Abstract
An overview of the most important gallium compounds in nuclear medicine and oncology in the two last decades, with an emphasis on the last decade (especially the last 5 years), is given in this review. You can also find here some recent knowledge about 68Ge/68Ga radionuclide generator modern automated synthesis modules. Gallium has long been known to concentrate in skeletal tissue, particularly regions of bone deposition and remodeling. Elemental gallium is a potent inhibitor of bone resorption that acts to maintain or restore bone mass. There are several medically useful gallium radionuclides that have made extensive contribution in both the diagnosis and therapy of diseases. A huge variety of monofunctional and bifunctional chelators have been developed that allow the formation of stable 68Ga(III) complexes and convenient coupling to biomolecules such as amino acids, peptides, nanoparticles, or even whole cells. Gallium pharmaceuticals can be divided into two groups according to radioactivity, i.e., radiopharmaceuticals – using radioactive Ga(III) isotopes, and conventional pharmaceuticals – using non-radioactive Ga(III) ion. The pharmaceuticals can also be divided according to the target site of a drug, i.e., those having an impact on soft tissue (most of the drugs) and hard tissue (bones and bone metastasis). In oncology and nuclear medicine, gallium and its compounds have been applied for imaging as well as therapy, and their importance in this field is still growing.
- List of abbreviations
- AE105-NH2
Asp-cyclohexylalanine-Phe-d-Ser-d-Arg-Tyr-Leu-Trp-Ser-NH2
- AMBA
DO3A-CH(2)CO-G-4-aminobenzoyl-Q-W-A-V-G-H-L-M-NH(2)
- ATP
adenosine triphosphate
- Cyclo-MG1
cyclo[γ-d-Glu-Ala-Tyr-d-Lys]-Trp-Met-Asp-Phe-NH2
- Cyclo-MG2
cyclo[γ-d-Glu-Ala-Tyr-d-Lys]-Trp-Nle-Asp-Phe-NH2
- dNTP
intracellular deoxyribonucleoside
- DOTA-TATE
DOTA-(Tyr3)-octreotate
- DOTA
1,4,7,10-tetraazacyclodecane-1,4,7,10-tetraacetic acid
- DOTANOC
DOTA-1-Nal3-octreotide
- DOTATOC
DOTA-Tyr3-octreotide
- DTPA
diethylenetriaminepentaacetic acid
- EDTA
ethylenediamine-N,N-diacetic acid
- EDTMP
ethylenediamine-N,N,N′,N′-tetrakismethylene phosphonate
- FDG
18-fluoro-deoxyglucose
- HER2
human epidermal growth factor receptor type 2
- HMPAO
hexamethylpropyleneamine oxime
- HYNIC
hydrazinonicotinicamide
- KP46
tris(8-quinolinolato)-gallium(III)
- NODAGA
triazacyclononane,1-glutaric acid-4,7-acetic acid
- NOTA
1,4,7-triazacyclononane-1,4,7-triacetic acid
- PET
positron emission tomography
- RADIGIS
radioisotope generator integrated system
- sCCK8
cholecystokinin 8
- SPECT
single photon emission computed tomography
- TF
transferrin
Introduction
Gallium has shown efficacy in the treatment of several apparently diverse disorders. These disorders can be broadly categorized as (i) accelerated bone resorption, with or without elevated plasma calcium; (ii) autoimmune diseases and allograft rejection; (iii) certain cancers; and (iv) infectious diseases (Lessa et al., 2012).
Gallium has several medically useful radionuclides that have made extensive contribution in both the diagnosis and therapy of diseases. In the late 1940s, 72Ga was localized in bones and was suggested to be potentially useful for treating bone tumors. After extending this theory, 67Ga was concluded as a better radioisotope, as 72Ga has a short half-life (T1/2, 14 h) and was only available with a low specific activity. In contrast, 67Ga with a longer T1/2 (78 h) could easily be made with no carrier added (i.e., this isotope can be isolated from cyclotron without any carrier atom). However, the imaging equipment at that time were not sophisticated enough to produce a good-quality clinical study with this radioisotope; therefore, the development in this field was not so pronounced. The introduction of a 68Ge/68Ga generator, in the early 1960s, rekindled interest in radiogallium for bone tumor localization. In the early 1970s, 67Ga reemerged, as its characteristics better matched with the newly developed instrument, the γ-camera, and the lower amount of carrier gallium that was added to obtain bone localization. The carrier-free nuclide was found, unexpectedly, to localize in certain soft tissue tumors, and a few years later in inflammatory processes. These two uses for 67Ga continue to this day (Welch and Redvanly, 2003).
A major advantage of a 68Ge/68Ga generator is its continuous source of 68Ga, independently from an on-site cyclotron. The increase in knowledge of purification and concentration of the eluate and the complex ligand chemistry has led to 68Ga-labeled pharmaceuticals with major clinical impact. 68Ga-labeled pharmaceuticals have the potential to cover all of today’s clinical options with 99mTc, with the concordant higher resolution of positron emission tomography (PET) in comparison with single photon emission computed tomography (SPECT). 68Ga-labeled analogues of octreotide (an octapeptide), such as DOTATOC, DOTANOC, and DOTA-TATE [where DOTA is 1,4,7,10-tetraazacyclodecane-1,4,7,10-tetraacetic acid, DOTANOC is DOTA-1-Nal3-octreotide, DOTATOC is DOTA-Tyr3-octreotide, and DOTA-TATE is DOTA-(Tyr3)-octreotate], are clinically applied in nuclear medicine. These analogues are now the most frequently applied of all of the 68Ga-labeled pharmaceuticals (Breeman et al., 2011).
The ability of non-radioactive Ga(III) complexes to serve as therapeutic agents in oncology also has to be mentioned. For example, Ga-chloride or Ga-nitrate has inhibitory effects on cancer cell growth. Ga(III) ion is also used as a complexing metal for sonosensitizers such as porphyrins. They are used for sonodynamic therapy, the ultrasound-dependent enhancement of cytotoxic activities of certain compounds (sonosensitizers) (Rosenthal et al., 2004).
An aim of this work is to provide a brief view on and an evaluation of the most important gallium complexes applied in oncology in the period of the last two decades. Developments and new trends in this field are also briefly discussed.
Gallium and its isotopes
Gallium is the second metal ion, after platinum, to be used in cancer treatment. It is because of its ability to inhibit DNA and protein synthesis, and the activity of a variety of enzymes involving serum alkaline phosphatase and ribonucleotide reductase (Collery et al., 2002).
The solution and coordination chemistries of Ga(III) are somewhat similar to those of Al(III) and In(III); however, they are very similar to those of Fe(III). The biochemical similarities of these two ions, particularly concerning protein and chelate binding, are likely responsible for many of the physiological activities of gallium. Blood contains thousands of dissolved components, including many proteins, small-molecule ligands, anions, anionic groups, metal ions, and complexes of all of these components, in addition to colloidal and cellular components. In vivo studies using subnanomolar 67Ga revealed that virtually whole gallium in blood is present in plasma, with traces in leukocytes (Bernstein, 1998).
Nearly all plasma gallium is tightly bound to the iron-transport protein, transferrin (TF). TF transports its metal load into cells through the TF receptor, a protein that can bind two TF molecules. This receptor binds most strongly to diferric TF, less strongly to monoferric TF, and weakly to apotransferrin (having no metal ions) at neutral pH levels. The complex of metal-bearing TF and TF receptor is taken into the cell by endocytosis; the endosome is then acidified to release the metal. Malignant cells generally have a very high TF receptor expression. Gallium binds even more avidly to the related protein lactoferrin, which can remove Ga from TF. A third iron-binding protein to which gallium can bind is ferritin. Ferritin serves as iron storage, is present in most cells to varying degrees, and is particularly concentrated in the Kupffer cells of the liver; it is also concentrated in some other tissue macrophages (Lessa et al., 2012).
66Ga has a half-life of 9.5 h and a high positron energy (4.15 MeV), and also has several γ-rays associated with the decay at energies >1 MeV. For this reason, it has been proposed as a radiodiagnostic agent. A 67Ga-γ-emitter is one of the most widely used single photon markers for the presence of inflammation and malignancy. It has a 78 h half-life and decays by electron capture, emitting γ-rays at 93.3, 184.6, and 300.2 keV. The γ-ray at 184.6 keV is detected by γ-camera or SPECT imaging. The common way 68Ge is used as the source of 68Ga, which is employed very extensively in PET applications, is as a convenient source of positrons (positron emitter). It can also be produced directly, if that is desired (Welch and Redvanly, 2003). The short half-life of 68Ga permits imaging applications with sufficient radioactivity while maintaining the patient’s dose to an acceptable level. Furthermore, owing to superior resolution, 68Ga-PET agents have the ability to replace the current SPECT agents in many applications (Smith et al., 2013). The 72Ga isotope (14.3 h half-life) can be made either with fast or thermal neutrons. 72Ga was used as the source of internal radiation because of the interest in this isotope as an irradiator of bone tumor (Brucer et al., 1953). The important nuclear characteristics of Ga isotopes are given in Table 1. Although the complexion properties of various isotopes of Ga(III) are the same, the characteristic radiation of particular isotopes is used for specific purposes, i.e., therapeutic (α or β radiation) and imaging (γ radiation).
Nuclear characteristics of Ga isotopes (Brucer et al., 1953; Welch and Redvanly, 2003).
| Characteristic | 67Ga | 68Ga | 66Ga | 72 Ga |
|---|---|---|---|---|
| Beam energy (MeV) | 12–22 | 12–22 | 8–12 | |
| γ Photon energy (keV) | 93, 184, 300 | – | 1039, 2750 | 835 |
| % Photons/disintegration | 38, 24, 22 | 178 | 114, 37, 23 | 96 |
| Electrons (keV) | 84, 92 | 1900 (β+) | 4153 (β+) | β- |
| Half-life | 78 h | 68 min | 9.5 h | 14.3 h |
| Production method | 68Zn(p,2n)67Ga | 68Ge daughter, 66Zn(α,2n)68Ge | 63Cu(α,n)66Ga | 72Ge |
| Contaminant | 66Ga, 65Zn | 68Ge | 67Ga | |
| Way of detection | γ-Camera, SPECT | PET | γ-Camera, PET | γ-Camera |
68Ge/68Ga generator and purification methods
Concerning gallium radioisotopes, this generator confers a huge advantage and makes a major impact on gallium radiopharmaceuticals. This fact is mentioned in almost all publications about 68Ga. The invention of this generator and 68Ga represent the very early applications of radionuclides to PET imaging at a time when even the wording of PET itself was not established (Rösch, 2013a).
In 1964, the first 68Ge/68Ga radionuclide generators were described. Although the generator design was by far not adequate in terms of today’s level of chemical, radiopharmaceutical, and medical expectations, it perfectly met the needs of molecular imaging of this period (Rösch, 2013b). Today, it faces a renaissance in terms of new 68Ge/68Ga radionuclide generators, sophisticated 68Ga radiopharmaceuticals, and state-of-the-art clinical diagnoses by means of PET/CT. Thanks to the pioneering achievement of radiochemists in Obninsk, Russia, a new type of 68Ge/68Ga generators became commercially available in the first years of the 21st century. Generator elutes based on hydrochloric acid provided ‘cationic’ 68Ga instead of ‘inert’ 68Ga-complexes, opening new pathways of Me(III)-based radiopharmaceutical chemistry (Rösch, 2013a). The increasing applications of generator-based 68Ga radiopharmaceuticals (for diagnosis alone, but increasingly for treatment planning, thanks to the inherent option as expressed by theranostics) now ask for further developments – toward the optimization of 68Ge/68Ga generators both from the chemical and regulatory points of view. Dedicated chelators may be required to broaden the feasibility of 68Ga labeling of more sensitive targeting vectors, and generator chemistry may be adopted to those chelators – or vice versa (Rösch, 2013b).
Generator-produced 68Ga is typically of suboptimal purity, mainly due to the breakthrough of the parent radionuclide 68Ge, and Fe(III) impurity (Belosi et al., 2013). Anionic, cationic, and fractional elution methods are well known (Mueller et al., 2013). Modern automated synthesis modules adopt both fractionation methods and purification methods to get rid of 68Ge breakthrough (Costa et al., 2013; Schultz et al., 2013).
There is also a good example of a 68Ge/68Ga generator combined with an automated 68Ga elute purification-concentration unit [radioisotope generator integrated system (RADIGIS)], specially designed for 68Ga processing (RADIGIS-68Ga) (Le, 2013). Another of the many examples of automated purification systems is described where [68Ga]Ga-AMBA [AMBA=DO3A-CH(2)CO-G-(4-aminobenzoyl)-Q-W-A-V-G-H-L-M-NH(2), one of bombesin analogues (Schroeder et al., 2011)] was HPLC purified and Sep-Pak purified, using the built-in radiodetector of the Tracerlab FX F-N synthesizer to monitor fractionated 68Ge/68Ga generator elution and purification (Cagnolini et al., 2010).
There is a number of fully automated systems to produce 68Ga precursors with various chelators already available and ready to use with an appropriate ligand.
Gallium pharmaceuticals
As was mentioned, Ga(III) and Fe(III) have some familiar similarities in biochemical behavior, and thus the free isotope ion binds to proteins in blood and tends to concentrate in areas of rapid cell division, which is typical for cancer cells, and in areas of higher concentration of leukocytes (such as inflammation sites). Ga(III) binds to TF, leukocyte lactoferrin, bacterial siderophores, inflammatory proteins, and cell membranes in neutrophils, both living and dead (Campain et al., 2010). These binding properties of Ga(III) are used in the diagnosis and therapy of both soft tissue and hard tissue diseases. The specificity of Ga(III) binding in the body can be properly modified through appropriate ligands and/or chelators. Although all the isotopes of Ga (66Ga, 67Ga, 68Ga) can be used for imaging using different imaging equipment (PET, SPECT, and γ-camera), 66Ga and 67Ga, thanks to being sources of γ-rays with high energy, are appropriate for imaging as well. Their complexation with a suitable ligand (and, in case of a need, with a chelator as well) can provide a more specific targeting of tumor cells and enhance the cytotoxicity by synergic effects (e.g., by complexing with an already known cytotoxic drug or by a sonosensitizer) (Maecke et al., 2005; Maecke and Andre, 2007; Fani et al., 2008; Roesch and Riss, 2010; Wadas et al., 2010). 67Gallium imaging has proved useful in high-grade lymphomas, either to evaluate lymphoma at the initial staging or to assess the response to therapy. The sensitivity of 67Ga imaging, however, is highly dependent on the cell type and the size and location of the lesion (Kostakoglu and Goldsmith, 2000). 67Ga imaging of lymphoma provides information about the presence or absence of an active lymphoma. Most important, the positive predictive value for the presence of active disease has consistently been shown to be greater than that for morphologic imaging techniques (such as CT and MRI), primarily owing to the presence of residual mass in treated lymphoma (Tumeh et al., 1987; Front et al., 1990; Gasparini et al., 1993; Front and Israel, 1995). However, 68Ga, used only for the diagnosis of diseases, can be modified as well into a pharmaceutically active molecular structure in the same way.
Renewed interest in 68Ga has recently arisen from several reasons. First, PET has developed during the last decade from a research tool into a powerful diagnostic and imaging tool for routine clinical applications. Second, 68Ge/68Ga generators have been developed that produce suitable elutes for labeling that can be converted into 68Ga-labeled pharmaceuticals for PET studies. Third, there are many DOTA peptides that can be labeled with 68Ga. Fourth, a variety of monofunctional and bifunctional chelators have been developed that allow the formation of stable 68Ga(III) complexes and convenient coupling to biomolecules. Fifth, the availability of PET radiolabeled pharmaceuticals by the introduction of 68Ga in radiopharmacy, independent of an on-site cyclotron, has opened new applications and possibilities. Coupling of 68Ga to small peptides and biomolecules has recently been reviewed (Maecke et al., 2005; Maecke and Andre, 2007; Fani et al., 2008; Roesch and Riss, 2010; Wadas et al., 2010), and 68Ga is potentially an alternative to 18F- and 11C-based radiopharmacy. Last but not least, equipment, including generators, purification and concentration of elute, techniques of radiolabeling, robotics, and PET cameras, have improved during the last decade.
Gallium pharmaceuticals can be divided into two groups according to their radioactivity. These are the radiopharmaceuticals employing radioactive 67,68Ga(III) isotopes, e.g., Ga(imidazole)3 and its derivates, as epidermal growth factor receptors (Garcia et al., 2009); GaCl3, for imaging of lymphoma, inflammatory processes, transmitting nerve impulses, and regulatory fluid in and out of cells (Collery et al., 2000; Silvola et al., 2011); Ga-folate, as diagnostic agent for receptor-positive tumor (Melpomeni et al., 2011); Ga-citrate, suitable for scintigraphy for the detection of a wide variety of diseases: Hodgkin’s, lung cancer, malignant lymphoma, and pancreatic cancer (Kunn et al., 1997; Liu et al., 2003; Lin et al., 2007); Ga-nitroimidazole and its derivative, for PET imaging of tumor hypoxia (Goldman, 1982; Juchau, 1989; Kunn et al., 1997; Fernández et al., 2013); and Ga- biphosphonate, for PET tracing of bone lesions (Fellner et al., 2012). There are also some conventional pharmaceuticals employing non-radioactive Ga(III) pharmaceuticals used in oncology, e.g., Ga-EDTMP, for therapy of bone metastasis (Su et al., 2005); Ga(8-quinolinate)3, for treating renal cell cancer (Collery et al., 2000; Jalilian et al., 2005; Rudnev et al., 2006); Ga-semicarbazones, with a wide spectrum of antitumor effects in the ovary, breast, and colon (Rudnev et al., 2006; Kalinowski et al., 2009; Gambino et al., 2011); Ga(maltol)3, for the treatment of several types of cancer, including liver cancer and lymphoma (Rudnev et al., 2006); Ga(nitrate)3, for the treatment of lymphomas, bone metastasis, and bladder cancer (Collery et al., 2002; Jakupec and Keppler, 2004); Ga-porphyrins, a sonosensitizer in photodynamic therapy (Rosenthal et al., 2004; Jalilian et al., 2005); Ga-pyrazole and its derivatives, as antitumor agents for the therapy of ovarian adenocarcinoma and human lung carcinoma (Tajiri et al., 1994; Balbi et al., 2011); and Ga-thiolate ligands, for their dose-dependent antiproliferative effect toward cancer cells (Gallego et al., 2011). Besides low molecular weight ligands (Gallego et al., 2011; Collery et al., 2002; Rosenthal et al., 2004; Garcia et al., 2009), protein biopolymers and even whole cells have been successfully employed in Ga(III) pharmaceuticals, as illustrated in Table 2. The structure and properties of such protein ligands are further illustrated and mentioned in Table 3. According to the target site of a drug, we can divide them into pharmaceuticals with an impact on hard tissue (bones and bone metastasis) and soft tissue (mainly the liver, spleen, ovaries, prostate, breasts, intestine and mucous tissue, various receptors in tumor cells, or any tissue undergoing inflammatory processes; i.e., most of the drugs), as will be discussed in the next two subsections, respectively. Gallium complexes in oncology can be used for imaging and/or therapy, which is discussed therein as well. The main advantage of radiopharmaceuticals over other drugs is that they are able to visualize metabolic pathways and the functions of cells and tissues.
Examples of protein ligands for Ga pharmaceuticals.
| Protein ligand | Usage | Gallium type | Other ions used | Chelators used | References |
|---|---|---|---|---|---|
| Streptokinase | SPECT imaging of thrombi in many cardiovascular diseases | 67,68Ga | 99mTc, 131I | DTPA-dianhydride | (Jalilian et al., 2009) |
| Bleomycin | SPECT imaging and/or therapy of neoplastic tissues | 68Ga | 111In, 57Co, 99mTc, radioferric salts, and 105Rh | – | (Jalilian et al., 2005) |
| Arg-Gly-Asp (RGD) and bombesin (BBN) analogues | Arg-Gly-Asp (RGD) peptide images integrin αVβ3 expression through PET; bombesin (BBN) analogues target the gastrin-releasing peptide receptor and visualize/treat prostate cancer | 67,68Ga | 111In, 177Lu, 18F, 64Cu | DOTA, NOTA | (Liu et al., 2009a,b; Schroeder et al., 2011; Fournier et al., 2012; Inkster et al., 2013; Sturzu et al., 2014; Varasteh et al., 2013) |
| Annexin V | Apoptosis-detecting radioligand, SPECT/PET imaging | 67,68Ga | 99mTc, 123,124,125I, 111In, 18F, 64Cu | DOTA | (Lahorte et al., 2004) |
| White blood cells | SPECT imaging of acute inflammation and infection | 67Ga | 111In, 99mTc | DOTA, HMPAO, oxine | (Roivainen et al., 2012) |
| Octreotide derivatives such as DOTANOC, DOTATOC | PET imaging methods in the diagnosis of thoracic and gastroenteropancreatic (GEP) neuroendocrine tumors (NETs) | 68Ga | 111In, 99Tc, 90Y, 177Lu | DOTA | (Breeman et al., 2011; Treglia et al., 2012) |
| AE105-NH2 | PET imaging of the urokinase-type plasminogen activator receptor (u-PAR) expression in tumor cells | 68Ga | 64Cu | DOTA, NODAGA | (Leung, 2004–2013; Persson et al., 2012) |
| Anti-HER2 nanobodies (2Rs15d) | PET tracer of high-specific-contrast imaging of HER2-positive tumors and breast cancer | 68Ga | 99mTc | NOTA | (Vaneycken et al., 2011; Xavier et al., 2013) |
| Cyclo-MG1 and cyclo-MG2 | A minigastrin analogue for PET imaging of gastrin receptor overexpressed in most tumor tissues, such as medullary thyroid carcinomas (>90%), astrocytomas (>65%), and stromal ovarian cancers (100%) | 68Ga | 111In, 99mTc | DOTA, EDDA, HYNIC | (Chopra, 2004–2013a,b) |
| sCCK8 derivatives | PET imaging of gastrin receptor overexpressed in most tumor tissues, such as medullary thyroid carcinomas (>90%), astrocytomas (>65%), and stromal ovarian cancers (100%) | 68Ga | 111In, 99mTc | DOTA, EDDA, HYNIC | (Chopra, 2004–2013a,b) |
Structure and properties of some protein ligands for Ga pharmaceuticals.
| Ligand | Structure | Properties of ligand |
|---|---|---|
| Streptokinase | C2100 H3278 N566 O669 S4 | A protein binding and activating human plasminogen; causes lysis of venous thrombi |
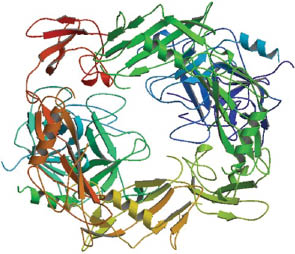 | ||
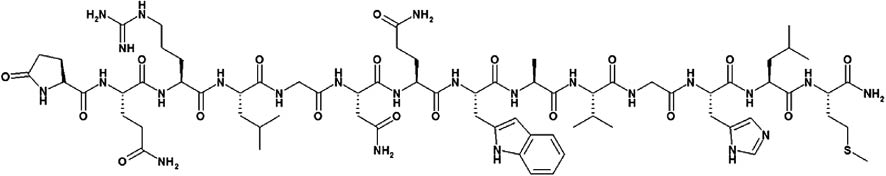
| Bleomycin | C55 H84 N17 O21 S3 | A glycopeptide antibiotic causing breaks in DNA |
 | ||
| Arg-Gly-Asp | C15 H27 N7 O8 | A short amino acid sequence; plays a role in many recognition systems involved in cell-to-cell and cell-to-matrix adhesion |
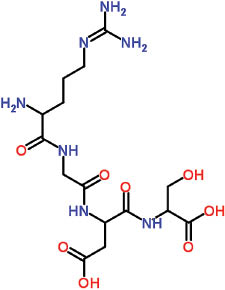 | ||
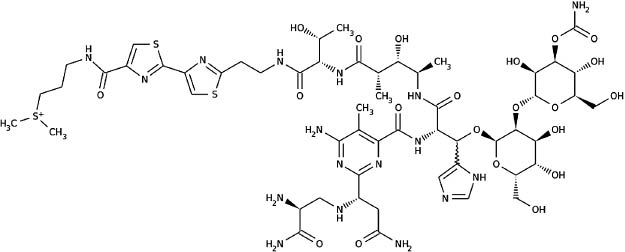
| Bombesin | C71 H110 N24 O18 S | A peptide that stimulates gastrin release from G cells |
 | ||
| Annexin V |  | A member of the calcium and phospholipid binding superfamily of Annexin proteins |
| White blood cells |  | Cells of the immune system involved in defending the body against infectious disease and foreign materials |
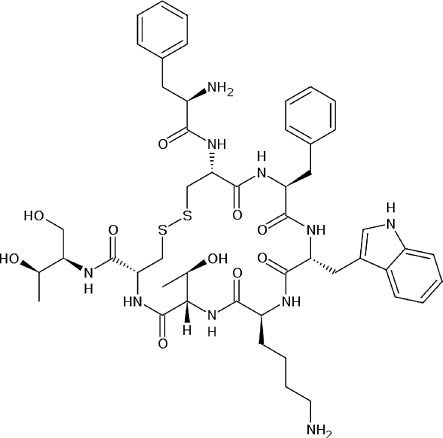
| Octreotide | C49 H66 N10 O10 S2 | An octapeptide that mimics natural somatostatin pharmacologically, although it is a more potent inhibitor of growth hormone, glucagon, and insulin than the natural hormone |
 |
Hard tissue gallium pharmaceuticals
Gallium has long been known to concentrate in skeletal tissue, particularly regions of bone deposition and remodeling. In growing bone, gallium is concentrated in the metaphysis, particularly in the hypertrophic cartilage zone (growth plate); it is also concentrated in regions of fracture healing. To a less extent, gallium accumulates on the endosteal and periosteal surfaces of diaphyseal bone (Bernstein, 1998). A possible way of Ga getting into bone is through calcium channels or by a simple diffusion. Only a minimum amount goes through the TF receptor (Bernstein, 1998; Liu et al., 2003).
Elemental gallium is a potent inhibitor of bone resorption that acts to maintain or restore bone mass. By virtue of these biological effects, gallium compounds are potentially useful treatments for a variety of diseases that are characterized by accelerated bone loss, including cancer-related hypercalcemia, bone metastases, Paget’s disease, and postmenopausal osteoporosis (Warrell, 1995).
Gallium nitrate was originally developed as an antineoplastic agent; however, further studies have revealed that this drug has extremely potent effects on the turnover of bone, and that low doses can be used to reduce bone resorption. The results of randomized double-blind studies have suggested that this drug has superior clinical efficacy relative to etidronate, calcitonin, and pamidronate for the acute control of cancer-related hypercalcemia (Warrell, 1997). Gallium nitrate injection (Ganite) has been approved by the US Food and Drug Administration as an intravenous treatment for patients with cancer-related hypercalcemia (Su et al., 2005).
67Ga whole-body scan is commonly used in nuclear medicine, and it is useful for finding bone tumors. 67Ga-nitrate or citrate is used as a carrier molecule. 67Ga is imaged with a γ-camera, a SPECT camera, or SPECT/CT hybrid machines.
The specificity of Ga pharmaceuticals toward the target is given by the ligand in Ga compound. For example, EDTMP (ethylenediamine-N,N,N′,N′-tetrakismethylene phosphonate) is a ligand with a high affinity for skeletal tissue, and its Ga(III) complex is used to treat bone metastasis (Su et al., 2005). Bone metastases are a serious aggravation for patients with cancer. Therefore, early recognition of bone metastases is of great interest for the further treatment of patients. Bisphosphonates are widely used for scintigraphy of bone lesions with 99mTc. Using the 68Ge/68Ga generator together with a macroyclic bisphosphonate, a comparable PET tracer comes into focus. The bisphosphonate DOTA-conjugated ligand reveals great potential for the diagnosis of bone metastases by means of PET/CT (Fellner et al., 2012).
Soft tissue gallium pharmaceuticals
Unlike in hard tissues, gallium penetrates soft tissues mainly through TF receptors. Isotopes such as 68Ga have suitable physical properties with a high positron yield reaching 89% of all disintegrations. Its half-life of 68 min matches the pharmacokinetics of many peptides and other small molecules owing to a fast blood clearance, quick diffusion, and target localization (Al-Nahhas et al., 2007). The peptide-binding properties and proper half-life of 68Ga indicates it suitability for soft tissues.
67Ga seems to behave as an analogue of the ferric ion, accumulating within the tumor cell by simple diffusion and possible penetration through the calcium channels. Many studies have demonstrated the usefulness of 67Ga scan in detecting Hodgkin’s disease (cancer of lymph tissue found in the lymph nodes, spleen, liver, and bone marrow) (Liu et al., 2003).
Whole-body scintigraphy with 67Ga-citrate (67Ga-citrate scan) has been one of the most widely used tumor-seeking radiopharmaceutical, with great value in detecting lung cancer and malignant lymphoma. Meanwhile, pancreatic cancer is detectable by 67Ga-citrate scan at low sensitivity (Kunn et al., 1997). 67Ga and labeled leukocytes are useful in the detection of an unknown infectious source. However, the delay in the diagnosis of a 67Ga-citrate scan (72 h for a whole-body scan) and the complicated labeling technique of a 67Ga-leukocyte scan are major drawbacks to their clinical use (e.g., higher time/preparation demands are not acceptable in case of acute inflammations). 67Ga-citrate scan was the first radionuclide method to be widely used in the imaging of inflammation. The mechanism of gallium uptake in infectious sites is not completely understood. Multiple factors are thought to contribute to the accumulation and retention of 67Ga in inflammatory lesions, including increased capillary permeability at the inflammatory sites, binding of gallium to tissue proteins such as lactoferrin, as well as direct leukocyte and bacterial uptake (Lin et al., 2007). Nowadays, 67Ga-citrate scan is supplanted by FDG scan in developed countries.
Chelating agents are molecules that have the ability to form more than one bond to a metal ion, thereby increasing the stability of the ion complex. Compounds such as DOTA, DTPA, DOTANOC, DOTATATE, chloride, or citrate acid are chelating agents of different strengths. Several studies showed that somatostatin receptor PET and PET/CT, using different radiopharmaceuticals (e.g., [68Ga]DOTANOC, [68Ga]DOTATOC, and [68Ga]DOTATATE), are accurate imaging methods in the diagnosis of thoracic (mainly pulmonary and thymic) and gastroenteropancreatic neuroendocrine tumors (Breeman et al., 2011).
Radiolabeled peptides are of increasing interest in nuclear oncology. Special emphasis has been given to the development of peptides labeled with positron emitters. Several bifunctional chelators based on 1,4,7-triazacyclononane-N,N′,N″-triacetic acid and 1,4,7,10-tetraazacyclododecane-N,N′,N″,N′″-tetraacetic acid macrocycles are available for coupling to peptides and other biomolecules. In addition to these hydrophilic chelators, a lipophilic tetradentate S(3)N legend was developed. The recent clinical experience with 68Ga-peptides includes imaging neuroendocrine tumors, particularly carcinoid [[68Ga]DOTA,Tyr(3)-octreotide, localizing neuroendocrine tumors with higher sensitivity than [111In]diethylenetriaminepentaacetic acid-octreotide], as well as neuroectodermal tumors such as phaeochromocytoma and paraganglioma or prostate cancer. In vitro and animal testing are still progressing alongside clinical studies, with promising results in the use of [68Ga]DOTA-rhenium-cyclized α-melanocyte stimulating hormone and [68Ga]DOTA-napamide in melanoma, [68Ga]DOTA-PEG(4)-BN(7-14) for the imaging of bombesin receptor-positive tumors, and [68Ga]ethylene dicysteine-metronidazole for imaging tumor hypoxia. In addition to tumors, the [68Ga]DOTA peptide inhibitor of vascular peptide protein 1 is being assessed for imaging inflammatory reaction. An additional value following a positive scan is the use of β-emitters labeled to the same peptides for radionuclide treatment (Pagou et al., 2009). The use of peptides for PET molecular imaging has undeniable advantages: the possibility of targeting through peptide-receptor interaction, small size and low-molecular weight conferring good penetration in the tissue or at cellular level, low toxicity, no antigenicity, and the possibility of a wide choice for radiolabeling (for the representative examples, see Table 2) (Morgat et al., 2013). In conclusion, the recent introduction of 68Ga-peptides could greatly contribute to the management of a wide range of clinical conditions, including tumors and inflammation (Pagou et al., 2009).
Complexation of gallium with organic ligands has been recognized as a promising strategy for creating tumor-inhibiting therapeutics with a number of advantages over gallium salts concerning oral bioavailability. Indeed, their evolved hydrolytic stability and membrane penetration ability render gallium complexes improved intestinal absorption functions compared with nitrate or chloride salt, which leads to increased plasma concentrations of gallium. Owing to such benefits, as well as better antiproliferative properties, two oral compounds, tris(8-quinolinolato)-gallium(III) (KP46) and tris(3-hydroxy-2-methyl-4H-pyran-4-onato)gallium(III) (gallium maltolate), have been selected from a series of gallium complexes for clinical development. KP46 has finished phase I trials, with the outcome of promising tolerability and evidence of clinical activity in renal cell carcinoma; however, it also reduces the viability of A549 human malignant lung adenocarcinoma cells. Gallium maltolate is a promising chemotherapeutic agent for the treatment of hepatocellular carcinoma. Likewise, bis(2-acetylpyridine-4,4-dimethyl-3-thiosemicarbazonato-N,N,S)gallium(III) tetrachlorogallate(III) (KP1089) was the first representative of the class of a-N-heterocyclic thiosemicarbazone complexes that has been assayed for antineoplastic activity in human tumor cell lines – ovary, breast, and colon (Collery et al., 2000; Arion et al., 2002; Chua et al., 2006; Rudnev et al., 2006; Chitambar et al., 2007; Timerbaev, 2009).
Another promising bioactive organic ligand is the nanobodies. Nanobodies are the smallest fully functional antigen-binding antibody fragments possessing ideal properties as probes for molecular imaging. The anti-human epidermal growth factor receptor type 2 (HER2) nanobody was labeled with 68Ga through a 1,4,7-triazacyclononane-1,4,7-triacetic acid (NOTA) derivative, and its use for HER2 iPET imaging was assessed. Preclinical validation showed high-specific-contrast imaging HER 2-positive tumors with no observed toxicity. [68Ga]NOTA-2Rs15d is ready for the first-in-human clinical trials (Xavier et al., 2013).
Over the years, increasing attention has been paid to pyrazoles in drug and related research owing to the ability of metal complexes to be anticancer (e.g., human colorectal cell lines, gastric cell lines, leukemia cells, and many others), antibacterial/parasitic, and antiviral agents (Tajiri et al., 1994).
The trivalent gallium cation is capable of inhibiting tumor growth, mainly because of its resemblance to ferric iron. It affects cellular acquisition of iron by binding to TF, and it interacts with the iron-dependent enzyme ribonucleotide reductase, resulting in reduced intracellular deoxyribonucleoside triphosphate (dNTP) pools and inhibition of DNA synthesis. The abundance of TF receptors and the upregulation of ribonucleotide reductase render tumor cells susceptible to the cytotoxicity of gallium. A remarkable clinical activity in lymphomas and bladder cancer has been documented in clinical studies employing intravenous gallium nitrate (Jakupec and Keppler, 2004).
Another way to treat cancer is through photodynamic therapy. Tetrapyrroles are multisided natural products that are of relevance in clinical medicine. Owing to their specific accumulation in tumor tissue, porphyrins, metalloporphyrins, and chlorins have been used in photodynamic therapy and optical imaging in connection with radionuclides (Zoller et al., 2013). Ga-porphyrins are used for sonodynamic therapy, the ultrasound-dependent enhancement of cytotoxic activities of certain compounds (sonosensitizers). The possible mechanisms of sonodynamic therapy include generation of sonosensitizer-derived radicals that initiate chain peroxidation of membrane lipids through peroxyl and/or alkoxyl radicals; the physical destabilization of the cell membrane by the sonosensitizer, thereby rendering the cell more susceptible to shear forces; or ultrasound-enhanced drug transport across the cell membrane (sonoporation) (Rosenthal et al., 2004). For example, photodynamic therapy using the new photosensitizer Ga-porphyrin complex could be applied for the treatment of pancreatic cancer (Tajiri et al., 1994). Tetrapyrroles are multisided natural products that are of relevance in clinical medicine. Owing to their specific accumulation in tumor tissue, porphyrins, metalloporphyrins, and chlorines have been used in photodynamic therapy and optical imaging, for example, labeled using 68Ga. A proof-of-concept PET study of 68Ga-labeled tetrapyrrole complexes suggests the suitability of these novel tracer candidates for PET imaging (Zoller et al., 2013). Other examples are metal nanoparticles that have become of great interest in research as photosensitizers in photodynamic therapy of cancer, drug delivery, and photodynamic antimicrobial therapy (Managa et al., 2014).
Conclusions
Early and specific tumor detection, and also therapy selection and response evaluation, are some challenges of personalized medicine (Morgat et al., 2013). The development of new radiopharmaceuticals and their availability are crucial factors influencing the expansion of clinical nuclear medicine. The contribution of Ga in this way is undeniable. It has earned its place in the treatment of bone tumors and bone metastases (e.g., Ga-nitrate), as well as in whole-body scan γ-scintigraphy (67Ga-citrate). The number of new 68Ga-based imaging agents for PET has increased greatly in the last decade. 68Ga has been used for labeling of a broad range of molecules (small organic molecules, peptides, short amino acid sequences, proteins, and oligonucleotides) as well as particles (nanoparticles, body cells, monoclonal antibodies), thus demonstrating its potential to become a PET analogue of the legendary generator-produced γ-emitting 99mTc but with the added value of higher sensitivity and resolution, as well as quantitation and dynamic scanning (Velikyan, 2013). This is exactly where Ga pharmaceuticals are heading today, focusing mainly on the 68Ga isotope while it is available right on site, in hospitals, thanks to the generator mentioned above; high purity and radiochemical yields (important factors for the development of an automated processing system); lower toxicity compared with 66,67Ga isotopes; dynamic scanning; and a number of chelators available for complexing with biomolecules. In a review on the continuing role of radionuclide generator systems for nuclear medicine, Knapp and Mirzadeh (1994) stated that ‘despite the availability of the 68Ge/68Ga generator application of 68Ga, radiopharmaceuticals may suffer from the complex ligand chemistry required for Ga(III) complexation to useful tissue-specific radiopharmaceuticals’. Indeed, at that time (1994), no 68Ga-labeled pharmaceuticals were in clinical studies. However, in 2011, two 68Ga-labeled pharmaceuticals – [68Ga]N-[(4,7,10-tricarboxymethyl-1,4,7,10-tetraazacyclododec-1-yl)acetyl]-d-phenylalanyl-l-cysteinyl-l-tyrosyl-d-tryptophanyl-l-lysyl-l-threoninyl-l-cysteinyl-l-threonine-cyclic(2-7)disulfide and [68Ga]pasireotide tetraxetan – for the diagnosis of gastroenteropancreatic endocrine tumors were found. However, these drugs are still not authorized by the European Medicines Agency but are in a pipeline. There is an urgent need to reduce the cost (i.e., time and money) of developing imaging agents for routine clinical use (Breeman et al., 2011). Generator-produced 68Ga and the development of small chelator-coupled peptides (and other small biomolecules) may open a new generation of freeze-dried, good manufacturing practice-produced, kit-formulated PET radiopharmaceuticals similar to 99Mo-/99mTc-generator-based, 99mTc-labeled radiopharmaceuticals (Maecke et al., 2005).
From this review, it is obvious that Ga has a wide spectrum of use in cancer therapy, mainly as an imaging agent for the early detection of cancer cells or malignant changes in the body, as a therapeutic agent for treating various cancers, and even as a sonosensitizer. Another example of the importance of radionuclide medicine lies in the early diagnosis of abscesses. Most reports agree that CT is the imaging method of choice for the diagnosis of intra-abdominal abscesses. However, in patients with a distortion of normal anatomy because of recent trauma or surgery, an early infection before the development of discrete fluid collections is more difficult to detect with CT. In these clinical situations, radionuclide scanning may be of great value (Lin et al., 2007). Ga(III) complexes with pharmaceutically active organic ligands may show additive or synergistic effects of both metal and ligands in one same compound. If the metal acts by a mechanism that is distinct from that of the ligands, different targets would be reached by the administration of the complex. In addition, organic ligands may be useful carriers of gallium into the cells. Although the pharmacological properties of gallium have been extensively investigated, there is not a great number of non-radioactive Ga(III) complexes that have been examined for their therapeutic potential (Lessa et al., 2012).
Acknowledgments
The authors thank the Toxicological and Antidoping Center of the Faculty of Pharmacy Comenius University for supporting this work. This work was also supported by a research grant from the Slovak Grant Agency (VEGA No. 1/0664/12) and by grants from the Faculty of Pharmacy Comenius University in Bratislava (FaF 33/2013 and FaF 38/2013).
References
Al-Nahhas, A.; Win, Z.; Szyszko, T.; Singh, A.; Khan, S.; Rubello, D. What can gallium-68 PET add to receptor and molecular imaging? Eur. J. Nucl. Med. Mol. Imaging2007, 34, 1897–1901.Suche in Google Scholar
Arion, V. B.; Jakupec, M. A.; Galanski, M.; Unfried, P.; Keppler, B. K. Synthesis, structure, spectroscopic and in vitro antitumour studies of a novel gallium(III) complex with 2-acetylpyridine 4N-dimethylthiosemicarbazone. J. Inorg. Biochem.2002, 91, 298–305.Suche in Google Scholar
Balbi, A.; Anzaldi, M.; Macciò, C.; Aiello, C; Mazzei, M.; Gangemi, R.; Castagnola, P.; Miele, M.; Rosno, C.; Viale, M. Synthesis and biological evaluation of novel pyrazole derivatives with anticancer activity. Eur. J. Med. Chem.2011, 46, 5293–5309.Suche in Google Scholar
Belosi, F.; Cicoria, F. G.; Lodi, F.; Malizia, C.; Fanti, S.; Boschi, S.; Marengo, M. Generator breakthrough and radionuclidic purification in automated synthesis of 68Ga-DOTANOC. Curr. Radiopharm. 2013, 6, 72–77.Suche in Google Scholar
Bernstein, L. R. Mechanisms of therapeutic activity for gallium. Pharm. Rev.1998, 50, 665–682.Suche in Google Scholar
Breeman, W. A. P.; de Blois, E.; Chan, H. S.; Konijnenberg, M; Kwekkeboom, D. J.; Krenning, E. P. 68Ga-labeled DOTA-peptides and 68Ga-labeled radiopharmaceuticals for positron emission tomography: current status of research, clinical applications, and future perspectives. Semin. Nucl. Med.2011, 41, 314–321.Suche in Google Scholar
Brucer, M.; Andrews, G. A.; Bruner, H. D. A study of gallium-72. Radiology1953, 61, 534–613.Suche in Google Scholar
Cagnolini, A.; Chen, J.; Ramos, K; Skedzielewski, T. M.; Lantry, L. E.; Nunn, A. D.; Swenson, R. E.; Linder, K. E. Automated synthesis, characterization and biological evaluation of [68Ga]Ga-AMBA, and the synthesis and characterization of natGa-AMBA and [67Ga]Ga-AMBA. Appl. Radiat. Isot. 2010, 68, 2285–2292.Suche in Google Scholar
Campain, M. E.; Hardziyenka, M.; Bruin, K.; van Eck-Smit, B. L. F.; de Bakker, J. M. T.; Verberne, H. J.; Tan, H. L. Early inflammatory response during the development of right ventricular heart failure in a rat model. Eur. J. Heart Fail. 2010, 12, 653–658.Suche in Google Scholar
Chitambar, C. R.; Purpi, D. P.; Woodliff, J.; Yang, M.; Wereley, J. P. Development of gallium compounds for treatment of lymphoma: gallium maltolate, a novel hydroxypyrone gallium compound, induces apoptosis and circumvents lymphoma cell resistance to gallium nitrate. J. Pharmacol. Exp.Ther. 2007, 322, 1228–1236.Suche in Google Scholar
Chopra, A. 111In/68Ga-labeled DOTA-conjugated cyclo[γ-d-Glu-Ala-Tyr-d-Lys]-Trp-Met-Asp-Phe-NH2 (cyclo-MG1), a minigastrin analog. Molecular Imaging and Contrast Agent Database (MICAD). National Center for Biotechnology Information: Bethesda, MD, USA, 2004–2013a.Suche in Google Scholar
Chopra, A. 68Ga-labeled DOTA-conjugated sCCK8[Phe2(p-CH2 SO3 H),Nle3,6], a sulfated cholecystokinin 8 (sCCK8) peptide derivative. Molecular Imaging and Contrast Agent Database (MICAD). National Center for Biotechnology Information: Bethesda, MD, USA, 2004–2013b.Suche in Google Scholar
Chua, M. S.; Bernstein, L. R.; Li, R.; So, S. K. S. Gallium maltolate is a promising chemotherapeutic agent for the treatment of hepatocellular carcinoma. Anticancer Res.2006, 26, 1739–1744.Suche in Google Scholar
Collery, P.; Lechenault, F.; Cazabat, A.; Juvin, E.; Khassanova, A.; Evangelou, A.; Kepller, B. Inhibitory effects of gallium chloride and tris (8-quinolinolato) gallium III on A549 human malignant cell line. Anticancer Res. 2000, 20, 955–958.Suche in Google Scholar
Collery, P.; Keppler, B.; Madoulet, C.; Desoize, B. Galium in cancer treatment. Crit. Rev. Oncol./Hematol.2002, 42, 283–296.10.1016/S1040-8428(01)00225-6Suche in Google Scholar
Costa, R. F.; Barboza, M. F.; Osso J. A. (67)Ga and (68)Ga purification studies: preliminary results. Rec. Res. Cancer Res.2013, 194, 89–97.Suche in Google Scholar
Fani, M.; Andre, J. P.; Maecke, H. R. 68Ga-PET: a powerful generator-based alternative to cyclotron-based PET radiopharmaceuticals. Contrast Media Mol. Imaging2008, 3, 67–77.Suche in Google Scholar
Fellner, M.; Biesalski, B.; Bausbacher, N.; Kubícek, V.; Hermann, P.; Rösch, F.; Thews, O. 68Ga-BPAMD: PET-imaging of bone metastases with a generator based positron emitter. Nucl. Med. Biol.2012, 39, 993–999.Suche in Google Scholar
Fernández, S.; Dematteis, S.; Giglio, J.; Cerecetto, H.; Rey, A. Synthesis, in vitro and in vivo characterization of two novel 68Ga-labelled 5-nitroimidazole derivatives as potential agents for imaging hypoxia. Nucl. Med. Biol. 2013, 40, 273–279.Suche in Google Scholar
Fournier, P.; Dumulon-Perreault, V.; Ait-Mohand, S.; Tremblay, S.; Bénard, F.; Lecomte, R.; Guérin, B. Novel radiolabeled peptides for breast and prostate tumor PET imaging: (64)Cu/and (68)Ga/NOTA-PEG-[d-Tyr(6),βAla(11),Thi(13),Nle(14)]BBN(6–14). Bioconjug. Chem. 2012, 23, 1687–1693.Suche in Google Scholar
Front, D.; Israel, O. The role of Ga-67 scintigraphy in evaluating the results of therapy of lymphoma patients. Sem. Nucl. Med. 1995, 25, 60–71.Suche in Google Scholar
Front, D.; Israel, O.; Epelbaum, R.; Ben Hajm, S.; Sapir, E. E.; Jerushalmi, J.; Kolodny, G. M.; Robinson, E. Ga-67 SPECT before and after treatment of lymphoma. Radiology 1990, 175, 515–519.Suche in Google Scholar
Gallego, B.; Kaluderović, M. R.; Kommera, H.; Paschke, R.; Hey-Hawkins, E.; Remmerbach, T. W.; Kaluderović, G. N.; Gómez-Ruiz, S. Cytotoxicity, apoptosis and study of the DNA-binding properties of bi- and tetranuclear gallium(III) complexes with heterocyclic thiolato ligands. Invest. New Drugs2011, 29, 932–944.Suche in Google Scholar
Gambino, D.; Fernández, M.; Santos, D.; Etcheverría, G. A.; Piro, O. E.; Pavan, F. R.; Leite, C. Q. F.; Tomaz, I.; Marques, F. Searching for gallium bioactive compounds: gallium(III) complexes of tridentate salicylaldehyde semicarbazone derivatives. Polyhedron, 2011, 30, 1360–1366.Suche in Google Scholar
Garcia, R.; Fouskova, P.; Gano, L.; Paulo, A.; Campello, P.; Toth, E.; Santos, I. A quinazoline-derivative DOTA-type gallium(III) complex for targeting epidermal growth factor receptors: synthesis, characterisation and biological studies. J. Biol. Inorg. Chem. 2009, 14, 261–271.Suche in Google Scholar
Gasparini, M. D.; Balzarini, L.; Castellani, M. R.; Tesoro Tess, J. D.; Maffioli, L. S.; Petrillo, R.; Ceglia, E.; Musumeci, R.; Buraggi, G. L. Current role of gallium scan and magnetic resonance imaging in the management of mediastinal Hodgkin’s lymphoma. Cancer 1993, 72, 577–582.Suche in Google Scholar
Goldman, P. The development of 5-nitroimidazoles for the treatment and prophylaxis of anaerobic bacterial infections. J. Antimicrob. Chemother. 1982, 10, 23–33.Suche in Google Scholar
Inkster, J.; Lin, K. S.; Ait-Mohand, S.; Gosselin, S.; Bénard, F.; Guérin, B.; Pourghiasian, M.; Ruth, T.; Schaffer, P.; Storr, T. 2-Fluoropyridine prosthetic compounds for the 18F labeling of bombesin analogues. Bioorg. Med. Chem. Lett., 2013, 23, 3920–3926.Suche in Google Scholar
Jakupec, M. A.; Keppler, B. K. Gallium in cancer treatment. Curr. Top. Med. Chem. 2004, 4, 1575–1583.Suche in Google Scholar
Jalilian, A. R.; Rowshanfarzad, P.; Sabet, M.; Novinrooz, A.; Raisali, G. Preparation of [66Ga]bleomycin complex as a possible PET radiopharmaceutical. J. Radioanal. Nucl. Chem. 2005, 264, 617–621.Suche in Google Scholar
Jalilian, A. R.; Mirazizi, F.; Nazem, H. Molecular-biological problems of drug design and mechanism of drug action: development of 67Ga-labeled streptokinase. Pharm. Chem. J. 2009, 43, 287–293.Suche in Google Scholar
Juchau, M. R. Bioactivation in chemical teratogenesis. Annu. Rev. Pharmacol. Toxicol. 1989,29, 165–167.Suche in Google Scholar
Kalinowski, D. S.; Quach, P.; Richardson, D. R. Thiosemicarbazones: the new wave in cancer treatment. Future Med. Chem. 2009, 1, 1143–1151.Suche in Google Scholar
Knapp, F. F.; Mirzadeh, S. The continuing important role of radionuclide generator systems for nuclear medicine. Eur. J. Nucl. Med.1994, 21, 1151–1165.Suche in Google Scholar
Kostakoglu, L.; Goldsmith, S. J. Positron emission tomography in lymphoma: comparison with computed tomography and gallium-67 single photon emission computed tomography. Clin. Lymphoma2000, 1, 67–74.10.3816/CLM.2000.n.007Suche in Google Scholar
Kunn, I.; Sumiya, H.; Taki, J.; Nakjima, K.; Yokoyama, K.; Kinuya, S.; Kinuya, K.; Ichikawa, A.; Konishi, S.; Michigishi, T.; et al. Intense Ga-67 uptake in adenosquamous carcinoma of the pancreas. Ann. Nucl. Med. 1997, 11, 41–43.Suche in Google Scholar
Lahorte, Ch. M. M.; Vanderheyden, J. -L.; Steinmetz, N.; Van de Wiele, Ch.; Dierckx, R. A.; Slegers, G. Apoptosis-detecting radioligands: current state of the art and future perspectives. Eur. J. Nucl. Med. Mol. Imaging2004, 31, 887–919.Suche in Google Scholar
Le, V. S. (68)Ga generator integrated system: elution-purification-concentration integration. Rec. Res. Cancer Res. 2013, 194, 43–75.Suche in Google Scholar
Lessa, J. A.; Parrilha, G. L.; Beraldo, H. Gallium complexes as new promising metallodrug candidates. Inorg. Chim. Acta2012, 393, 53–63.Suche in Google Scholar
Leung, K. 68Ga-1,4,7-Triazacyclononane,1-glutaric acid-4,7-acetic acid-Asp-cyclohexylalanine-Phe-d-Ser-d-Arg-Tyr-Leu-Trp-Ser-NH2 (AE105-NH2). Molecular Imaging and Contrast Agent Database (MICAD). National Center for Biotechnology Information: Bethesda, MD, USA, 2004–2013.Suche in Google Scholar
Lin, W. -Y.; Hung, G. -U.; Chao, T. -H. The value of Tc-99m (V) dimercaptosuccinic acid in detecting intra-abdominal infection: compared with gallium scan. Ann. Nucl. Med. 2007, 21, 285–291.Suche in Google Scholar
Liu, F. -Y.; Shiau, Y. -C.; Yen, R. -F.; Wang, J. -J.; Ho, S. -T.; Kao, C. -H. Comparison of gallium-67 citrate and technetium-99m tetrofosmin scan to detect Hodgkin’s disease. Ann. Nucl. Med. 2003, 17, 439–442.Suche in Google Scholar
Liu, Z.; Niu, G.; Wang, F.; Chen, X. 68Ga-labeled NOTA-RGD-BBN peptide for dual integrin and GRPR-targeted tumor imaging. Eur. J. Nucl. Med. Mol. Imaging2009a, 36, 1483–1494.10.1007/s00259-009-1123-zSuche in Google Scholar
Liu, Z.; Niu, G.; Shi, J.; Liu, S.; Wang, F.; Chen, X. (68)Ga-labeled cyclic RGD dimers with Gly3 and PEG4 linkers: promising agents for tumor integrin αvβ3 PET imaging. Eur. J. Nucl. Med. Mol. Imaging2009b, 36, 947–957.10.1007/s00259-008-1045-1Suche in Google Scholar
Maecke, H. R.; Andre, J. P. 68Ga-PET radiopharmacy: a generator-based alternative to 18F-radiopharmacy. Ernst Schering Res. Found. Workshop2007, 64, 215–242.Suche in Google Scholar
Maecke, H. R.; Hofmann, M.; Haberkorn, U. (68)Ga-labeled peptides in tumor imaging. J. Nucl. Med. 2005, 46, 172–178.Suche in Google Scholar
Managa, M.; Antunes, E.; Nyokong, T. Conjugates of platinum nanoparticles with gallium tetra – (4-Carboxyphenyl) porphyrin and their use in photodynamic antimicrobial chemotherapy when in solution or embedded in electrospun fiber. Polyhedron2014, 76, 94–101.Suche in Google Scholar
Melpomeni, F.; Xuejuan, W.; Guillaume, N.; Christelle, M.; Raynal, I.; Port, M.; Maecke, H. R. Development of new folate-based PET radiotracers: preclinical evaluation of 68Ga-DOTA-folate conjugates. Eur. J. Nucl. Med. Mol. Imaging2011, 38, 108–119.Suche in Google Scholar
Morgat, C.; Hindié, E.; Mishra, A. K.; Allard, M.; Fernandez, P. Gallium-68: chemistry and radiolabeled peptides exploring different oncogenic pathways. Cancer Biother. Radiopharm. 2013, 28, 85–97.Suche in Google Scholar
Mueller, D.; Klette, I.; Baum, R. P. Purification and labeling strategies for (68)Ga from (68)Ge/(68)Ga generator eluate. Rec. Res. Cancer Res. 2013, 194, 77–87.Suche in Google Scholar
Pagou, M.; Zerizer, I.; Al-Nahhas, A. Can gallium-68 compounds partly replace (18)F-FDG in PET molecular imaging? Hell. J. Nucl. Med. 2009, 12, 102–105.Suche in Google Scholar
Persson, M.; Madsen, J.; Østergard, S.; Ploug, M.; Kjaer, A. 68Ga-labeling and in vivo evaluation of a uPAR binding DOTA- and NODAGA-conjugated peptide for PET imaging of invasive cancers. Nucl. Med. Biol.2012, 39, 560–569.Suche in Google Scholar
Roesch, F.; Riss, P. J. The renaissance of the Ge/Ga radionuclide generator initiates new developments in Ga radiopharmaceutical chemistry. Curr. Top. Med. Chem. 2010, 10, 1633–1668.Suche in Google Scholar
Roivainen, A.; Jalkanen, S.; Nanni, C. Gallium-labelled peptides for imaging of inflammation. Eur. J. Nucl. Med. Mol. Imaging2012, 39, 68–77.10.1007/s00259-011-1987-6Suche in Google Scholar
Rösch, F. Past, present and future of 68Ge/68Ga generators. Appl. Radiat. Isot. 2013a, 76, 24–30.Suche in Google Scholar
Rösch, F. (68)Ge/(68)Ga generators: past, present, and future. Rec. Res. Cancer Res. 2013b, 194, 3–16.Suche in Google Scholar
Rosenthal, I.; Sostaric, J. Z.; Riesz, P. Sonodynamic therapy – a review of the synergistic effects of drugs and ultrasound. Ultrason. Sonochem.2004, 11, 349–363.Suche in Google Scholar
Rudnev, A. V.; Foteeva, L. S.; Kowol, Ch.; Berger, R.; Jakupec, M. A.; Arion, V. B.; Timerbaev, A. R.; Keppler, B. K. Preclinical characterization of anticancer gallium(III) complexes: solubility, stability, lipophilicity and binding to serum proteins. J. Inorg. Biochem.2006, 100, 1819–1826.Suche in Google Scholar
Schroeder, R. P.; van Weerden W. M.; Krenning, E. P.; Bangma, Ch.; Berndsen, S.; Grievink-de Ligt, Ch.; Groen, Ch.; Reneman, S.; de Blois, E.; Breeman, W. A.; et al. Gastrin-releasing peptide receptor-based targeting using bombesin analogues is superior to metabolism-based targeting using choline for in vivo imaging of human prostate cancer xenografts. Eur. J. Nucl. Med. Mol. Imaging2011, 38, 1257–1266.Suche in Google Scholar
Schultz, M. K.; Mueller, D.; Baum, R. P.; Watkins, G. L.; Breeman, W. A. A new automated NaCl based robust method for routine production of gallium-68 labeled peptides. Appl. Radiat. Isot. 2013, 76, 46–54.Suche in Google Scholar
Silvola, J. M. U.; Laitinen, L.; Sipilä, H. J.; Laine, V. J. O.; Leppänen, P.; Ylä-Herttuala, S.; Knuuti, J.; Roivainen, A. Uptake of 68gallium in atherosclerotic plaques in LDLR-/- ApoB100/100 mice. EJNMMI Res. 2011, 1, 1–8.Suche in Google Scholar
Smith, D. L.; Breeman, W. A.; Sims-Mourtada, J. The untapped potential of Gallium 68-PET: the next wave of 68Ga-agents. Appl. Radiat. Isot. 2013, 76, 14–23.Suche in Google Scholar
Sturzu, A.; Sheikh, S.; Echner, H.; Nägele, T.; Deeg, M.; Amin, B.; Schwentner, C.; Horger, M.; Ernemann, U.; Heckl, S. Rhodamine-marked bombesin: a novel means for prostate cancer fluorescence imaging. Invest. New Drugs2014, 32,37–46.Suche in Google Scholar
Su, M.; Yunping Qiu, M. S.; Wei Jia, M. S. A pilot study of antitumor effect of gallium ethylenediaminetetramethylene phosphonate [Ga(III)-EDTMP] in tumor-bearing rats. Adv. Ther.2005, 22, 297–306.Suche in Google Scholar
Tajiri, H.; Kuroki, M.; Niwa, H. Photodynamic therapy using a new photosensitizer gallium porphyrin complex in the treatment of experimental pancreatic cancer. Dig. Endosc. 1994, 6, 28–33.Suche in Google Scholar
Timerbaev, A. R. Advances in developing tris(8-quinolinolato)gallium(III) as an anticancer drug: critical appraisal and prospects. Metallomics2009, 1, 193–198.10.1039/b902861gSuche in Google Scholar
Treglia, G.; Castaldi, P.; Rindi, G.; Giordano, A.; Rufini, V. Diagnostic performance of Gallium-68 somatostatin receptor PET and PET/CT in patients with thoracic and gastroenteropancreatic neuroendocrine tumours: a meta-analysis. Endocrine2012, 42, 80–87.Suche in Google Scholar
Tumeh, S. S.; Rosenthal, D. S.; Kaplan, W. D.; English, R. J.; Holman, B. L. Lymphoma: evaluation with Ga-67 SPECT. Radiology. 1987, 167, 111–114.Suche in Google Scholar
Vaneycken, I.; Devoogdt, N.; Van Gassen, N.; Vincke, C.; Xavier, C.; Werney, U.; Muyldermans, S.; Lahoutte, T.; Caveliers, V. Preclinical screening of anti-HER2 nanobodies for molecular imaging of breast cancer. FASEB J. 2011, 25, 2433–2446.Suche in Google Scholar
Varasteh, Z.; Velikyan, I.; Lindeberg, G.; Sörensen, J.; Larhed, M.; Sandström, M.; Selvaraju, R. K.; Malmberg, J.; Tolmachev, V.; Orlova, A. Synthesis and characterization of a high-affinity NOTA-conjugated bombesin antagonist for GRPR-targeted tumor imaging. Bioconjug. Chem.2013, 24, 1144–1153.Suche in Google Scholar
Velikyan, I. The diversity of (68)Ga-based imaging agents. Rec. Res. Cancer Res. 2013, 194, 101–131.Suche in Google Scholar
Wadas, T. J.; Wong, E. H.; Weisman, G. R. Coordinating radiometals of copper, gallium, indium, yttrium, and zirconium for PET and SPECT imaging of disease. Chem. Rev. 2010, 110, 2858–2902.Suche in Google Scholar
Warrell, R. P., Jr. Handbook of Metal-Ligand Interactions in Biological Fluids – Bioinorganic Medicine. Marcel Dekker Incorporated: New York, 1995.Suche in Google Scholar
Warrell, R. P., Jr. Gallium nitrate for the treatment of bone metastases. Cancer1997, 80, 1680–1685.10.1002/(SICI)1097-0142(19971015)80:8+<1680::AID-CNCR19>3.0.CO;2-WSuche in Google Scholar
Welch, M. J.; Redvanly, C. S. Handbook of Radiopharmaceuticals: Radiochemistry and Applications, John Wiley & Sons, Ltd: UK, 2003.10.1002/0470846380Suche in Google Scholar
Xavier, C.; Vaneycken, I.; D’huyvetter, M.; Heemskerk, J.; Keyaerts, M.; Vincke, C.; Devoogdt, N.; Muyldermans, S.; Lahoutte, T.; Caveliers, V. Synthesis, preclinical validation, dosimetry, and toxicity of 68Ga-NOTA-anti-HER2 nanobodies for iPET imaging of HER2 receptor expression in cancer. J. Nucl. Med. 2013, 54, 776–784.Suche in Google Scholar
Zoller, F.; Riss, P. J.; Montforts, F. P.; Kelleher, D. K.; Eppard, E.; Rösch, F. Radiolabelling and preliminary evaluation of 68Ga-tetrapyrrole derivatives as potential tracers for PET. Nucl. Med. Biol. 2013, 40, 280–288.Suche in Google Scholar
©2014 by De Gruyter
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Artikel in diesem Heft
- Frontmatter
- Review
- Gallium compounds in nuclear medicine and oncology
- Research Articles
- Reaction of digermanes and related Ge-Si compounds with trifluoromethanesulfonic acid: synthesis of helpful building blocks for the preparation of Ge-Ge(Si)-catenated compounds
- Coordination propensity of N-protected amino acids and sterically congested heterocyclic β-diketones toward BuSnCl3: preparation and structural features of some new seven coordinated organic-inorganic hybrid formulations of monobutyltin(IV)
- The structures of strontium xanthates Sr(S2COR)2·xROH (R=Et, Pri, But)
- Synthesis, structural characterization and antibacterial activity of triorganotin ferrocenecarboxylates
- Short Communications
- Molecular structure of the functionalized bismuth alkoxide Bi[OC(CH2 NMe2)3]3
- Synthesis and crystal structure of Pb(II) complex with 1-phenyl-3-methyl-4-benzoyl-5-pyrazolone
- [n-Bu2 NH2]3[SnPh3(SeO4)2]: the first triorganotin(IV) complex with terminally coordinated selenato ligands
Artikel in diesem Heft
- Frontmatter
- Review
- Gallium compounds in nuclear medicine and oncology
- Research Articles
- Reaction of digermanes and related Ge-Si compounds with trifluoromethanesulfonic acid: synthesis of helpful building blocks for the preparation of Ge-Ge(Si)-catenated compounds
- Coordination propensity of N-protected amino acids and sterically congested heterocyclic β-diketones toward BuSnCl3: preparation and structural features of some new seven coordinated organic-inorganic hybrid formulations of monobutyltin(IV)
- The structures of strontium xanthates Sr(S2COR)2·xROH (R=Et, Pri, But)
- Synthesis, structural characterization and antibacterial activity of triorganotin ferrocenecarboxylates
- Short Communications
- Molecular structure of the functionalized bismuth alkoxide Bi[OC(CH2 NMe2)3]3
- Synthesis and crystal structure of Pb(II) complex with 1-phenyl-3-methyl-4-benzoyl-5-pyrazolone
- [n-Bu2 NH2]3[SnPh3(SeO4)2]: the first triorganotin(IV) complex with terminally coordinated selenato ligands

