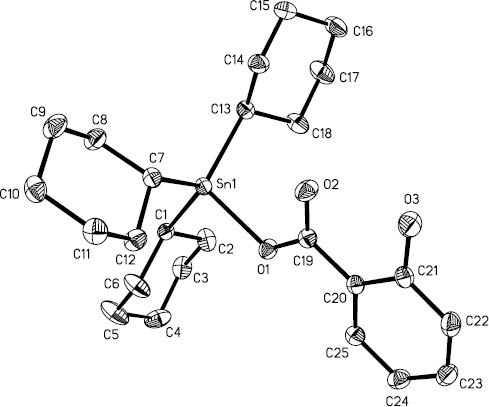
The molecular structure of 1.
Hydrogen atoms are omitted for clarity.
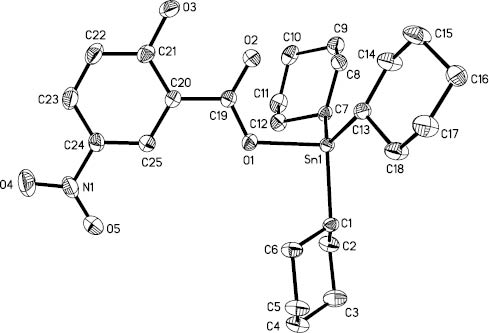
The molecular structure of 2.
Hydrogen atoms are omitted for clarity.

The molecular structure of 3.
Hydrogen atoms are omitted for clarity.
Selected bond lengths (Å) and angles (°) for 1–3.
| 1 | 2 | 3 | |
|---|---|---|---|
| Bond (Å) | |||
| Sn(1)-O(1) | 2.0890 (16) | 2.115 (3) | 2.126 (3) |
| Sn(1)-C(1) | 2.162 (2) | 2.154 (4) | 2.144 (4) |
| Sn(1)-C(7) | 2.154 (2) | 2.151 (4) | 2.156 (4) |
| Sn(1)-C(13) | 2.155 (2) | 2.156 (4) | 2.143 (4) |
| C(19)-O(1) | 1.293 (3) | 1.292 (5) | 1.267 (5) |
| C(19)-O(2) | 1.235 (3) | 1.236 (5) | 1.248 (4) |
| Angles (°) | |||
| O(1)-Sn(1)-C(1) | 94.74 (7) | 94.66 (14) | 90.80 (12) |
| O(1)-Sn(1)-C(7) | 105.94 (8) | 99.85 (14) | 100.60 (13) |
| O(1)-Sn(1)-C(13) | 107.49 (8) | 101.60 (15) | 102.66 (16) |
| C(1)-Sn(1)-C(7) | 117.80 (8) | 115.76 (16) | 119.10 (15) |
| C(1)-Sn(1)-C(13) | 113.05 (8) | 111.52 (15) | 112.47 (19) |
| C(7)-Sn(1)-C(13) | 114.92 (9) | 125.61 (17) | 122.40 (17) |
Compounds 2 and 3 crystallize in the monoclinic space group C2/c and P21/n, respectively. The structures of 2 and 3 are similar to compound 1, and the tin atom also has a distorted tetrahedral geometry with the angles in the range of 94.66(14)°–125.61(17)° for 2 and 90.80(12)°–122.40(17)° for 3, respectively. In 2 and 3, there is a long Sn(1)···O(4)i contact (3.090(4) Å for 2 and 3.039(4) Å for 3) (symmetry operation i=1.5-x, 0.5+y, 0.5-z for 2 and 1.5-x, -0.5+y, 1.5-z for 3) between the tin atom and the O(4) atom of nitro (N(1)O(4)O(5)) from an adjacent molecule. Through the intermolecular interaction molecules are connected to each other into a one-dimensional supramolecular chain (Figures 4 and 5). The atom O(4)i exerts a steric influence on the atom Sn(1) from the opposite side of the atom O(1), and thus contributes to the distortion of the tetrahedral geometry around the Sn atom, by opening up the C-Sn(1)-C angles (111.52(15)°–125.61(17)° for 2 and 112.47(19)°-122.40(17)° for 3) and contracting the O(1)-Sn(1)-C angles (94.66(14)°–101.60(15)° for 2 and 90.80(12)°–102.66(16)° for 3). The hydroxy group (O(3)H) in each molecule of compounds 1–3 forms an intramolecular hydrogen bond with the carboxylate carbonyl oxygen atom (O(2)) of the same ligand (Table 2), and the O(2)-C(19)-C(20)-C(21)-O(3) in each molecule is essentially planar.

The zigzag chain of 2 formed by the intermolecular Sn···O interaction.

The zigzag chain of 3 formed by the intermolecular Sn···O interaction.
Hydrogen bond O(3)-H(3)···O(2) distances in compounds 1–3.
| O(3)-H(3) (Å) | H(3)···O(2) (Å) | O(3)···O(2) (Å) | O(3)-H(3)···O(2) (°) | |
|---|---|---|---|---|
| 1 | 0.82 | 1.860 | 2.579 (4) | 145.8 |
| 2 | 0.82 | 1.799 | 2.526 (4) | 147.0 |
| 3 | 0.82 | 1.778 | 2.511 (3) | 147.8 |
Antibacterial activity
The antibacterial activity of compounds 1–3, tricyclohexyltin hydroxide, the free acids and the reference drugs, penicillin sodium (sodium (2S,5R,6R)-3,3-dimethyl-7-oxo-6-[(phenylacetyl)amino]-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylate) and cefazolin sodium (sodium (6R,7R)-7-amino-8-oxo-3-[[(1H-1,2,3-triazol-4-yl)-sulphanyl]methyl]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate), is listed in Table 3. The results showed that compounds 1–3 against Escherichia coli are active, and the activity is better than that of the substrate (Cy3SnOH), the ligand (free acid), and penicillin sodium, but is weaker than that of cefazolin sodium. Compared with the reported tricyclohexyltin carboxylates such as 2-phenyl-1,2,3-triazole-4-carboxylate [minimum inhibitory concentration (MIC)=13.50 μg mL-1] (Tian et al., 2005), ferrocenecarboxylate (MIC=23.98 μg mL-1) (Dong et al., 2014), and phenoxyacetate (MIC=25.22 μg mL-1) (Zhang et al., 2015), these salicylates are more active. The activity of the three compounds decreased in the order 3>2>1 under experimental conditions. Thus, the carboxylate ligand of trioganotin carboxylates appears to play an important role in determining their antibacterial activity, and further structure modification of organotin compounds by selecting a suitable carboxylate ligand is valuable for enhancing activity.
Antibacterial activity (MIC, μg mL-1) of the compounds.
| Compound | Escherichia coli |
|---|---|
| 1 | 6.04 |
| 2 | 5.40 |
| 3 | 3.85 |
| (c-C6H11)3SnOH | 31.23 |
| 2-HOC6H4COOH | 25.74 |
| 5-NO2-2-HOC6H3COOH | 24.04 |
| 3,5-(NO2)2-2-HOC6H2COOH | 15.15 |
| Penicillin sodium | 8.03 |
| Cefazolin sodium | 2.01 |
MIC, Minimum inhibitory concentration.
Conclusions
Three tricyclohexyltin salicylates have been synthesized and characterized. In 1–3, the carboxylate is in monodentate coordination to the tin atom, and the tin atom possesses a distorted tetrahedral geometry. In 2 and 3, molecules are connected to each other into a zigzag supramolecular chain by the intermolecular Sn···O interaction between the tin atom and the oxygen atom of nitro from an adjacent molecule. The compounds have good activity against Escherichiacoli, and can be considered as antibacterial compounds to further study.
Experimental details
General
All chemicals used in the syntheses were of analytical grade and purchased from commercial sources (Sinopharm Chemical Reagent Company Limited, Shanghai, China) and used as received. Carbon and hydrogen analyses were determined using a Perkin Elmer 2400 Series II elemental analyzer (Perkin Elmer, Waltham, MA, USA). IR spectra were recorded on a Nicolet Nexus 470 FT-IR spectrophotometer using KBr disks in the range of 4000–400 cm-1 (Thermo Nicolet Corporation, Madison, WI, USA). 1H NMR spectral data were collected using a Bruker Avance DPX300 NMR spectrometer (Bruker Corporation, Switzerland) with CDCl3 as solvent and tetramethylsilane as internal standard.
Synthesis of the complexes
Tricyclohexyltin salicylate (1):
To a suspension of tricyclohexyltin hydroxide (0.77 g, 2 mmol) in 50 mL of toluene was added salicylic acid (0.28 g, 2 mmol). Under electromagnetic stirring, the reaction mixture was heated under reflux for 5 h with a Dean-Stark separator, and then allowed to cool to room temperature. The solution was filtered and the solvent was removed under reduced pressure by a rotary evaporator. The resulting white solid was recrystallized from methanol and dried in a vacuum dryer for 24 h to afford a colorless crystal of 1 (0.92 g, 91%). M.p. 105–106°C. Anal. calcd. for C25H38O3Sn (%): C, 59.43; H, 7.58. Found: C, 59.54; H, 7.52. IR (KBr): 3195 [br, ν(OH)], 1632 [ν(COO-)as], 1306 [ν(COO-)s] cm-1. 1H NMR (CDCl3) δ: 1.25–1.99 (m, 33H, c-C6H11), 6.86–6.99 (m, 2H, Ar-H-3 and H-5), 7.42 (dt, J=8.2, 1.8 Hz, 1H, Ar-H-4), 7.80 (dd, J=8.2, 1.8 Hz, 1H, Ar-H-6), 11.49 (s, 1H, OH) ppm.
Tricyclohexyltin 5-nitrosalicylate (2):
This compound was prepared in the same way as 1 by the reaction of tricyclohexyltin hydroxide (0.77 g, 2 mmol) with 5-nitrosalicylic acid (0.37 g, 2 mmol). Yield 0.95 g (86%). M.p. 112–113°C. Anal. calcd. for C25H37NO5Sn (%): C, 54.57; H, 6.78; N, 2.55. Found: C, 54.39; H, 6.56, N, 2.52. IR (KBr): 3210 [br, ν(OH)], 1638 [ν(COO-)as], 1320 [ν(COO-)s] cm-1, 1H NMR (CDCl3) δ: 1.37–2.09 (m, 33H, c-C6H11), 7.03 (d, J=9.0 Hz, 1H, Ar-H-3), 8.27 (dd, J=9.0, 2.0 Hz, 1H, Ar-H-4), 8.79 (d, J=2.0 Hz, 1H, Ar-H-6), 12.57 (s, 1H, OH) ppm.
Tricyclohexyltin 3,5-dinitrosalixylate (3):
Complex 3 was prepared by the same procedure as 1 by the reaction of tricyclohexyltin hydroxide (0.77 g, 2 mmol) with 3,5-dinitrosalicylic acid (0.46 g, 2 mmol). Yield 1.01 g (85%). M.p. 134–135°C. Anal. calcd. for C25H36N2O7Sn (%): C, 50.44; H, 6.10; N, 4.71. Found: C, 50.39; H, 6.05, N, 4.72. IR (KBr): 3208 [br, ν(OH)], 1635 [ν(COO-)as], 1315 [ν(COO-)s] cm-1. 1H NMR (CDCl3) δ: 1.34–2.04 (m, 33H, c-C6H11), 8.87 (d, J=3.0 Hz, 1H, Ar-H-6), 9.02 (d, J=3.0 Hz, 1H, Ar-H-4), 12.69 (s, 1H, OH) ppm.
X-ray crystallography
The colorless single crystals of 1–3 were obtained from methanol by slow evaporation at room temperature. Diffraction measurements were performed on a Bruker Smart Apex imaging-plate area detector fitted with graphite monochromatized MoKα radiation (0.71073 Å) using the φ and ω scan technique at 295(2) K. Empirical corrections for absorption effects were made using the SADABS program (Sheldrick, 1996). The structures were solved by direct methods and refined by a full-matrix least-squares procedure based on F2 using SHELXL-97 (Sheldrick, 2008). The non-hydrogen atoms were refined anisotropically and hydrogen atoms were placed at calculated positions in the riding model approximation, with C-H=0.93 Å for aromatic and formyl H atoms, C-H=0.97 Å for methylene H atoms, C-H=0.98 Å for methine H atoms, and O-H=0.82 Å for hydroxy H atoms. Crystal data, collection procedures, and refinement results are shown in Table 4. The crystallographic data of compounds 1–3 have been deposited with the Cambridge Crystallographic Data Centre as supplementary publication numbers CCDC 1471305, 1471306, and 1471307.
Crystallographic data and structure refinement for 1–3.
| Compound | 1 | 2 | 3 |
|---|---|---|---|
| Empirical formula | C25H38O3Sn | C25H37NO5Sn | C28H39N2O7Sn |
| Formula weight | 505.24 | 550.25 | 634.30 |
| Crystal system | Monoclinic | Monoclinic | Monoclinic |
| Space group | P21/c | C2/c | P21/n |
| a (Å) | 17.0790 (12) | 16.2724 (12) | 10.7198 (15) |
| b (Å) | 9.3944 (7) | 17.4651 (12) | 18.013 (3) |
| c (Å) | 15.8293 (11) | 18.5199 (13) | 15.025 (2) |
| α (°) | 90 | 90 | 90 |
| β (°) | 92.028 (2) | 92.762 (2) | 94.962 (2) |
| γ (°) | 90 | 90 | 90 |
| Volume (Å3) | 2425.1 (3) | 5257.2 (6) | 2890.4 (7) |
| Z | 4 | 8 | 4 |
| Dc (g·cm-3) | 1.384 | 1.390 | 1.458 |
| μ (mm-1) | 1.075 | 1.005 | 0.930 |
| F(000) | 1048 | 2272 | 1308 |
| θ range | 2.50–25.99 | 1.71–26.00 | 1.77–26.00 |
| Crystal size (mm) | 0.46×0.42×0.36 | 0.36×0.36×0.22 | 0.24×0.16×0.12 |
| Unique reflections | 20 551 (Rint=0.023) | 16 613 (Rint=0.027) | 22 123 (Rint=0.033) |
| Reflections [I>2σ(I)] | 4769 | 5174 | 5678 |
| Goodness of fit on F2 | 1.050 | 1.034 | 1.025 |
| R indices [I>2σ(I)] | R=0.025, wR=0.065 | R=0.044, wR=0.118 | R=0.043, wR=0.103 |
| R indices (all data) | R=0.029, wR=0.067 | R=0.058, wR=0.128 | R=0.054, wR=0.110 |
| Δρmax, Δρmin/(e,·Å-3) | 0.478, -0.338 | 0.969, -0.353 | 1.040, -0.614 |
Antibacterial activity
The antibacterial activity of compounds 1–3 against Escherichia coli was determined by a microcalorimetric method according to the literature (Zhang et al., 2004, 2015). A 2277 Thermal Activity Monitor (Thermometric AB, Sweden) was used to determine the power-time curves of bacterial growth at 310 K. Based on the data of power-time curves and theoretical model, the growth rate constants were calculated (Zhang et al., 2004). The relationship between the growth rate constants (μ) and concentration (C) of organotin medicine was fitted by using a computer (see the μ-C curves in Figures S-1–S-7 in the online supplementary material). When the growth rate constant was 0, the MIC was confirmed.
Funding source: Natural Science Foundation of Shandong Province
Award Identifier / Grant number: ZR2013BM007
Funding source: Shandong Province
Award Identifier / Grant number: BS2014CL015
Funding statement: This work was supported by Shandong Provincial Natural Science Foundation, China (ZR2013BM007), the Experimental Teaching Reform Project of Qufu Normal University (No. sj201401), and the Research Award Fund for Outstanding Young Scientists in Shandong Province (BS2014CL015).
Acknowledgments
This work was supported by Shandong Provincial Natural Science Foundation, China (ZR2013BM007), the Experimental Teaching Reform Project of Qufu Normal University (No. sj201401), and the Research Award Fund for Outstanding Young Scientists in Shandong Province (BS2014CL015).
References
Amir, M. K.; Khan, S.; Rehman, Z.; Shah, A; Butler, I. S. Anticancer activity of organotin carboxylates. Inorg. Chim. Acta2014, 423, 14–25.10.1016/j.ica.2014.07.053Suche in Google Scholar
Basu Baul, T. S.; Tiekink, E. R. T. Bis(2-hydroxybenzoato-O,O’)dimethyltin. Acta Crystallogr. Sect. C1996, 52, 1959–1961.10.1107/S0108270196005057Suche in Google Scholar
Basu Baul, T. S.; Dhar, S.; Pyke, S. M.; Tiekink, E. R. T.; Rivarola, E.; Butcher, R.; Smith, F. E. Synthesis and characterization of triorganotin(IV) complexes of 5-[(E)-2-(aryl)-1-diazenyl]-2-hydroxybenzoic acids. J. Organomet. Chem.2001, 633, 7–17.10.1016/S0022-328X(01)01024-5Suche in Google Scholar
Davies, A. G.; Gielen, M.; Pannell, K. H.; Tiekink, E. R. T. Tin Chemistry: Fundamentals, Frontiers, and Applications; John Wiley & Sons: Chichester, UK, 2008.10.1002/9780470758090Suche in Google Scholar
Deacon, G. B.; Phillips, R. J. Relationships between the carbon-oxygen stretching frequencies of carboxylate complexes and the type of carboxylate coordination. Coord. Chem. Rev.1980, 33, 227–250.10.1016/S0010-8545(00)80455-5Suche in Google Scholar
Dong, Y.; Yu, Y.; Tian, L. Synthesis, structural characterization and antibacterial activity of triorganotin ferrocenecarboxylates. Main Group Met. Chem.2014, 37, 91–95.10.1515/mgmc-2014-0027Suche in Google Scholar
Gielen, M.; Boualam, M.; Mahieu, B.; Tiekink, E. R. T. Crystal structure and in vitro antitumour activity of dibutylbis(5-chloro-2-hydroxybenzoato)tin(IV). Appl. Organomet. Chem.1994, 8, 19–23.10.1002/aoc.590080105Suche in Google Scholar
Hadjikakou, S. K.; Hadjiliadis, N. Antiproliferative and anti-tumor activity of organotin compounds. Coord. Chem. Rev.2009, 253, 235–249.10.1016/j.ccr.2007.12.026Suche in Google Scholar
Hu, S.; Zhou, C.; Cai, Q. Average Van der Waals radii of atoms in crystals. Acta Phys. Chim. Sin.2003, 19, 1073–1077.10.3866/PKU.WHXB20031118Suche in Google Scholar
Li, W.; Du, D.; Liu, S.; Zhu, C.; Sakho, A. M.; Zhu, D.; Xu, L. Self-assembly of a novel 2D network polymer: syntheses, characterization, crystal structure and properties of a four-tin-nuclear 36-membered diorganotin(IV) macrocyclic carboxylate. J. Organomet. Chem.2010, 695, 2153–2159.10.1016/j.jorganchem.2010.06.001Suche in Google Scholar
Mao, W.; Bao, K.; Feng, Y.; Wang, Q.; Li, J.; Fan, Z. Synthesis, crystal structure, and fungicidal activity of triorganotin1-methyl-1H-imidazole-4-carboxylates. Main Group Met. Chem. 2015, 38, 27–30.10.1515/mgmc-2014-0040Suche in Google Scholar
Molloy, K. C.; Purcell, T. G.; Hahn, E.; Schumann H.; Zuckerman, J. J. Organotin biocides. Crystal and molecular structure of tricyclohexylstannyl 3-indolylacetate, incorporating the first monodentate carboxylate group bonded to a triorganotin. Organometallics1986, 5, 85–89.10.1021/om00132a014Suche in Google Scholar
Narula, S. P.; Bharadwaj, S. K.; Sharda, Y.; Day, R. O.; Howe, L.; Holmes, R. R. Dimeric structures of di-n-butyltin(IV) ortho-substituted dibenzoates. Organometallics1992, 11, 2206–2211.10.1021/om00042a039Suche in Google Scholar
Sheldrick, G. M. SADABS: Program for Empirical Absorption Correction of Area Detector Data; University of Göttingen: Göttingen, Germany, 1996.Suche in Google Scholar
Sheldrick, G. M. A short history of SHELX. Acta Crystallogr.2008, A64, 112–122.10.1107/S0108767307043930Suche in Google Scholar PubMed
Smith, P. J.; Day, R. O.; Chandrasekhar, V.; Holmes, J. M.; Holmes, R. R. Chain structures of trimethyltin esters of salicylic acid and o-anisic acid. Tin-119m Moessbauer study of a series of trimethyltin and triphenyltin carboxylates. Inorg.Chem.1986, 25, 2495–2499.10.1021/ic00235a005Suche in Google Scholar
Song, X.; Cahill, C.; Eng, G. Crystallographic report: triorganotin 2-(p-chlorophenyl)- 3-methylbutyrates. Appl. Organomet. Chem.2003, 17, 743–744.10.1002/aoc.492Suche in Google Scholar
Szorcsik, A.; Nagy, L.; Sletten, J.; Szalontai, G.; Kamu, E.; Fiore, T.; Pellerito, L.; Kalman, E. Preparation and structural studies on dibutyltin(IV) complexes with pyridine mono- and dicarboxylic acids. J. Organomet. Chem.2004, 689, 1145–1154.10.1016/j.jorganchem.2003.11.040Suche in Google Scholar
Tian, L.; Sun, Y.; Li, H.; Zheng, X.; Cheng, Y.; Liu, X.; Qian, B. Synthesis, characterization and biological activity of triorganotin 2-phenyl-1,2,3-triazole- 4-carboxylates. J. Inorg. Biochem. 2005, 99, 1646–1652.10.1016/j.jinorgbio.2005.05.006Suche in Google Scholar PubMed
Tian, L.; Cao, H.; Wang, S.; Sun, Y.; Liu, Z. Synthesis, characterization and cytotoxic activity of tricyclohexyltin carboxylates derived from cyclic dicarboxylic anhydrides. J. Coord. Chem.2013, 66, 624–637.10.1080/00958972.2013.767446Suche in Google Scholar
Tian, L.; Wang, X.; Zheng, X.; Dong, Y.; Liu, Z. Synthesis, structure and in vitro anti-tumor activity of triorganotin (4R)-3-[(2S)-(5-oxo-2-pyrrolidinyl)carbonyl]-4-thiazolidinecarboxylates. Chinese J.Inorg.Chem.2014, 30, 2087–2092.Suche in Google Scholar
Tian, L.; Kong, L.; Zhang, C. Synthesis, structure and in vitro cytotoxic activity of two organotin complexes of 2-phenyl-1,2,3-triazole-4-carboxylic acid. Main Group Met. Chem.2015a, 38, 83–91.10.1515/mgmc-2015-0010Suche in Google Scholar
Tian, L.; Yu, H.; Zheng, X.; Liu, X. Synthesis, crystal structure and cytotoxic activity of tricyclohexyltin complexes of benzenedioxyacetic acids. Appl. Organomet. Chem.2015b, 29, 725–729.10.1002/aoc.3357Suche in Google Scholar
Tiekink, E. R. T. Structural chemistry of organotin carboxylates: a review of the crystallographic literature. Appl. Organomet. Chem. 1991, 5, 1–23.10.1002/aoc.590050102Suche in Google Scholar
Tiekink, E. R. T. The rich diversity in tin carboxylate structures. Trends Organomet. Chem.1994, 1, 71–116.Suche in Google Scholar
Vollano, J. F.; Day, R. O.; Rau, D. N.; Chandrasekhar, V.; Holmes, R. R. Intramolecularly formed pentacoordinated structures of triphenyltin esters of salicylic acid, o-anisic acid, and p-methylthiobenzoic acid. Inorg. Chem.1984, 23, 3153–3160.10.1021/ic00188a026Suche in Google Scholar
Wang, X.; Liu, X.; Tian, L. Synthesis, characterization and in vitro cytotoxic activity of bis(triorganotin) 2,6-pyridinedicarboxylates. Main Group Met. Chem.2014, 37, 143–147.10.1515/mgmc-2014-0033Suche in Google Scholar
Zhang, H.; Yu, X.; Li, X.; Pan, X. A study of promotive and fungistatic actions of steroidal saponin by microcalorimetric method. Thermochim. Acta2004, 416, 71–74.10.1016/j.tca.2003.11.033Suche in Google Scholar
Zhang, H.; Yu, H.; Liu X.; Tian, L. Synthesis, structural characterization and antibacterial activity of tricyclohexyltin aryloxyacetates. Main Group Met. Chem.2015, 38, 157–164.10.1515/mgmc-2015-0025Suche in Google Scholar
Supplemental Material:
The online version of this article (DOI: 10.1515/mgmc-2016-0014) offers supplementary material, available to authorized users.
©2016 Walter de Gruyter GmbH, Berlin/Boston
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Artikel in diesem Heft
- Frontmatter
- Research Articles
- Synthesis, spectroscopic, and theoretical studies of tin(II) complexes with biologically active Schiff bases derived from amino acids
- A density functional theory insight into the structure and reactivity of diphenyltin(IV) derivative of glycylphenylalanine
- Synthesis, crystal structure, and antibacterial activity of tricyclohexyltin salicylates
- Fungal strain Aspergillus flavus F3 as a potential candidate for the removal of lead (II) and chromium (VI) from contaminated soil
- Two SrII coordination compounds based on tetrazole-carboxylate ligands
- Short Communications
- Crystal structure of the triphenyltin(IV) chloride dimethyl N-cyanodithioiminocarbonate adduct
- Triorganotin carboxylates – synthesis and crystal structure of 2-methyl-1H-imidazol-3-ium catena-O,O′-oxalatotriphenylstannate
- Organotin(IV) scorpionates – X-ray structure and crystal packing of TpSn(Cl)2(n-Bu) [Tp=hydrotris(pyrazol-1-yl)borate]
Artikel in diesem Heft
- Frontmatter
- Research Articles
- Synthesis, spectroscopic, and theoretical studies of tin(II) complexes with biologically active Schiff bases derived from amino acids
- A density functional theory insight into the structure and reactivity of diphenyltin(IV) derivative of glycylphenylalanine
- Synthesis, crystal structure, and antibacterial activity of tricyclohexyltin salicylates
- Fungal strain Aspergillus flavus F3 as a potential candidate for the removal of lead (II) and chromium (VI) from contaminated soil
- Two SrII coordination compounds based on tetrazole-carboxylate ligands
- Short Communications
- Crystal structure of the triphenyltin(IV) chloride dimethyl N-cyanodithioiminocarbonate adduct
- Triorganotin carboxylates – synthesis and crystal structure of 2-methyl-1H-imidazol-3-ium catena-O,O′-oxalatotriphenylstannate
- Organotin(IV) scorpionates – X-ray structure and crystal packing of TpSn(Cl)2(n-Bu) [Tp=hydrotris(pyrazol-1-yl)borate]

