Abstract
A new Cd(II) complex, [Cd(PMBP)2(CH3OH)2] (PMBP = 1-phenyl-3-methyl-4-benzoyl-5-pyrazolone), has been synthesized and characterized by elemental analysis, IR spectroscopy, thermal gravimetric analysis, fluorescence analysis, and X-ray single-crystal diffraction. In the complex, a slightly distorted octahedral geometry of cadmium ion is coordinated by six oxygen atoms, and the cadmium ion locates in the center position. The complex is a monomeric species, which is extended into 2D supramolecular architecture by strong intermolecular hydrogen bonds. Moreover, the intramolecular hydrogen bonds and C-H···π interactions are also found in the complex so that the 2D supramolecular network is consolidated.
Introduction
The design and synthesis of supramolecular coordination complexes have attracted much attention because of their application in various fields, such as catalysis, luminescence, magnetism, and so on (Janiak, 2003; Cook et al., 2013; Guo et al., 2013; Höke et al., 2013). Supramolecular complexes can be constructed not only by metal-organic frameworks (Janiak, 1997; Fang et al., 2011), but also by monomeric complexs (Zheng et al., 2014) using weak interactions. Some supramolecular complexes containing 1-phenyl-3-methyl-4-benzoyl-5-pyrazolone (PMBP) ligand have been synthesized (Dey et al., 2003; Yang et al., 2010; Cheng et al., 2014). When our work was at the final stage, we became aware of the analogous complex [Cd(PMBP)2(EtOH)2] (Sun et al., 2013), which is also a supramolecular complex. HPMBP has potential coordination ability as a bidentate ligand, which can be used as a chelating ligand and an effective extractant (Lahiri et al., 2003; Zhao et al., 2006). Moreover, HPMBP has two O atoms, two N atoms, and two phenyl rings, and its hydrogen bonds network and other weak interactions can be formed (Cheng et al., 2014). The Cd(II) atom, as a IIB group metal ion, has a 4d10 electron configuration and displays flexible coordination numbers (Sun et al., 2005; Hu and Li, 2010; Nie et al., 2010; Calatayud et al., 2013; Sharif and Najafi, 2013). In addition, some Cd(II) complexes show good fluorescent (Nie et al., 2010; Pathak et al., 2012; Calatayud et al., 2013) and antimicrobial (Zaky et al., 2012) properties. Herein, we report a new Cd(II) compound with PMBP ligand, [Cd(PMBP)2(CH3OH)2] (1) and its properties.
Results and discussion
Infrared spectral studies
The IR spectrum of [Cd(PMBP)2(CH3OH)2] (1) was compared with that of the free HPMBP ligand. The strong absorption bands in the range of 3418–3545 cm-1 can be ascribed to O-H stretching, which indicates the existence of methanol in this complex. The band at 3063 cm-1 can be assigned to the C-H stretching of phenyl ring. The C=O absorbtion band of free HPMBP is 1645 cm-1, which is shifted to 1611 cm-1 in the complex, demonstrating that C=O is coordinated to the central Cd(II) atom. The new band at 550 cm-1 is close to the peak at 500 cm-1 of the Mn-O stretching vibration (Yang et al., 2010), which may be attributed to the Cd-O stretching. According to Akama and Tong (1996), the assignments of some other IR bands are as follows: The framework vibrations of phenyl ring are found at 1533 and 1481 cm-1. The band at 1456 cm-1 is characteristic peak for the methyl of the pyrazolone ring, but it can also be assigned to the phenyl ring. Methanol methyl absorption shows strong peak at 1435 cm-1. The rocking of -CH3 is observed at 1018 cm-1. The spectra of the pyrazolone ring show two characteristic bands at 1398 and 1362 cm-1. The characteristic peak of C-H (in-plane bending) is 1229 cm-1, which overlaps with the peak of ν(C=C) and ν (C-CH3). The ν (C-N) stretching vibration peak is at 1153 cm-1. The bands at 999, 947, 916, and 907 cm-1 are attributable to the stretching vibration of the (C-C6H5). The two peaks at 793 and 735 cm-1 can be verified as the absorptions of C-H (out-of-plane bending).
Structural analysis
X-ray structural investigation reveals that the complex [Cd(PMBP)2(CH3OH)2] (1) crystallizes in the monoclinic system with P21/c space group and exhibits monomeric species. Furthermore, the whole molecule displays central symmetry and the Cd atom acts as the symcenter. The Cd(II) ion is coordinated by six O atoms (Figure 1), four O atoms from two deprotonated HPMBP ligands and another two O atoms from two methanol molecules. Thus, a slightly distorted octahedral structure is formed and the Cd(II) ion occupies the center position. The analogous complex [Cd(PMBP)2(EtOH)2] (Sun et al., 2013) has the same coordination mode as complex 1, but there exist obvious differences between the two complexes, as listed in Table 1. As shown in Figure 1, three planes are founded around the Cd atom. The bond angles of O1-Cd-O2, O1i-Cd-O2, O1-Cd-O2i, and O1i-Cd-O2i (symmetry code: i=1-x, -y, 1-z.) are 83.32(6), 96.68(6), 96.68(6) and 83.32(6)°, respectively, with the sum to be 360°, which shows that the four O atoms (O1, O2, O1i and O2i) are coplanar. The same circumstance occurs in two other planes (see Table 2). Given that the Cd(II) ion is chelated by two PMBP ligands, two new six-membered rings (Cd-O1-C9-C8-C11-O2 and Cdi-O1i-C9i-C8i-C11i-O2i, symmetry code: i=1-x, -y, 1-z.) are accordingly generated. The bond distances for Cd-O1, Cd-O2 and Cd-O3 are 2.2256(16), 2.2823(16) and 2.3105(16) Å, respectively, which compare well with those found in some other Cd (II) complexes (Nie et al., 2010; Xue et al., 2012; Sharif and Najafi, 2013; Sun et al., 2013), whose steric configuration are also showing octahedral structure. Among the ligand PMBP, the C-O (C9-O1 and C11-O2) bond lengths are 1.267(3) and 1.256(3) Å, respectively, which clearly indicate that a double-bond character is exhibited between the O and C atoms (bond length of C=O is approximately 1.20 Å).
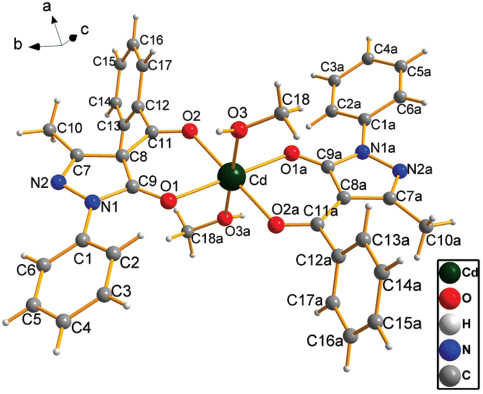
Coordination environments of the Cd atom.
Differences between [Cd(PMBP)2(CH3OH)2] and [Cd(PMBP)2(EtOH)2].
| Formula | [Cd(PMBP)2(CH3OH)2] | [Cd(PMBP)2(EtOH)2] |
| Temperature (K) | 173(2) | 298(2) |
| Crystal system | Monoclinic | Triclinic |
| Space group | P21/c | P-1 |
| Z | 2 | 1 |
| a (Å) | 10.819(2) | 9.1410(10) |
| b (Å) | 16.367(3) | 10.3201(13) |
| c (Å) | 9.5314(19) | 10.9079(14) |
| α (°) | 90.00 | 106.7810 |
| β (°) | 107.095(3) | 107.613(2) |
| γ (°) | 90.00 | 104.6090 |
Selected angles (°) for the compound.
| O1-Cd1-O1i | 179.999(1) |
| O2i -Cd1-O2 | 179.999(1) |
| O1-Cd1-O3i | 90.52(6) |
| O1i -Cd1-O3i | 89.48(6) |
| O2i -Cd1-O3i | 93.02(6) |
| O2-Cd1-O3i | 86.98(6) |
| O1-Cd1-O3 | 89.48(6) |
| O1i -Cd1-O3 | 90.52(6) |
| O2i -Cd1-O3 | 86.98(6) |
| O2-Cd1-O3 | 93.02(6) |
| O3i -Cd1-O3 | 180.0 |
| C9-O1-Cd1 | 124.35(14) |
| C11-O2-Cd1 | 129.13(15) |
| C18-O3-Cd1 | 123.57(16) |
Symmetry code: (i) 1-x, -y, 1-z.
In complex 1, strong intermolecular hydrogen bonds (Figure 2, Table 3) are observed between the nitrogen atom (N2) and the hydrogen atom (H3) of methanol [O3-H3···N2, 2.769(3) Å, 176°], which link the [Cd(PMBP)2(CH3OH)2] unit to form a 2D supramolecular architecture. Intramolecular hydrogen bonds [C2-H2···O1, 2.890(3) Å, 114°] (Figure 2, Table 3) are also observed in the PMBP ligand. In addition, C-H···π interaction [C5-H5···Cg1(C12ii-C17ii), symmetry code: ii = 1-x, 1/2+y, 1/2-z. H···π = 2.69 Å] exists between neighboring PMBP ligands (Figure 3), and another two C-H···π interactions [C18-H18C···Cg2 (Cd1iii-O1iii-C9iii-C8iii-C11iii-O2iii), symmetry code: iii = 1-x, -y, 1-z. H···π = 2.79 Å and C18–H18C···Cg3 (Cd1aiv–O1aiv–C9aiv–C8aiv–C11aiv-O2aiv), symmetry code: iv = x, y, z. H···π = 2.79 Å] are also found in the molecule itself (Figure 3). By virtue of intramolecular hydrogen bonds and the C-H···π interactions, the stability of the 2D supramolecular complex is enhanced.
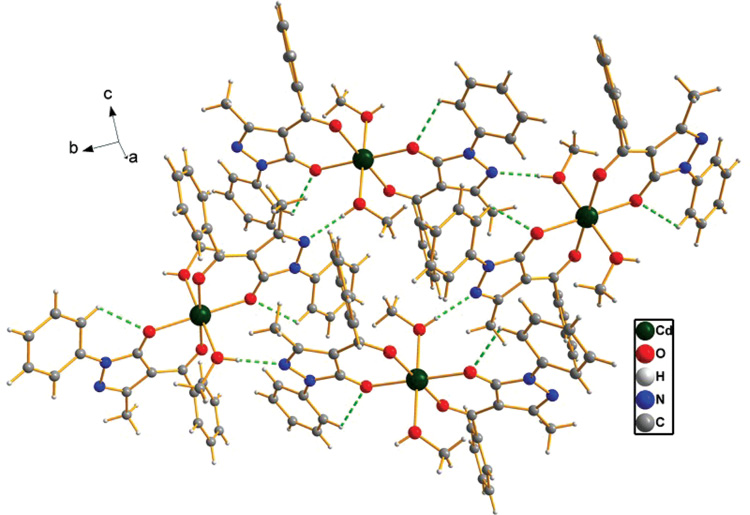
Hydrogen bonds (shown as green dashed lines) of the title complex.
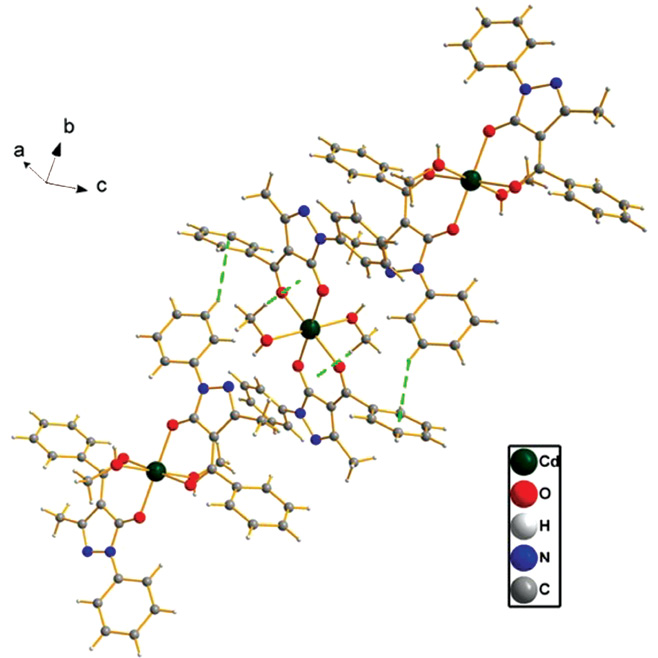
C-H···π interactions of the title complex.
H-bonding geometry parameters (Å and °) for the compound.
| Type | D-H···A | D-H (Å) | H···A (Å) | D···A (Å) | D-H···A (°) |
|---|---|---|---|---|---|
| Inter- | O3-H3···N2i | 0.84 | 1.93 | 2.769(3) | 176 |
| Intra- | C2-H2···O1 | 0.95 | 2.37 | 2.890(3) | 114 |
Symmetry code: (i) x, 1/2-y, 1/2+z.
Thermal gravimetric analysis
Thermal gravimetric analysis of this compound was performed under N2 gas between 25 and 800°C, and the TGA curve is shown in Figure 4, which indicates that the complex decomposes in two stages. The first decomposition was in the range of 106–202°C, with a weight loss of 7.7755%, corresponding to the departure of two methanol molecules (calculated, 8.7651%). At the second step (336–750°C), the PMBP ligands were destroyed gradually.
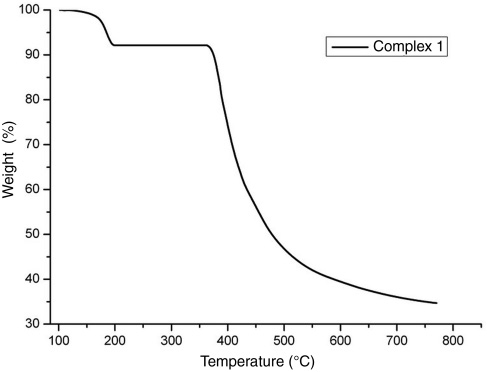
TG curve of the complex.
Fluorescence properties
The luminescent properties of complex 1 and HPMBP ligand were investigated in solid-state at room temperature. The emission peaks are 455 nm for complex 1 (λex=214 nm) and 453 nm (λex=212 nm) for the HPMBP ligand. The emission peak of complex 1 is very close to that of the free HPMBP ligand, which shows that the emission shift is neither metal-to-ligand charge transfer nor ligand-to-metal charge transfer in nature, and the emission maybe mainly caused by the PMBP ligand.
Conclusion
In summary, one new Cd(II) complex based on 1-phenyl-3-methyl-4-benzoyl-5-pyrazolone ligand has been synthesized and structurally characterized. The molecule, [Cd(PMBP)2(CH3OH)2], is centrosymmetric, and the cadmium ion acts as the center of symmetry. Two PMBP ligands are symmetrical around the cadmium ion. The complex features a 2D network, which was built from monomeric [Cd(PMBP)2(CH3OH)2] species, interconnected via strong intermolecular hydrogen bonds, and enhanced by intramolecular hydrogen bonds and the C-H···π interactions.
Experimental section
General
All reagents were purchased commercially and used without further purification. Elemental analyses of carbon, hydrogen, and nitrogen were performed with a Perkin-Elmer 2400 II element analyzer (PerkinElmer, Waltham, MA, USA). Fourier transform IR (FTIR) spectra were recorded on an IR Affinity-1 FTIR spectrophotometer (Shimadzu Corporation, Kyoto, Japan) (range: 400–4000 cm-1) as KBr pellets. TGA was conducted with a Mettler5MP/PF7548/MET/400W instrument (Mettler Toledo Company, Zurich, Switzerland) in a N2 atmosphere in the temperature range of 25–800°C (heating rate=10°C/min). Fluorescence spectra were recorded on a Varian Cary Eclipse fluorescence spectrometer (Varian, Inc., Palo Alto, CA, USA) in the solid-state at room temperature. Crystal structure was determined on a Bruker SMART APEX II CCD X-ray diffractometer (Bruker AXS, Karlsruhe, Germany).
Preparation of [Cd(PMBP)2(CH3OH)2]
A mixture of Cd(NO3)2·4H2O (0.1234 g, 0.4 mmol) and 1-phenyl-3-methyl-4-benzoyl-5-pyrazolone (0.3340 g, 1.2 mmol) in an ethanol/methanol (1:1) solution (40 mL) was refluxed for 5 h and filtered. Light-yellow block crystals were obtained after 4 days. Yield: 63.93% based on Cd(NO3)2 salt. Elemental analysis calculated (%) for C36H34N4O6Cd: C, 59.15; H, 4.69; N, 7.66. Found: C, 59.19; H, 4.56; N, 7.72. IR (KBr, cm-1): 3545s, 3474s, 3462s, 3447s, 3418s, 3063m, 1611s, 1533m, 1481s, 1456s, 1435s, 1398m, 1362m, 1229w, 1153w, 1132w, 1061m, 1018m, 999w, 947m, 916w, 907w, 843m, 793w, 768m, 735w, 702m, 660m, 611m, 550m.
X-ray crystallography
Single-crystal X-ray diffraction analysis was conducted by a Bruker SMART APEX II CCD X-ray diffractometer at 173(2) K using graphite monochromated MoKα radiation (λ=0.71073 Å). Semi-empirical absorption correction was applied using SADABS program (Sheldrick, 1997a). The structure was solved by direct methods and refined with the full-matrix least-squares technique using the SHELXS-97 and SHELXL-97 programs (Sheldrick, 1997b,c). Selected angles are given in Table 2. Detailed crystal data and structure refinement are listed in Table 4. Crystallographic data has been deposited with the Cambridge Crystallographic Centre as supplementary publication number CCDC-977202.
Crystal data and refinement parameters.
| Empirical formula | C36H34N4O6Cd |
| Formula weight | 731.08 |
| Crystal dimensions (mm) | 0.14×0.17×0.18 |
| Crystal system | Monoclinic |
| Space group | P21/c |
| a (Å) | 10.819(2) |
| b (Å) | 16.367(3) |
| c (Å) | 9.5314(19) |
| α (°) | 90.00 |
| β (°) | 107.095(3) |
| γ (°) | 90.00 |
| V (Å3) | 1613.2(5) |
| Z | 2 |
| Dc (g·cm-3) | 1.505 |
| μ (mm-1) | 0.730 |
| F(000) | 748 |
| T (K) | 173(2) |
| λ (Å) | MoKα(0.71073) |
| θ Range (°) | 1.97–25.00 |
| Measured reflections | 7997 |
| Unique reflections | 2850 |
| Observed reflections | 2540 |
| No. of parameters refined | 216 |
| R1, WR2 [I>2σ(I)] | 0.0294, 0.0749 |
| R1, WR2 [all data] | 0.0326, 0.0772 |
| GOOF | 1.082 |
| Largest peak and hole (e·Å-3) | 0.737, -0.742 |
Acknowledgments
We are grateful for the financial support provided by the National Natural Science Foundation of China (Grant No. 81201346), the Natural Science Foundation of Shandong Province (Grant No. ZR2014BL006), and A Project of Shandong Province Higher Educational Science and Technology Program (Grant No. J14LC19).
References
Akama, Y.; Tong, A. Spectroscopic studies of the keto and enol tautomers of 1-phenyl-3-methyl-4-benzoyl-5-pyrazolone. Microchem. J.1996, 53, 34–41.Suche in Google Scholar
Calatayud, D. G.; López-Torres, E.; Mendiola, M. A. A fluorescent dissymmetric thiosemicarbazone ligand containing a hydrazonequinoline arm and its complexes with cadmium and mercury. Eur. J. Inorg. Chem.2013, 80–90.10.1002/ejic.201200815Suche in Google Scholar
Cheng, Y.-Z.; Tang, Y.; Yan, F. Synthesis and crystal structure of Pb(II) complex with 1-phenyl-3-methyl-4-benzoyl-5-pyrazolone. Main Group Met. Chem.2014, 37, 101–106.Suche in Google Scholar
Cook, T. R.; Zheng, Y.-R.; Stang, P. J. Metal-organic frameworks and self-assembled supramolecular coordination complexes: comparing and contrasting the design, synthesis, and functionality of metal-organic materials. Chem. Rev.2013, 113, 734–777.Suche in Google Scholar
Dey, S. K.; Bag, B.; Dey, D. K.; Gramlich, V.; Li, Y.; Mitra, S. Synthesis and characterization of copper(II) and zinc(II) complexes containing 1-phenyl-3-methyl-4-benzoyl-5-pyrazolone. Z. Naturforsch. 2003, 58b, 1009–1014.Suche in Google Scholar
Fang, X.-Q.; Deng, Z.-P.; Huo, L.-H.; Wan, W.; Zhu, Z.-B.; Zhao, H. Gao, S. New family of silver(I) complexes based on hydroxyl and carboxyl groups decorated arenesulfonic acid: syntheses, structures, and luminescent properties. Inorg. Chem.2011, 50, 12562–12574.Suche in Google Scholar
Guo, X.-G.; Yang, W.-B.; Wu, X.-Y.; Zhang, Q.-K.; Lin, L.; Yu, R.; Chen, H.-F.; Lu, C.-Z. Auxiliary ligand-directed synthesis of a series of Cd(II)/Co(II) coordination polymers with methylenebis-(3,5-dimethylpyrazole): syntheses, crystal structures, and properties. Dalton Trans.2013, 42, 15106–15112.Suche in Google Scholar
Hu, Y. L.; Li, T. B. Crystal structure and third-order nonlinear optical property of a cadmium complex constructed by 1,3-dithiole-2-thione-4,5-dithiolato ligand. Mol. Cryst. Liq. Cryst.2010, 533, 172–177.Suche in Google Scholar
Höke, T.; Herdtweck, E.; Bach, T. Hydrogen-bond mediated region- and enantioselectivity in a C-H amination reaction catalysed by a supramolecular Rh(II) complex. Chem. Commun.2013, 49, 8009–8011.Suche in Google Scholar
Janiak, C. Functional organic analogues of zeolites based on metal-organic coordination frameworks. Angew. Chem. Int. Ed.1997, 36, 1431–1434.Suche in Google Scholar
Janiak, C. Engineering coordination polymers towards applications. Dalton Trans. 2003, 2781–2804.10.1039/b305705bSuche in Google Scholar
Lahiri, S.; Wu, X. L.; Yang, W.; Xu, Y.; Yuan, S. Solvent extraction of 46Sc with PMBP. J. Radioanal. Nucl. Chem.2003, 257, 431–432.Suche in Google Scholar
Nie, F.-M.; Li, M.; Li, G.-X. Mono- and dinuclear cadmium(II) complexes based on polybenzimidazole-containing tetradentate and heptadentate ligands: synthesis, structures and fluorescent properties. J. Mol. Struct.2010, 977, 45–50.Suche in Google Scholar
Pathak, R. K.; Hinge, V. K.; Mahesh, K. Rai, A.; Panda, D.; Rao, C. P. Cd2+ complex of a triazole-based calix[4]arene conjugate as a selective fluorescent chemosensor for cys. Anal. Chem.2012, 84, 6907–6913.Suche in Google Scholar
Sharif, M. A.; Najafi, G. R. Synthesis, structure and characterization of a helical seven-coordinated pyridine-2,6-dicarboxylate-bridged cadmium(II) complex. Acta Chim. Slov.2013, 60, 138–143.Suche in Google Scholar
Sheldrick, G. M. SADABS: program for bruck area detector absorption correction. University of Göttingen, Germany, 1997a.Suche in Google Scholar
Sheldrick, G. M. SHELXS-97: program for crystal structure solution. University of Göttingen, Germany, 1997b.Suche in Google Scholar
Sheldrick, G. M. SHELXL-97: program for crystal structure refinement. University of Göttingen, Germany, 1997c.Suche in Google Scholar
Sun, X.-Z.; Huang, Z.-L.; Wang, H.-Z.; Ye, B.-H.; Chen, X.-M. Syntheses and crystal structures of cadmium complexes with thiophenedicarboxylate and bipyridine-like ligands. Z. Anorg. Allg. Chem.2005, 631, 919–923.Suche in Google Scholar
Sun, Y.-X.; Lu, R.-E.; Lan, Q.-Y.; Zhang, X.-Y.; Ma, F.-X. Synthesis and crystal structure of a new 2D-supramolecular complex: [Cd(C17H13N2O2)2(C2H6O)2]. Asian J. Chem.2013, 25, 7115–7117.Suche in Google Scholar
Xue, L. W.; Han, Y. J.; Zhao, G. Q.; Feng, Y. X. Synthesis, structure, and antimicrobial activity of a cadmium(II) complex derived from N,N’-bis(5-chlorosalicylidene)propane-1,3-diamine. Russ. J. Coord. Chem.2012, 38, 24–28.Suche in Google Scholar
Yang, L.; Shao, C.; Wang, Z.; Liu, J.; Zhou, L. Crystal structure and thermodecomposition kinetics of Mn(II) complex with 1-phenyl-3-methyl-4-benzoyl-5-pyrazolone. J. Chem. Crystallogr.2010, 40, 58–63.Suche in Google Scholar
Zaky, R. R.; Yousef, T. A.; Ibrahim, K. M. Co(II), Cd(II), Hg(II) and U(VI)O2 complexes of o-hydroxyacetophenone[N-(3-hydroxy-2-naphthoyl)] hydrazone: physicochemical study, thermal studies and antimicrobial activity. Spectrochim. Acta. A2012, 97, 683–694.Suche in Google Scholar
Zhao, J.; Bai, Y.; Li, D.; Li, W. Extraction of rare earths(III) from nitrate medium with di-(2-ethylhexyl) 2-ethylhexyl phosphonate and synergistic extraction combined with 1-phenyl-3-methyl-4-benzoyl-pyrazolone-5. Sep. Sci. Technol.2006, 41, 3047–3063.Suche in Google Scholar
Zheng, Z.; Yu, Z.-P.; Yang, M.-D.; Jin, F.; Ye, L.-N.; Fang, M.; Zhou, H.-P.; Wu, J.-Y.; Tian, Y.-P. Silver(I) supramolecular complexes generated from isophorone-based ligands: crystal structures and enhanced nonlinear optical properties through metal complexation. Dalton Trans.2014, 43, 1139–1150.Suche in Google Scholar
©2015 by De Gruyter
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Artikel in diesem Heft
- Frontmatter
- Research Articles
- Synthesis, spectroscopic characterization and crystal structures of diorganotin (IV) complexes of 2-N-propyl and 2-N-benzyl-amino-1-cyclopentene-1-carbodithioates
- Crystal growth, characterization and dielectric studies of silica gel-grown polydistrontiumdimalate pentahydrate: a 2D porous metal-organic framework
- Synthesis, spectroscopic [IR, (1H, 13C, 27Al) NMR] and mass spectrometric studies of aluminium(III) complexes containing O- and N-chelating Schiff bases
- Synthesis, crystal structure, and fungicidal activity of triorganotin(IV) 1-methyl-1H-imidazole-4-carboxylates
- Self-assembly of zinc(II) tetraaza macrocyclic complex and 1,3,5-cyclohexanetricarboxylic acid
- Synthesis and crystal structure of a new 2D supramolecular Cd(II) complex with 1-phenyl-3-methyl-4-benzoyl-5-pyrazolone ligand
- Antimony and antimony oxide@graphene oxide obtained by the peroxide route as anodes for lithium-ion batteries
- Short Communications
- Unexpected photochemical reactions of 2-aryl- 2-ethynyltrisilane derivative
- Synthesis and structure of zinc dichloride bis(t-butylhydrazine) monohydrate
Artikel in diesem Heft
- Frontmatter
- Research Articles
- Synthesis, spectroscopic characterization and crystal structures of diorganotin (IV) complexes of 2-N-propyl and 2-N-benzyl-amino-1-cyclopentene-1-carbodithioates
- Crystal growth, characterization and dielectric studies of silica gel-grown polydistrontiumdimalate pentahydrate: a 2D porous metal-organic framework
- Synthesis, spectroscopic [IR, (1H, 13C, 27Al) NMR] and mass spectrometric studies of aluminium(III) complexes containing O- and N-chelating Schiff bases
- Synthesis, crystal structure, and fungicidal activity of triorganotin(IV) 1-methyl-1H-imidazole-4-carboxylates
- Self-assembly of zinc(II) tetraaza macrocyclic complex and 1,3,5-cyclohexanetricarboxylic acid
- Synthesis and crystal structure of a new 2D supramolecular Cd(II) complex with 1-phenyl-3-methyl-4-benzoyl-5-pyrazolone ligand
- Antimony and antimony oxide@graphene oxide obtained by the peroxide route as anodes for lithium-ion batteries
- Short Communications
- Unexpected photochemical reactions of 2-aryl- 2-ethynyltrisilane derivative
- Synthesis and structure of zinc dichloride bis(t-butylhydrazine) monohydrate

