Abstract
Objectives
To evaluate the accuracy of mean platelet volume (MPV) testing in the neonatal population and assess the applicability of the optical platelet volume measurement method on Sysmex XN9000 in this demographic.
Methods
From June 2023 to June 2024, we analyzed complete blood count (CBC) data from 1,070 neonates with infections to assess MPV testing reliability using instrument alerts and histograms. Additionally, 284 neonates without infections or hematologic diseases were included to evaluate the Sysmex optical platelet volume indicator (PLT-F-Y) reliability. Finally, four cases of neonatal sepsis were examined to determine the correlation between PLT-F-Y and neonatal sepsis.
Results
In neonates with infections, 24.67 % of MPV data did not accurately reflect platelet volume, and this issue was not confined to cases with low platelet counts. The PLT-F-Y indicator demonstrated superior accuracy in measuring platelet volume, with a reference range of approximately 42.6–64.2 mean fluorescence intensity (MFI) for neonates. Additionally, PLT-F-Y correlated with the progression of neonatal sepsis.
Conclusions
MPV is not a “perfect” indicator for newborns, whereas PLT-F-Y may serve as a potential better alternative.
Introduction
Sepsis is a systemic inflammatory response syndrome caused by pathogenic microorganisms such as bacteria. Critically ill patients often experience insufficient organ perfusion in addition to systemic inflammatory response and primary infectious lesions [1]. With over 18 million severe sepsis cases globally each year, and an annual rise of 1.5–8.0 %, the condition remains highly prevalent [2]. In China, prospective multicenter research reported a sepsis incidence of 77.6 % (843/1,087 cases), and a septic shock rate of 38.7 % (423/1,087 cases) [3]. Due to undeveloped immune systems of newborns, neonatal sepsis (NS) poses higher risks, particularly among very low birth weight infants and premature infants, ranging from 1 to 10 in every 1,000 neonates and 1–70 %, respectively [4].
Confirmation of NS typically relies on body fluid cultivation, which is time-consuming and prone to false negatives, limiting its clinical utility [5]. Other diagnostic methods include white blood cell counting and inflammatory markers such as C-reactive protein (CRP). While CRP is effective for ruling out infections, it has limited sensitivity in the early stages of infection [6].
Platelet-related parameters in complete blood count, such as platelet (PLT) count, mean platelet volume (MPV), and plateletcrit (PCT), are measured using the Sysmex XN9000 analyzer [7]. Previous studies have reported a close relationship between MPV and NS, with MPV potentially indicating the occurrence, progression, and recovery of the condition [8]. As NS worsens, platelet volume increases. However, MPV is not an ideal marker due to limitations with traditional impedance method. On blood analyzer, traditional impedance method may misclassify small red blood cells (RBCs), RBC debris, and varying-sized platelets, leading to inaccurate MPV measurement [9]. In neonates, Capillary blood and the high production of macroplatelets during infections further complicate accurate MPV measurement. Our study of 1,070 infectious newborns found that MPV could not be measured in 24.67 % of cases, with alarm information in PLT parameters indicating unavoidable issues. Of 479 cases with undetermined MPV values, 76 had “PLT aggregation”, 118 had “thrombocytopenia” and 285 had “unequal size of RBC” (Table 1), with some histograms showing alarms despite MPV values, which were unreliable. These findings highlight the limitations of current MPV detection methods and suggest a need for more reliable approaches for measuring neonatal platelet volume.
Reasons why MPV cannot be detected. Erythrocyte effect was the most common.
| Instrument warning | Case quantity | Proportion | Total amount |
|---|---|---|---|
| Platelet aggregation | 76 | 15.87 % | 479 |
| Low platelet count | 118 | 24.64 % | |
| Erythrocyte effect | 285 | 59.49 % |
Since 2000s, a novel method for measuring PLT count, known as Fluorescence PLT (PLT-F), has been invented and applied on Sysmex XN9000 blood analyzer. This technique utilizes a new fluorescent dye with high specificity, staining only platelets and minimizing interference from reticulocytes and erythrocytes [10]. The platelet count is still determined using flow cytometry, where the X-axis of PLT-F histogram represents side scatter (SSC), and the Y-axis represents forward scattered light (FSC). Since FSC reflects particle volume, we are wondering whether the Y-axis value of PLT-F histogram (PLT-F-Y) can serve as an indicator of platelet volume and aid in predicting the onset, progression, and recovery of NS.
Materials and methods
Establishment of a normal reference range for PLT-F-Y in newborns
According to Reference intervals of blood cell analysis in children (WS/T 779–2021), Peripheral blood samples of 285 newborns without infections under 28 days old were collected. PLT counts were examined using the PLT-F channel on Sysmex XN9000 blood analyzer (Sysmex, Japan). The Y-axis values of the PLT-F histogram were recorded and analyzed using SPSS statistics to determine the normal reference range.
The inclusion criteria for normal newborns were as follows:
full-term infants without infections or hematological diseases,
white blood cell counts, hemoglobin levels and platelet counts within normal ranges,
MPV within the normal range and no triggering of histogram alarm.
This research involving human subjects adhered to all relevant national regulations, institutional policies and was conducted in accordance with the principles of the Helsinki Declaration (as revised in 2008). The study was approved by the Biomedical Ethics Committee of West China Second University Hospital. Participants were informed of their right to withdraw from the study at any time without penalty. All personal information was kept confidential and stored securely.
Verify the relationship between PLT-F-Y and NS
Neonates with sepsis were grouped as the “sepsis” group, while a matching number of healthy neonates served as control group. Blood samples were collected at various stages of neonatal sepsis and from normal infantes at the same time. PLT counts and PLT-F-Y values were examined using the PLT-F channel. In the meantime, CRP concentrations were also assessed. GraphPad prism 9 was used to analyze the relationship among these metrics.
PLT size observation by an automatic smear reader
Peripheral blood smears of collected blood samples were prepared using an automatic blood smear preparer (SP-50, Sysmex). These smears were then examined with an automatic smear reader (DI-60, Sysmex), which captured images of platelets of varying sizes using its camera.
Results
Normal reference range of neonatal PLT-F-Y was established
According to the guidance of the China National Accreditation Service for Conformity Assessment, 240 infection-free neonates were involved in this establishment. Half of them were boys while others were girls. The reference range for boy neonates was 41.8–64.8 MFI, and for girl neonates was 42.8–63.5 MFI. No significant gender differences were found (p=0.46) (Table 2). Thus, the data were combined, yielding a reference range of 42.6–64.2 MFI for neonates (Table 3).
Both sets of data were normally distributed.
| Gender | Case quantity | Normal distribution test (Kolmogorov test) | Reference range | Significance | |
|---|---|---|---|---|---|
| PLT-F-Y | MPV | ||||
| Male neonates | 147 | p>0.05 | 41.8–64.8 | 9.0–12.9 | p>0.05 |
| Female neonates | 137 | p>0.05 | 43.8–63.5 | 9.2–12.5 | |
-
There was no significance between reference ranges of two genders.
This set of data was normally distributed.
| Object | Case quantity | Normal distribution test (Kolmogorov test) | Reference range | |
|---|---|---|---|---|
| PLT-F-Y | MPV | |||
| Neonates | 284 | p>0.05 | 42.6–64.2 | 9.2–12.8 |
-
The reference ranges of PLT-F-Y and MPV, were successfully obtained.
Values of PLT-F-Y had a good correlation with those of MPV
A total of 200 neonatal blood samples were analyzed to ensure normal histogram distributions. The correlation between the traditional MPV measurement and the newly established PLT-F-Y method was assessed using paired T-test. Results showed that the correlation coefficient was 0.942, which indicated a strong relationship between PLT-F-Y and MPV when histograms were normal. This suggests that PLT-F-Y may serve as a viable alternative for MPV determination [11].
There was a correlation between PLT-F-Y and neonatal sepsis
In cases of neonatal sepsis, different stages of the disease were monitored during hospitalization. Weekly measurements of MPV, PLT-F-Y, and CRP were taken. A strong correlation was observed between PLT-F-Y values and the severity of neonatal sepsis. PLT-F-Y values increased with worsening infection severity and decreases as recovery progressed, aligning with previous studies (Figure 1) [8]. These results indicate that PLT-F-Y closely correlates with the stages of NS.
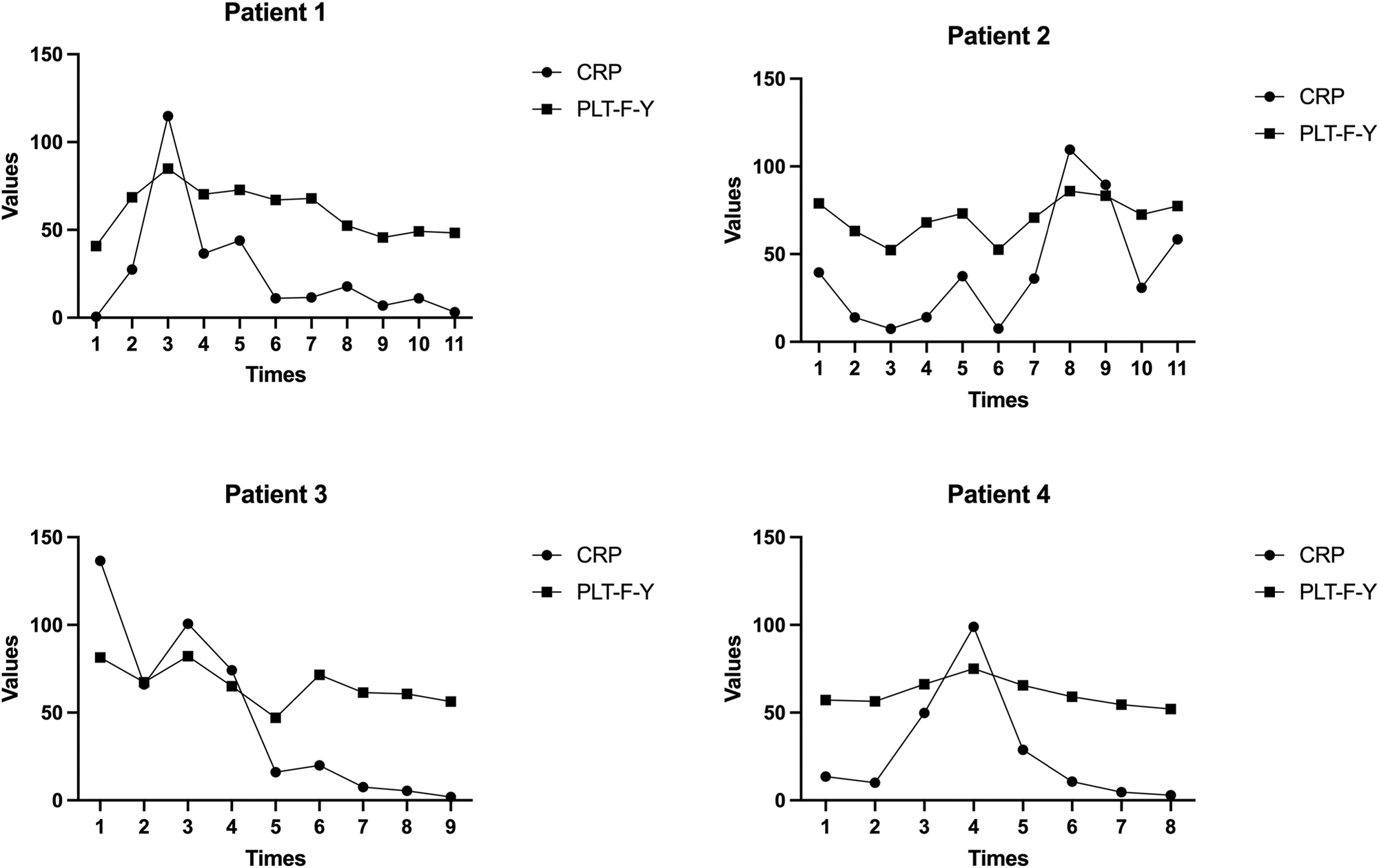
Four cases of neonatal sepsis were collected. PLT-F-Y and CRP showed the same trend of change. As the value of CRP increased, PLT-F-Y increased. As the condition gradually improved, both CRP and PLT-F-Y decreased simultaneously.
In addition, blood samples were collected from neonates without sepsis to serve as a control group, while neonates with NS were designated as experimental group. Measurements of MPV and PLT-F-Y were taken for both groups. Compared to experimental group, values of PLT-F-Y were lower in control group, which was consistent with MPV changing trend (Figure 2). Both PLT-F-Y and MPV were effective indicators for predicting and monitoring neonatal sepsis. However, there was a significant issue with MPV detection, with a 40 % failure rate (Table 4). This suggests that PLT-F-Y may provide a more stable and reliable measurement.
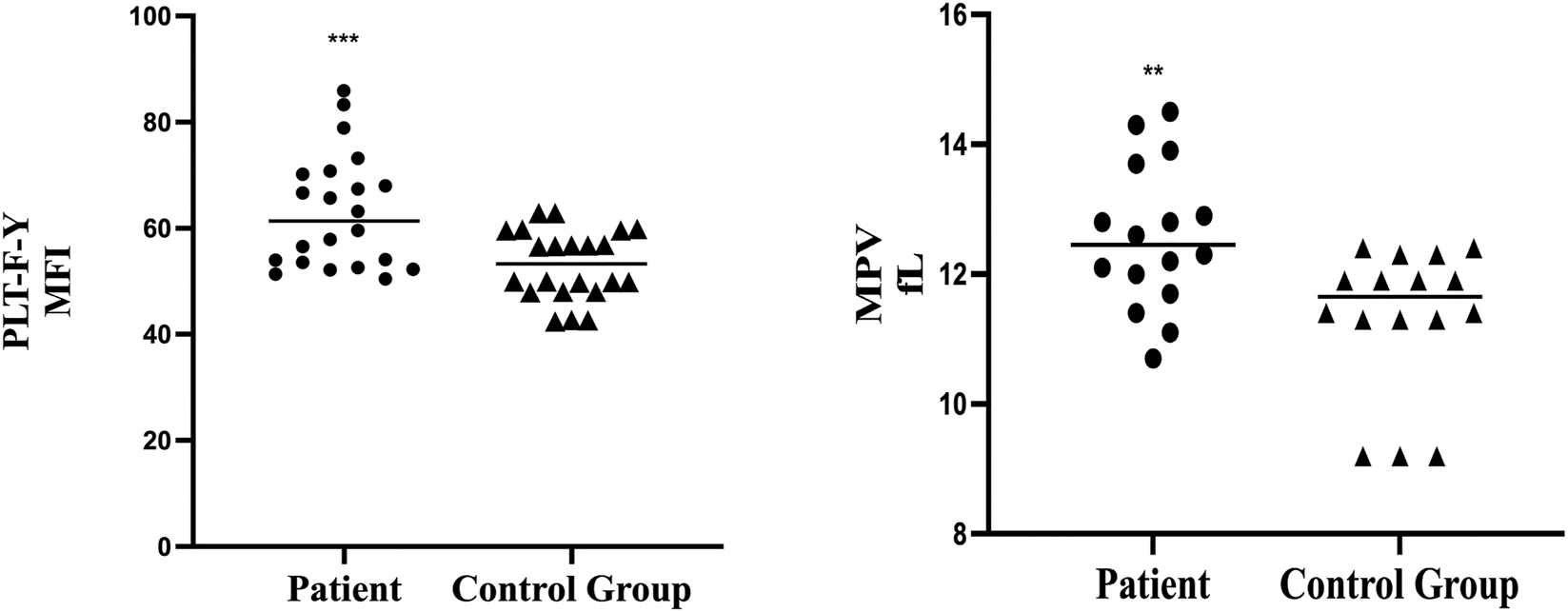
Comparison of patients and control group in values of PLT-F-Y and MPV. Values of PLT-F-Y and MPV were significantly higher in patient group.
Comparison of the probability that two indicators cannot be detected in the four cases.
| Indicator | Having results | No result | Proportion (no result) |
|---|---|---|---|
| MPV | 23 | 16 | 41.02 % |
| PlT-F-Y | 39 | 0 | 0.00 % |
-
The proportion of the former was much higher than the latter. PLT-F-Y was always detectable on Sysmex XN-9000.
We investigated whether PLT-F-Y could predict the prognosis in patients with NS by comparing values in discharge and during follow-up visits. Our analysis revealed that patients with higher PLT-F-Y values at discharge had more frequent follow-up visits compared to those with lower PLT-F-Y values (Figure 3, p<0.05). Unfortunately, a patient with persistently high PLT-F-Y levels ultimately passed away, suggesting that elevated PLT-F-Y level is associated with poor prognosis. These findings indicate that PLT-F-Y is a valuable indicator for predicting the prognosis of NS.
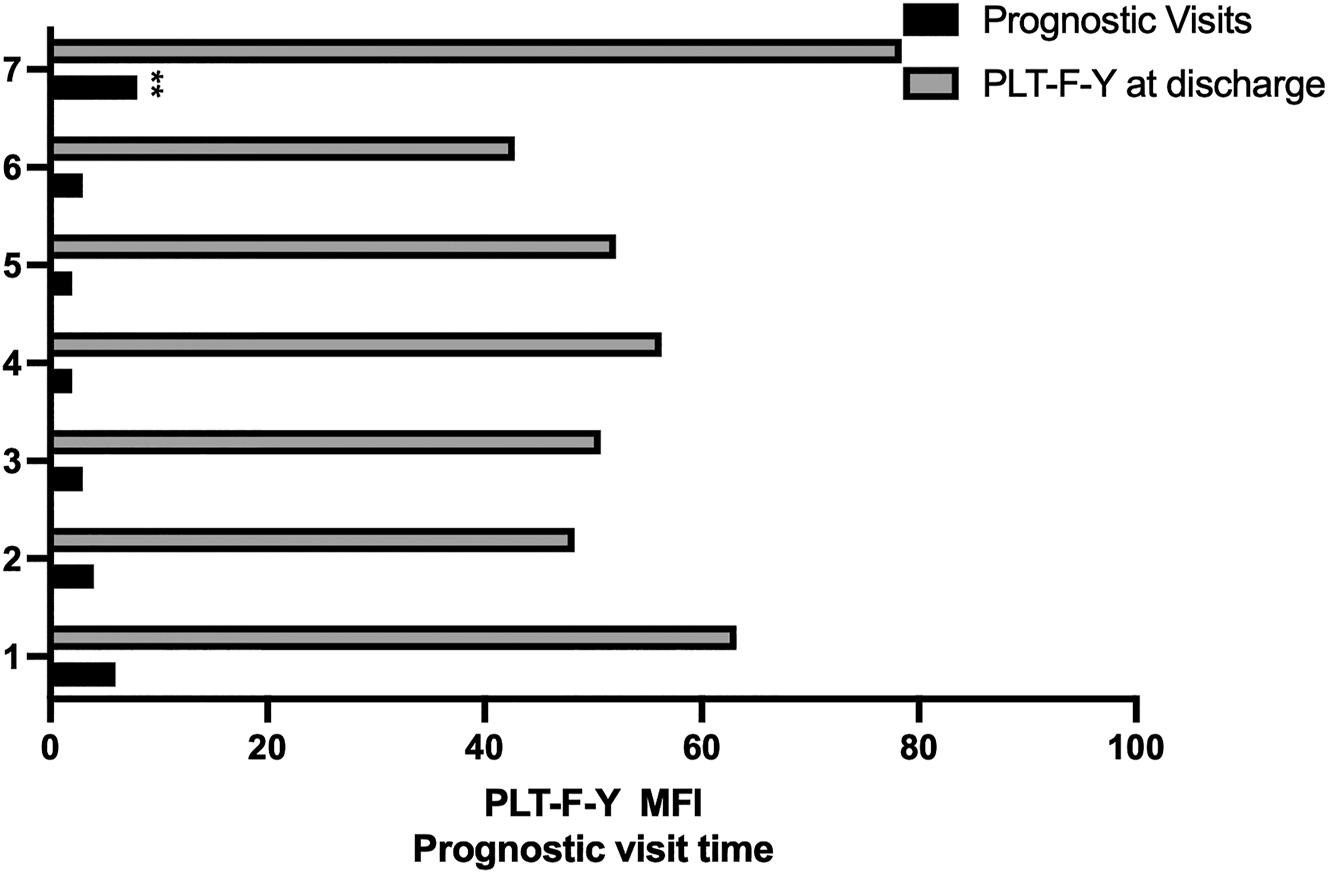
Relationship between PLT-F-Y and prognostic visits. The higher the PLT-F-Y value at discharge was the more prognostic visit times patient suffered.
Observation of platelet sizes in different stages
Blood samples from the “before treatment”, “in treatment” and “post-treatment” stages were collected. Smears were prepared, stained by Wright-Giemsa method, and examined with an automatic smear. Results showed that PLT sizes were larger before treatment. As treatment progressed, platelet volumes decreased, confirming that PLT volume reduced with the improvement of NS (Figure 4).
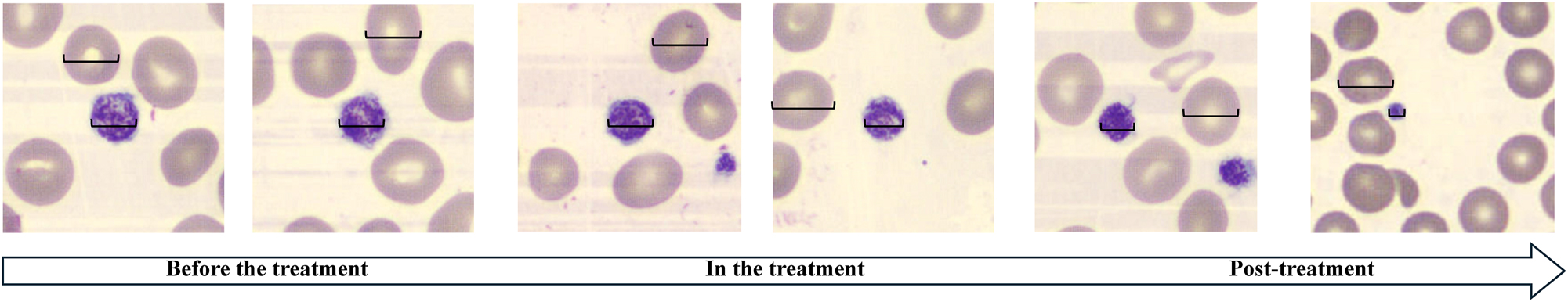
PLT size observation at different stages of neonatal sepsis. As the treatment progressed, the volume of PLT decreased from large to normal.
Discussion
Neonatal sepsis is a critical condition that poses a severe threat to newborns, making early assessment essential. Although there are several diagnostic methods for neonatal sepsis, many are time-consuming [2]. Mean platelet volume is fundamental indicator in blood routine test and has been shown to correlate well with neonatal sepsis. Previous research indicates a significant increase in MPV values in infants with sepsis [12], suggesting that MPV may be a potential indicator for predicting the disease. However, MPV measurements can be unreliable due to factors such as uneven red blood cell sizes, the presence of immature red blood cells, or severe infection, which may interfere with accurate readings by blood analyzers. This underscores the need for alternative assessment method. The PLT-F-Y channel on the Sysmex XN9000 represents a novel approach for PLT determination, utilizing nucleic acid fluorescence staining to specifically differentiate platelets from other blood components. This method provides a scatter diagram on the analyzer, where the Y-axis is the set of forward scattered light, reflecting the average size of PLT [13]. Given this, PLT-F-Y values could potentially serve as an indicator for measuring MPV. To determine whether the value of PLT-F-Y was increasing or decreasing, a normal range of PLT-F-Y was established.
Our study demonstrated that as the severity of infection worsened, the PLT-F-Y value increased. This change can be attributed to the alterations in the coagulation cascade characteristic of sepsis. During sepsis, multiple pro- and anti-inflammatory cytokines are released, which can lead to thrombus formation and the subsequent depletion of fibrinolytic factors and fibrinogen, resulting in further platelet destruction [14]. Additionally, P-selectin, which is expressed on the surface of platelets during systemic inflammation, enhances platelet adhesion to white blood cells and promotes platelet aggregation [15]. In neonatal sepsis, platelet depletion and thrombus formation due to active endothelium contribute to thrombocytopenia [16]. Meanwhile, CRP, which peaks around 50 h, aids in complement binding to foreign or damaged cells, playing a role in the inflammatory response. Although a non-specific marker, CRP can be a help in diagnosing sepsis, which is consistent with our findings [17]. Furthermore, Platelet activation is a significant physiological feature of NS. The increased production of young, larger platelets in the bone marrow leads to elevated PLT-F-Y values on the Sysmex XN9000, indicating its potential to predict the onset and progression of NS.
As mentioned above, MPV measurements in neonates can sometimes be challenging in our laboratory. One reason is the sample type; neonatal samples are often collected from capillary blood, which can be contaminated with tissue fluid due to compression. This contamination may lead the analyzer to misidentify tissue fluid components as platelets, thus affecting the accuracy of platelet-related indicators like MPV [18]. Additionally, anemia in neonates can further impact MPV readings. Overall, multiple factors can influence the accuracy of MPV detection.
The prognosis of NS is a critical concern for clinicians. Our study found that patients with higher PLT-F-Y values at discharge required more frequent follow-up visits, and a patient with persistently high PLT-F-Y levels unfortunately passed away. This observation aligns with previous research indicating that more severe NS is associated with higher MPV values. Therefore, PLT-F-Y may be able to provide a valuable prognostic indicator. If a patient’s PLT-F-Y value exceeds the reference upper limit at discharge, clinician should exercise heighted vigilance. Overall, platelet volume appears to have a strong correlation with the prognosis of NS.
In our study, we noted an unusual case where, despite the progression of sepsis and consistently low platelet counts on different days, values of MPV remained within the normal range. Interestingly, the PLT-F-Y values were consistent with the MPV values. As the CRP values decreased, PLT-F-Y and MPV values elevated. We hypothesized that after the resolution of infection, the platelet production function in the bone marrow recovered well, leading to the recovery of PLT count, resulting in the increase of MPV.
The study still has some limitations. Firstly, observational studies are influenced by confounding variables, which may affect true overall effect. Secondly, there is an incomplete data on some patients’ treatment processes, thus limiting our ability to further track the mortality and recurrence rates. Lastly, relationship between PLT-F-Y and other infectious diseases may be discussed in the future.
Funding source: Health and Family Planning Commission of Sichuan Province
Award Identifier / Grant number: 24QNMP025
Acknowledgments
We thank Dr. Maoting Xie from Department of Research & Sciences (Sysmex) for supporting us with data export from Sysmex XN9000.
-
Research ethics: Research involving human subjects complied with all relevant national regulations, institutional policies and is in accordance with the tenets of the Helsinki Declaration (as revised in 2008) and has been approved by the Biomedical Ethics Committee of West China Second University Hospital.
-
Informed consent: Informed consent was obtained from all individuals included in this study, or their legal guardians or wards.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Use of Large Language Models, AI and Machine Learning Tools: None declared.
-
Conflict of interest: The authors state no conflict of interest.
-
Research funding: This work was supported by Health Commission of Sichuan Province Medical Science and Technology Program (No. 24QNMP025).
-
Data availability: Not applicable.
References
1. Kellum, JA, Formeck, CL, Kernan, KF, Gómez, H, Carcillo, JA. Subtypes and mimics of sepsis. Crit Care Clin 2022;38:195–211. https://doi.org/10.1016/j.ccc.2021.11.013.Suche in Google Scholar PubMed PubMed Central
2. Celik, IH, Hanna, M, Canpolat, FE, Mohan, P. Diagnosis of neonatal sepsis: the past, present and future. Pediatr Res 2022;91:337–50. https://doi.org/10.1038/s41390-021-01696-z.Suche in Google Scholar PubMed PubMed Central
3. Cao, Y, Jiang, S, Sun, J, Hei, M, Wang, L, Zhang, H, et al.. Assessment of neonatal intensive care unit practices, morbidity, and mortality among very preterm infants in China. JAMA Netw Open 2021;4:e2118904. https://doi.org/10.1001/jamanetworkopen.2021.18904.Suche in Google Scholar PubMed PubMed Central
4. Yadav, P, Yadav, SK. Progress in diagnosis and treatment of neonatal sepsis: a review article. J Nepal Med Assoc 2022;60:318–24. https://doi.org/10.31729/jnma.7324.Suche in Google Scholar PubMed PubMed Central
5. Gopal, N, Chauhan, N, Jain, U, Dass, SK, Sharma, HS, Chandra, R. Advancement in biomarker based effective diagnosis of neonatal sepsis. Artif Cells Nanomed Biotechnol 2023;51:476–90. https://doi.org/10.1080/21691401.2023.2252016.Suche in Google Scholar PubMed
6. Ruan, L, Chen, GY, Liu, Z, Zhao, Y, Xu, GY, Li, SF, et al.. The combination of procalcitonin and C-reactive protein or presepsin alone improves the accuracy of diagnosis of neonatal sepsis: a meta-analysis and systematic review. Crit Care 2018;22:316. https://doi.org/10.1186/s13054-018-2236-1.Suche in Google Scholar PubMed PubMed Central
7. Dixon, LR. The complete blood count: physiologic basis and clinical usage. J Perinat Neonatal Nurs 1997;11:1–18. https://doi.org/10.1097/00005237-199712000-00003.Suche in Google Scholar PubMed
8. Milas, GP, Karageorgiou, V, Bellos, I. Mean platelet volume and neonatal sepsis: a systematic review and meta-analysis of diagnostic accuracy. J Matern Fetal Neonatal Med 2022;35:5324–36. https://doi.org/10.1080/14767058.2021.1879039.Suche in Google Scholar PubMed
9. Park, MJ, Park, PW, Seo, YH, Kim, KH, Park, SH, Jeong, JH, et al.. The relationship between iron parameters and platelet parameters in women with iron deficiency anemia and thrombocytosis. Platelets 2013;24:348–51. https://doi.org/10.3109/09537104.2012.699641.Suche in Google Scholar PubMed
10. Kim, H, Hur, M, Lee, GH, Kim, SW, Moon, HW, Yun, YM. Performance of platelet counting in thrombocytopenic samples: Comparison between Mindray BC-6800Plus and Sysmex XN-9000. Diagnostics (Basel) 2021;12:68. https://doi.org/10.3390/diagnostics12010068.Suche in Google Scholar PubMed PubMed Central
11. Schober, P, Boer, C, Schwarte, LA. Correlation coefficients: appropriate use and interpretation. Anesth Analg 2018;126:1763–8. https://doi.org/10.1213/ane.0000000000002864.Suche in Google Scholar PubMed
12. Hanaganahalli, SB, Sreeram, S, Bompada, M, Kuppannagari, SK, Suresh, PK, Philipose, CS. Is MPV a predictive marker for neonatal sepsis? A pilot study. J Pediatr Hematol Oncol 2018;40:548–52. https://doi.org/10.1097/mph.0000000000001272.Suche in Google Scholar
13. Chen, HT, Fu, LM, Huang, HH, Shu, WE, Wang, YN. Particles small angle forward-scattered light measurement based on photovoltaic cell microflow cytometer. Electrophoresis 2014;35:337–44. https://doi.org/10.1002/elps.201300189.Suche in Google Scholar PubMed
14. Akhmaltdinova, L, Kolesnichenko, S, Lavrinenko, A, Kadyrova, I, Avdienko, O, Panibratec, L. Influence of pathogen type on neonatal sepsis biomarkers. Int J Inflam 2021;2021:1009231. https://doi.org/10.1155/2021/1009231.Suche in Google Scholar PubMed PubMed Central
15. Tunjungputri, RN, van de Heijden, W, Urbanus, RT, de Groot, PG, van der Ven, A, de Mast, Q. Higher platelet reactivity and platelet-monocyte complex formation in Gram-positive sepsis compared to Gram-negative sepsis. Platelets 2017;28:595–601. https://doi.org/10.1080/09537104.2016.1252837.Suche in Google Scholar PubMed
16. Iba, T, Levy, JH. Inflammation and thrombosis: roles of neutrophils, platelets and endothelial cells and their interactions in thrombus formation during sepsis. J Thromb Haemost 2018;16:231–41. https://doi.org/10.1111/jth.13911.Suche in Google Scholar PubMed
17. Mair, RJ, Joy, AH, Finney, H, Weaver, A. Creating an algorithm for requesting C-reactive protein in the emergency department. Eur J Emerg Med 2010;17:125–6. https://doi.org/10.1097/mej.0b013e3283366ceb.Suche in Google Scholar
18. Surma, M, Sawicki, T, Piskuła, M, Wiczkowski, W. Relationship between the consumption of fermented red beetroot juice and levels of perfluoroalkyl substances in the human body’s fluids and blood parameters. Int J Mol Sci 2023;24:13956. https://doi.org/10.3390/ijms241813956.Suche in Google Scholar PubMed PubMed Central
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- Original Articles
- A comparative study between the Chrono-log 700 and the Sysmex CS-2100i analyzers for assessing ristocetin cofactor activity in patients with von Willebrand disease
- Evaluation of hemolysis index thresholds for 18 biochemistry assays: implications for laboratory-developed tests in the era of the IVDR
- Elevation of plasma podoplanin in Kawasaki disease
- Epidemiology characteristics of Epstein-Barr virus among children in Hangzhou from 2019 to 2023
- Mean platelet volume vs. optical platelet volume measurement: which is a better indicator for neonatal sepsis?
Artikel in diesem Heft
- Frontmatter
- Original Articles
- A comparative study between the Chrono-log 700 and the Sysmex CS-2100i analyzers for assessing ristocetin cofactor activity in patients with von Willebrand disease
- Evaluation of hemolysis index thresholds for 18 biochemistry assays: implications for laboratory-developed tests in the era of the IVDR
- Elevation of plasma podoplanin in Kawasaki disease
- Epidemiology characteristics of Epstein-Barr virus among children in Hangzhou from 2019 to 2023
- Mean platelet volume vs. optical platelet volume measurement: which is a better indicator for neonatal sepsis?


