Abstract
No single studies on the Burmese Red Serows, a little known bovid to date yet considered as nationally endangered species, have been conducted in Bangladesh. As part of a systematic wildlife inventory and monitoring project, this study utilized 48 camera traps to elucidate their occurrence, activity pattern and possible sympatric association with other artiodactyls species inhabiting Baraiyadhala National Park. The study found 25 independent serow events with relative abundance index (RAI) of 0.89. The number of individuals seems low, but this is by far the country’s stronghold population. The Red Serows are mostly nocturnal and showed moderate temporal activity with barking dear (∆1 = 0.59) and wild boar (∆1 = 0.62) in this area. Anthropogenic pressures due to tourists (RAI = 4.8) and poachers (RAI = 1.7) were highly evident and must have negative impact on overall wildlife of the national park. The study suggests similar studies in adjacent protected area (Hazarikhil Wildlife Sanctuary), habitat protection and restoration program, and awareness raising program targeting forest dependent communities for long term conservation of this species.
1 Introduction
Burmese Red Serow (Capricornis rubidus) is a confirmed resident in China, India, Myanmar and has a doubtful occurrence in Bangladesh as per IUCN global Red List assessment (Shepherd 2022). However, the national Red List of Bangladesh assessed only one species of serow which is Red Serow alias C. rubidus (IUCN Bangladesh 2015). There are about four to seven species of serows distributed over eastern and southeastern Asia and still there are disputes regarding classification and distribution of these ungulates across their range (Chen et al. 2019; Grubb 2005; Mori et al. 2019).
Despite controversy over the serow species’ classification, a recent molecular phylogenetic analysis of the Capricornis genus indicates that the serow species found in Bangladesh is possibly the Red Serow (C. rubidus) (Mori et al. 2019). However, it is speculated that more than one species of serow could inhabit Bangladesh. The Red Serow is a globally vulnerable bovid (Shepherd 2022) and very little is known about its population status, behavioral ecology, and habitat use pattern in most of the countries that make up its range. Bangladesh is no exception, and so far, it has only handful and sporadic appearances from mixed evergreen forests of the Chittagong Hill Tracts and the mixed evergreen forests of transboundary northeastern regions and is regarded as nationally endangered species (IUCN Bangladesh 2015; Khan 2018).
In recent times, with the advancement of camera trapping studies, there have been few reports of Red Serows from northeast region and Chittagong Hill Tracts. However, our understanding of its overall population, activity pattern, habitat use pattern with other sympatric species, and threats is still very limited. In this study, we did not attempt to address the issues with serow classification; rather, we reported on a population that had not been studied before in order to precise its relative abundance, activity patterns and co-occurrence with other ungulates.
2 Materials and methods
2.1 Study area
Baraiyadhala National Park (BNP) (22°42′53.47″N and 91°38′20.17″E) (Figure 1) is a stretch of 2933.61 ha mixed evergreen forest with scattered bamboo and bushy areas situated under Chittagong North Forest Division. This protected forest is ecologically diverse because of steep and undulating terrain with many waterfalls and hilly streams (Harun-ur-Rashid et al. 2018; Hasan et al. 2021; Karim and Ahsan 2016). Most of the streams are seasonal and dry up during the winter season. The climatic condition is tropical moist, and rainfall is frequent and heavy during the monsoon ranging between 115.2 and 561.1 mm with the total rainfall 1891 mm. The average maximum annual temperature was 31.50 °C and the minimum 20.67 °C. 29 mammalian species belonging to 26 genera, 17 families and 9 orders were recorded from this national park (Karim and Ahsan 2016). However, the presence of Dhole (Cuon alpinus), Bengal Fox (Vulpes bengalensis), Asiatic Brush-tailed Porcupine (Atherurus macrourus) and Indian Hare (Lepus nigricollis) was reported based on the local knowledge. A recently conducted camera trap study found 27 mammalian species from BNP (Kamrul Hasan pers. comm).
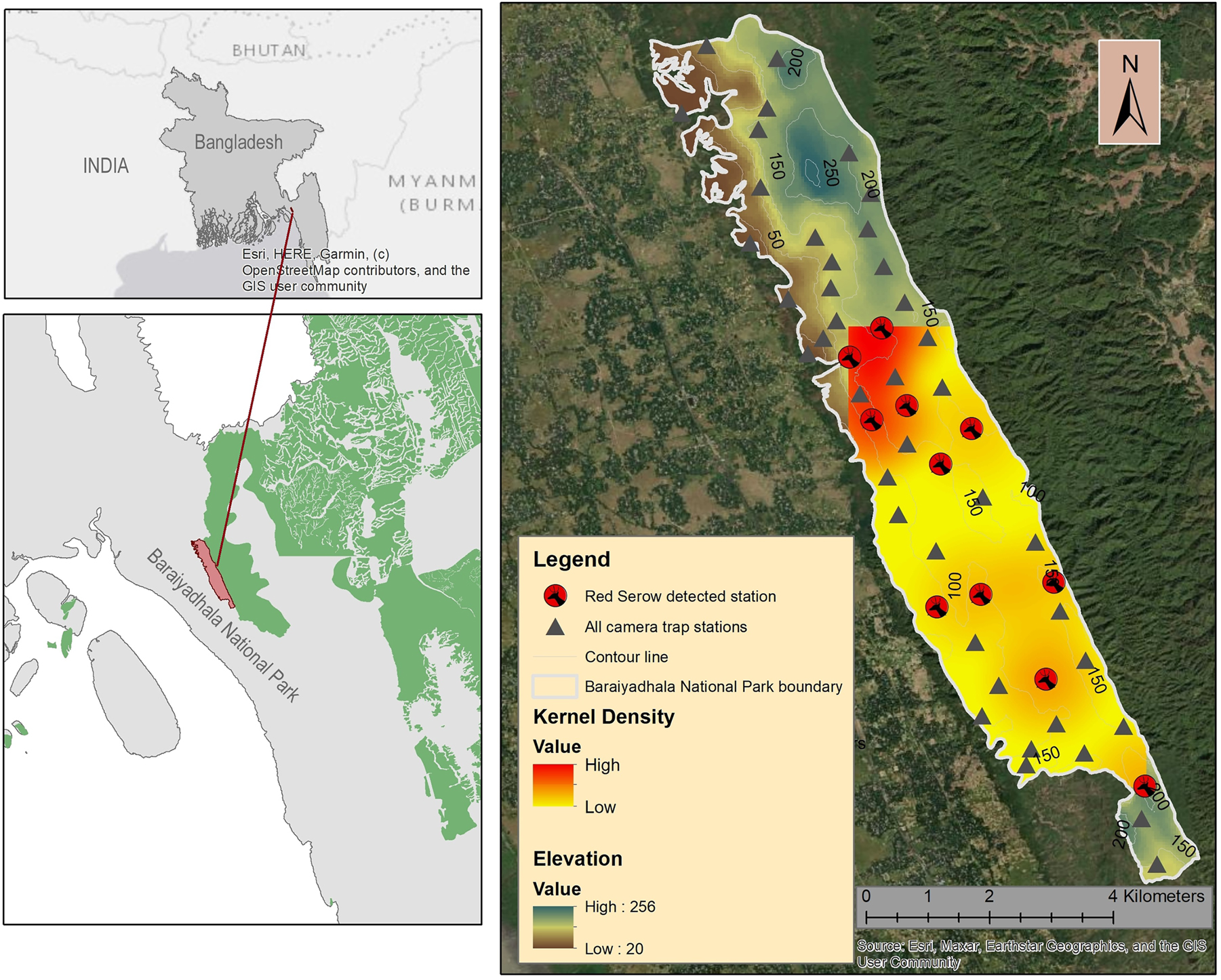
Study area and camera trap sites, indicating camera trap sites with Burmese Red Serow detection and sites where serows were not detected.
2.2 Data collection and analysis
To elucidate the overall diversity of wildlife inhabiting the national park, we conducted camera trap surveys from January 2022 to March 2023. A total of 48 camera trap stations (Figure 1) were selected based on the outcome of a reconnaissance survey conducted earlier. Camera trap stations were selected based on signs (tracks, footprints, and remains) left by animals along trails, forest floors or near waterbodies. A single motion-triggered camera trap (CamPark Hunting Trail Camera, T40A) was set around 20–40 cm above the ground (Chen et al. 2019; Palei et al. 2016). Cameras were deployed at different habitats and spaced at least 200 m apart. However, for some stations we couldn’t strictly maintain the distance due to inaccessible terrain. We did not use any lure or bait during the survey. Cameras were checked every two weeks to download memory cards and replace batteries.
The sum of the effective days for each camera at each station during its operational period served as the basis for calculating the total sampling effort. Photo captured at least 30 min apart were regarded as independent events (Guo et al. 2017). Different identifiable individuals (based on body size, horn size, scrotum etc.) were treated as a separate event even though they appeared in the same photo or the photos were taken within 30 min (O’Brien et al. 2003). Each photograph was scrutinized before been included in the dataset. We extracted independent detections of Burmese Red Serow, Barking Deer, and Wild Boar from image data acquired during the survey.
To estimate the abundance of Burmese Red Serow, RAI (relative abundance index) was calculated based on the following formula (O’Brien et al. 2003; Palei et al. 2016, 2022):
where, ‘A’ represents the total number of captures of a species by all cameras, and ‘N’ equals to the camera trap days during the study period.
We computed activity pattern of Burmese Red Serow and compared with two sympatric artiodactyls Barking Deer and Wild Boar to see the overlapping pattern in their daily activity using the package “Overlap” in R (R Core Team 2022). The estimated coefficient of overlap is denoted by “Dhat1” values (∆1) ranging from 0 (no overlap) and 1 (complete overlap) between two corresponding species (Ridout and Linkie 2009; Singh et al. 2022). We obtained 95 % confidence intervals as percentile intervals from 2000 bootstrap samples. To gather insights on hunting and serow occurrence, 120 individuals from two nearby ethnic Tripura communities, each with more than 100 families, were interviewed for the study. All statistics were performed in R version 4.2.1 (R Core Team 2022).
3 Results
We obtained 25 serow events captured from 11 spatially distinct locations with an effort of 2793 camera trap nights (Figure 2). Four female and 11 male serows were recognized from the photographs however, it was not certain which sexes the other ten individuals belonged to. All detections consisted of a single serow, apart from two incidents that entailed two individuals. About 65 % of detections (15 events) were in steep hilly habitats and the rest were in forest floor.

Camera trapped photo of Red Serow from the study area. The top image depicts an adult and a juvenile, and the bottom image is of an adult male.
The RAI of serow was 0.89, while the RAI of Barking Deer and Wild Boar was 9.92 and 5.01 respectively. The Burmese Red Serow appeared to be nocturnal and showed peak temporal activities after the dusk. Barking Deer and Wild Boar did not show any specific temporal preferences (Figure 3).
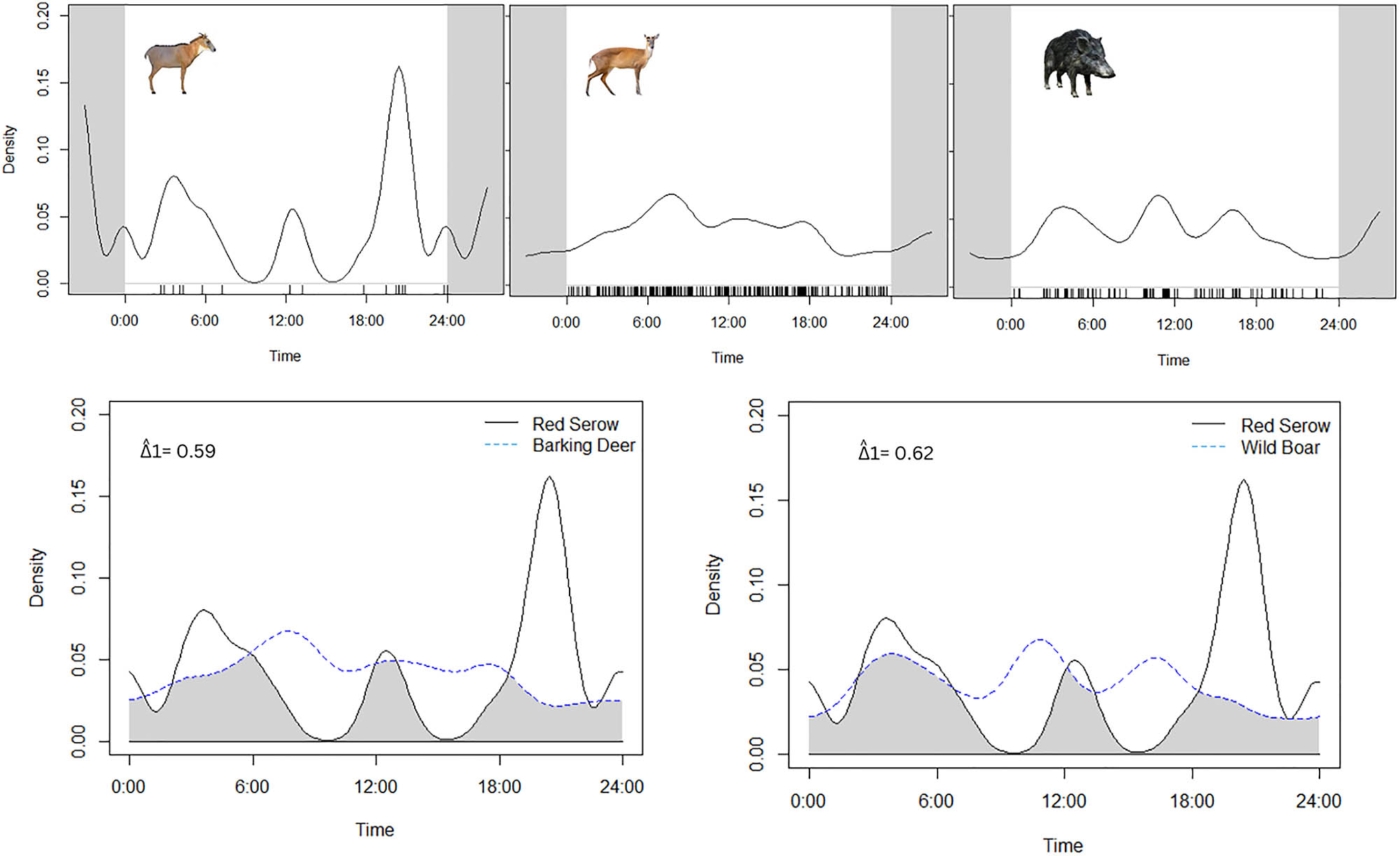
Temporal activity pattern and overlap estimates for focal species Burmese Red Serow and sympatric Barking Deer and Wild Boar.
In terms of overlapping temporal activity with two sympatric artiodactyls, Burmese Red Serow showed moderate overlap with both the Barking Deer (∆1 = 0.59; CI: 0.31–0.63) and Wild Boar (∆1 = 0.62; CI: 0.57–0.90) (Figure 3).
A total of 308 individual events of anthropogenic activities (categorized as villagers, tourists, poachers, livestock, and pet animals) were captured. Tourists have the higher RAI (4.8) followed by villagers (3.9) and poachers (1.7).
4 Discussion
Camera trapping studies provide valuable information on poorly studied cryptic, threatened, and sympatric wildlife species (Chen et al. 2019; Li et al. 2022; Singh et al. 2022; Zakir et al. 2020). This study for the first time reveals the country’s strongholds for Burmese Red Serow with vital insights on spatial and temporal activity pattern with sympatric artiodactyls in Baraiyadhala National Park. For taking conservation action and monitoring future changes, it is critical to know spatial as well as ecological information of a threatened species (Singh et al. 2022). Our study confirmed at least 25 individuals of Burmese Red Serow from BNP. The small number of detections (RAI = 0.82) of the Red Serow suggests that this species likely occurs at a low density in the study area. The local community people living nearby, and national park officials also reported similar perceptions on the occurrence of this species. Our camera trapped photos identified 49 individual poachers, which indicates intense hunting pressures for wildlife in this area. We speculated that Barking Deer, Serow, Wild Boar, Jungle Fowls, and pheasants are some of the targeted species.
Red Serow’s preferred nocturnal active period in our study site which was consistent with results obtained from Mt. Gaoligong National Nature Reserve, Yunnan Province, China (Chen et al. 2019). Aligned with other studies (Bhattacharya et al. 2012; Kobayashi and Takatsuki 2012; Liu et al. 2013; Seki and Hayama 2021) our results confirmed that the studied serows co-exist with other ungulates (Wild Boar and Barking Deer) by showing moderate temporal activity overlap in the study site. Such comparative studies of sympatric species are crucial in understanding co-evolutionary behavioural adaptations, activity pattern (Chen et al. 2019; Seki and Hayama 2021), niche separation (Feeroz 2012; Zhou et al. 2014), diet (Zhou et al. 2014) and foraging preference (Schweiger et al. 2015; Sugimoto et al. 2016), predator avoidance (Kittle et al. 2008), competition and other coexistence mechanisms.
Although our study was confined to the BNP, we propose conducting a comparable investigation in adjoining Hazarikhill Wildlife Sanctuary, which has long been recognized as a serow habitat by local people and forest department staffs but has yet to initiate a systematic camera trapping study. Baraiyadhala National Park (2933.61 ha) and adjacent Hazarikhill Wildlife Sanctuary (1177.53 ha), Sitakunda Ecopark and Botanical Garden (808 ha) altogether can be considered as a wider landscape for conserving existing wildlife, specially serow population. Since the ecological state in this area is favorable for serows, strict hunting restrictions, community awareness and restoration programs to improve the denuded forests could be worthwhile as initial efforts.
Funding source: Bangladesh Forest Department
Award Identifier / Grant number: SUFAL Innovation Grant (SIG-Phase 1)
Acknowledgments
We sincerely thank Bangladesh Forest Department for their assistance in this study. Special thanks to Proshenjit Debbarman, Ripon Biswas and Indrojit Bonik for executing the field work.
-
Research ethics: The work was conducted based on the permission from Bangladesh Forest Department (FD/SUFAL/SIG/17/2020/53).
-
Author contributions: Md. Kamrul Hasan as a principal investigator obtained research grant, designed the study and field work, assisted with conducting analyses, and contributed to revising the manuscript. Ashis Kumar Datta executed the analysis, assisted with study design, and wrote the manuscript.
-
Competing interests: The authors declare that they have no conflicts of interest regarding this article.
-
Research funding: The study was financially supported by Bangladesh Forest Department through SUFAL innovation grant (SIG-Phase 1) of The World Bank.
-
Data availability: The raw data can be obtained on request from the corresponding author.
References
Bhattacharya, T., Bashir, T., Poudyal, K., Sathyakumar, S., and Saha, G.K. (2012). Distribution, occupancy and activity patterns of goral (Nemorhaedus goral) and serow (Capricornis thar) in Khangchendzonga Biosphere Reserve, Sikkim, India. Mamm. Stud. 37: 173–181, https://doi.org/10.3106/041.037.0302.Suche in Google Scholar
Chen, Y., Xiao, Z., Zhang, L., Wang, X., Li, M., and Xiang, Z. (2019). Activity rhythms of coexisting Red serow and Chinese serow at Mt. Gaoligong as identified by camera traps. Animals 9: 1071, https://doi.org/10.3390/ani9121071.Suche in Google Scholar PubMed PubMed Central
Feeroz, M.M. (2012). Niche separation between sympatric pig-tailed macaque (Macaca leonina) and rhesus macaque (M. mulatta) in Bangladesh. J. Primatol. 1: 2, https://doi.org/10.4172/2167-6801.1000106.Suche in Google Scholar
Grubb, P. (2005) Artiodactyla: Bovidae: Caprinae. In: Wilson, D.E., and Reeder, D.M. (Eds.). Mammal species of the world: a taxonomic and geographic reference. Johns Hopkins University Press, Baltimore, Maryland, USA, pp. 703–706.Suche in Google Scholar
Guo, W., Cao, G., and Quan, R.-C. (2017). Population dynamics and space use of wild boar in a tropical forest, Southwest China. Glob. Ecol. Conserv. 11: 115–124, https://doi.org/10.1016/j.gecco.2017.04.005.Suche in Google Scholar
Harun-ur-Rashid, M., Islam, S., and Kashem, S.B. (2018). Floristic diversity (Magnoliids and Eudicots) of Baraiyadhala National Park, Chittagong, Bangladesh. Ban. J. Plant Taxon. 25: 273–288, https://doi.org/10.3329/bjpt.v25i2.39532.Suche in Google Scholar
Hasan, M.K., Hridoy, T.H., Rahman, T., Saha, A., Siddik, M.F.A., and Datta, A.K. (2021). First record of a toxic frog Odorrana livida (Blyth, 1856) in Bangladesh with notes on its skin toxin and natural history. Herpetol. Notes 14: 517–520.Suche in Google Scholar
IUCN Bangladesh (2015). Red List of Bangladesh. Volume 2: Mammals. IUCN, International Union for Conservation of Nature, Bangladesh Country Office, Dhaka, Bangladesh.Suche in Google Scholar
Karim, R. and Ahsan, F. (2016). Mammalian fauna and conservational issues of the Baraiyadhala National Park in Chittagong, Bangladesh. Open J. Forest. 6: 123, https://doi.org/10.4236/ojf.2016.62011.Suche in Google Scholar
Khan, M.M.H. (2018). Photographic guide to the wildlife of Bangladesh. Arannayk Foundation, Dhaka, Bangladesh, p. 488.Suche in Google Scholar
Kittle, A.M., Fryxell, J.M., Desy, G.E., and Hamr, J. (2008). The scale-dependent impact of wolf predation risk on resource selection by three sympatric ungulates. Oecologia 157: 163–175, https://doi.org/10.1007/s00442-008-1051-9.Suche in Google Scholar PubMed
Kobayashi, K. and Takatsuki, S. (2012). A comparison of food habits of two sympatric ruminants of Mt. Yatsugatake, central Japan: sika deer and Japanese serow. Acta Theriol. 57: 343–349, https://doi.org/10.1007/s13364-012-0077-x.Suche in Google Scholar
Li, J., Xue, Y., Liao, M., Dong, W., Wu, B., and Li, D. (2022). Temporal and spatial activity patterns of sympatric wild ungulates in Qinling Mountains, China. Animals 12: 1666, https://doi.org/10.3390/ani12131666.Suche in Google Scholar PubMed PubMed Central
Liu, X., Wu, P., Songer, M., Cai, Q., He, X., Zhu, Y., and Shao, X. (2013). Monitoring wildlife abundance and diversity with infra-red camera traps in Guanyinshan Nature Reserve of Shaanxi Province, China. Ecol. Indicat. 33: 121–128, https://doi.org/10.1016/j.ecolind.2012.09.022.Suche in Google Scholar
Mori, E., Nerva, L., and Lovari, S. (2019). Reclassification of the serows and gorals: the end of a never ending story? Mammal Rev. 49: 256–262, https://doi.org/10.1111/mam.12154.Suche in Google Scholar
O’Brien, T.G., Kinnaird, M.F., and Wibisono, H.T. (2003). Crouching tigers, hidden prey: Sumatran tiger and prey populations in a tropical forest landscape. Anim. Conserv. 6: 131–139, https://doi.org/10.1017/s1367943003003172.Suche in Google Scholar
Palei, H.S., Pradhan, T., Sahu, H.K., and Nayak, A.K. (2016). Estimating mammalian abundance using camera traps in the tropical forest of Similipal Tiger Reserve, Odisha, India. Proc. Zool. Soc. 69: 181–188, https://doi.org/10.1007/s12595-015-0143-x.Suche in Google Scholar
Palei, N.C., Rath, B.P., Kumar, S., and Palei, H.S. (2022). Occurrence and activity pattern of endangered Dhole (Cuon alpinus) in Debrigarh Wildlife Sanctuary, Odisha, India. Proc. Zool. Soc. 75: 134–138, https://doi.org/10.1007/s12595-021-00391-5.Suche in Google Scholar
R Core Team. (2022). R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, Available at: <https://www.R-project.org/>.Suche in Google Scholar
Ridout, M.S. and Linkie, M. (2009). Estimating overlap of daily activity patterns from camera trap data. J. Agricul. Biol. Environ. Stat. 14: 322–337, https://doi.org/10.1198/jabes.2009.08038.Suche in Google Scholar
Schweiger, A.K., Schütz, M., Anderwald, P., Schaepman, M.E., Kneubühler, M., Haller, R., and Risch, A.C. (2015). Foraging ecology of three sympatric ungulate species – behavioural and resource maps indicate differences between chamois, ibex and red deer. Move. Ecol. 3: 1–12, https://doi.org/10.1186/s40462-015-0033-x.Suche in Google Scholar PubMed PubMed Central
Seki, Y. and Hayama, S.I. (2021). Habitat selection and activity patterns of Japanese serows and sika deer with currently sympatric distributions. Animals 11: 3398, https://doi.org/10.3390/ani11123398.Suche in Google Scholar PubMed PubMed Central
Shepherd, C.R. (2022). Capricornis rubidus (amended version of 2021 assessment). The IUCN Red List of Threatened Species 2022:e.T3815A214430673, Available at: <https://doi.org/10.2305/IUCN.UK.20221.RLTS.T3815A214430673.en> (Accessed 01 May 2023).Suche in Google Scholar
Singh, H., Sharief, A., Joshi, B.D., Kumar, V., Mukherjee, T., Chandra, K., Bhardwaj, N., Thakur, M., and Sharma, L.K. (2022). Multi-species occupancy modeling suggests interspecific interaction among the three ungulate species. Sci. Rep. 12: 17602, https://doi.org/10.1038/s41598-022-20953-7.Suche in Google Scholar PubMed PubMed Central
Sugimoto, T., Aramilev, V.V., Nagata, J., and McCullough, D.R. (2016). Winter food habits of sympatric carnivores, Amur tigers and Far Eastern leopards, in the Russian Far East. Mammal. Biol. 81: 214–218, https://doi.org/10.1016/j.mambio.2015.12.002.Suche in Google Scholar
Zakir, T., Debbarma, H., and Akash, M. (2020). Dhole Cuon alpinus in Satchari National Park: on the first verifiable evidence from northeast Bangladesh. Mammalia 84: 587–593, https://doi.org/10.1515/mammalia-2019-0050.Suche in Google Scholar
Zhou, Q., Wei, H., Tang, H., Huang, Z., Krzton, A., and Huang, C. (2014). Niche separation of sympatric macaques, Macaca assamensis and M. mulatta, in limestone habitats of Nonggang, China. Primates 55: 125–137, https://doi.org/10.1007/s10329-013-0385-z.Suche in Google Scholar PubMed
© 2024 Walter de Gruyter GmbH, Berlin/Boston
Artikel in diesem Heft
- Frontmatter
- Ecology
- Occurrence and temporal activity pattern of Burmese Red Serow (Capricornis rubidus, Bovidae) in Baraiyadhala National Park, Bangladesh: insights from a camera trapping study
- Understanding habitat suitability and road mortality for the conservation of the striped hyaena (Hyaena hyaena) in Batna (East Algeria)
- Striped hyena Hyaena hyaena (Linnaeus 1758): feeding ecology based on den prey remains in a pastoralist landscape, southern Kenya
- Harpy eagle kill sample provides insights into the mandibular ontogenetic patterns of two-toed sloths (Xenarthra: Choloepus)
- Drivers of Indian pangolin (Manis crassicaudata) mortality in Central and Western Pakistan
- Food habits of invasive masked palm civets (Paguma larvata) in northern Japan
- First predation event of an anuran by Holochilus chacarius in the Pantanal wetland, central portion of South America
- Potential seed dispersal of cumbaru (Dipteryx alata) by fruit-eating bats (Artibeus sp.) in a Brazilian urban context
- Biogeography
- New and unusual records of Glironia venusta (Didelphimorphia, Didelphidae) in Brazil
- Bats (Mammalia: Chiroptera) from two priority areas for biodiversity conservation in the Brazilian Amazon and range extension for Carollia benkeithi (Phyllostomidae)
- A multidisciplinary approach unveils the distribution of the Alpine long-eared bat Plecotus macrobullaris (Vespertilionidae) in Italy
- First record of Great Himalayan leaf-nosed bat, Hipposideros armiger (Hipposideridae) from Bangladesh
- Ethology
- Insights into marking behavior of giant anteaters: a camera trap study in the Rupununi savannahs, Guyana
Artikel in diesem Heft
- Frontmatter
- Ecology
- Occurrence and temporal activity pattern of Burmese Red Serow (Capricornis rubidus, Bovidae) in Baraiyadhala National Park, Bangladesh: insights from a camera trapping study
- Understanding habitat suitability and road mortality for the conservation of the striped hyaena (Hyaena hyaena) in Batna (East Algeria)
- Striped hyena Hyaena hyaena (Linnaeus 1758): feeding ecology based on den prey remains in a pastoralist landscape, southern Kenya
- Harpy eagle kill sample provides insights into the mandibular ontogenetic patterns of two-toed sloths (Xenarthra: Choloepus)
- Drivers of Indian pangolin (Manis crassicaudata) mortality in Central and Western Pakistan
- Food habits of invasive masked palm civets (Paguma larvata) in northern Japan
- First predation event of an anuran by Holochilus chacarius in the Pantanal wetland, central portion of South America
- Potential seed dispersal of cumbaru (Dipteryx alata) by fruit-eating bats (Artibeus sp.) in a Brazilian urban context
- Biogeography
- New and unusual records of Glironia venusta (Didelphimorphia, Didelphidae) in Brazil
- Bats (Mammalia: Chiroptera) from two priority areas for biodiversity conservation in the Brazilian Amazon and range extension for Carollia benkeithi (Phyllostomidae)
- A multidisciplinary approach unveils the distribution of the Alpine long-eared bat Plecotus macrobullaris (Vespertilionidae) in Italy
- First record of Great Himalayan leaf-nosed bat, Hipposideros armiger (Hipposideridae) from Bangladesh
- Ethology
- Insights into marking behavior of giant anteaters: a camera trap study in the Rupununi savannahs, Guyana

