Diagnostic validation of the GAAD algorithm for hepatitis B virus-related hepatocellular carcinoma surveillance in Vietnam
-
Nguyen Thi Thanh Hai
, Ngoc Anh Doi
Abstract
Objectives
Chronic hepatitis B infection is a major cause in Vietnam of developing hepatocellular carcinoma (HCC). HCC surveillance for hepatitis B patients is recommended to detect tumors early for effective treatment and survival extension. The novel Elecsys®GAAD algorithm (Roche Diagnostic) was generated by using serum PIVKA-II and alpha-fetoprotein (AFP) measurements with age and biological sex factors for more effective HCC screening expectations. This study was conducted to validate the GAAD algorithm for HCC surveillance in Vietnamese hepatitis B patients.
Methods
A cross-sectional study with 337 hepatitis B patients divided into 142 HCC and 195 non-HCC including benign liver tumors, liver fibrosis and cirrhosis was performed in Vietnam. The Elecsys®GAAD score was computed by Elecsys®AFP, Elecsys®PIVKA-II and patient’s age, gender. The clinical evaluation of the GAAD algorithm was accessed by sensitivity, specificity at recommended cut-off, and the AUC of the receiver operating characteristic curve.
Results
The GAAD showed moderate performance with an AUC of 0.767 (95 % CI 0,716–0,818), but was more effective than AFP alone in the general detection of HCC, and more useful than PIVKA-II in the detection of HCC at an early stage with tumors ≤ 2 cm and in distinguishing benign liver tumor from HCC. It did not provide more benefit than PIVKA-II to detect HCC at all stages or cirrhosis background.
Conclusions
The study showed that GAAD had moderate performance, but appeared to be more effective than AFP alone in general HCC surveillance and may be superior to PIVKA-II for detecting early-stage HCC with tumors ≤2 cm, and for distinguishing HCC from benign liver tumors in chronic hepatitis B patients.
Introduction
Hepatitis B is a major global health problem. The burden of infection is highest in the WHO Western Pacific Region and the WHO African Region, where 116 million and 81 million people, respectively, are chronically infected (WHO). In Vietnam, the prevalence of current HBV infection (HBsAg+) is about 10 % in the general population [1], [2], [3]. Chronic infection with hepatitis B virus (HBV) is the most common risk factor for hepatocellular carcinoma (HCC) [4], one of the most common causes of cancer-related deaths worldwide and Vietnam [5], [6], [7]. The majority of HCC patients including HBV-related HCC in Vietnam were diagnosed at advanced stages, and the prognosis remains poor, with a median survival of less than 6 months [7]. Patients diagnosed with early-stage HCC may prolong overall survival and can achieve 5-year survival exceeding 70 % with various options including surgical resection, local ablative therapy, or liver transplantation [8], 9]. Therefore, HCC surveillance for early detection is essential for the management of HBV-infected patients.
Liver ultrasound is the most commonly used as the standard method for HCC surveillance. Ultrasound presented a sensitivity of 84 % for HCC at any stage and 63 % for early-stage HCC for HBV-infected patients [10]. However, the sensitivity of ultrasound can be influenced by several patient factors, especially in obese patients or/and advanced cirrhosis [10]. Cross-sectional imaging modalities, such as CT or MRI, would have high accuracy for HCC diagnosis, however, these methods are expensive and may not be useful for surveillance [10]. The combination of biomarkers and ultrasound has been used to increase the sensitivity of early HCC detection. The most common biomarker is alpha-fetoprotein (AFP) with the sensitivity for any stage HCC is approximately 60 %, but sensitivity for early-stage tumors is only 32–49 %, and false-negative and false-positive findings occur at about 20–40 % with a cut-off value of 20 ng/mL [11]. AFP may be increased in benign chronic liver disease, particularly in patients with significant elevations of transaminases like hepatitis B and in other malignancies such as cholangiocarcinoma [12]. Recently, other serum markers such as protein induced by vitamin K absence-II (PIVKA-II) or des-gamma-carboxyl-prothrombin (DCP) have been approved by the FDA for predicting the risk of HCC but not for HCC surveillance [13], 14]. This is an abnormal prothrombin protein that is generated as a result of a defect in the posttranslational carboxylation of the immature prothrombin in malignant hepatic cells, and 4.8-fold increased risk of HCC development with elevated ≥7.5 ng/mL [13], 14]. Several previous studies in cirrhosis patients found sensitivities of PIVKA-II ranging from 23 to 57 % compared to 14–71 % for AFP in HCC detection [13], 15], 16] and increased to 91 % by using a combination of the two markers [17]. Additionally, the performance of the biomarkers varies with geographic region, etiologies, and patient racial characteristics [17], 18]. Therefore, novel makers are developing to increase the effectiveness of HCC screening in distinct areas with different races and etiologies.
Recently, the GAAD score (Roche®Diagnostics) was generated as a statistical model for HCC screening in high-risk patients such as chronic hepatitis B disease. The GAAD score is derived from Gender, Age, AFP and Des-carboxy-prothrombin (DCP or PIVKA-II). The performance of the GAAD score was presented to be a higher effective model for the detection of HCC than single markers in multicenter in China, Germany, Thailand, Hong Kong, and Japan [19], 20], but has not as yet been evaluated in Vietnam, particularly in chronic hepatitis B patients. Thus, this study was conducted with the aims: 1. to evaluate the performance of the GAAD score for the detection of HCC in Vietnamese HBV infected cohorts, 2. to compare the performance of the GAAD score in detecting HCC to single tumor markers AFP and PIVKA-II.
Materials and methods
Patients collection
This study was conducted as a retrospective and prospective study, which enrolled a total of 337 subjects with chronic hepatitis B aged ≥18 years admitted to the Vietnam National Hospital of Tropical Diseases in routine examination every 6 months. This included 142 first-detected HCC patients and 195 non-HCC patients.
In the non-HCC group, there were 56 patients with liver cirrhosis (LC), 47 patients with moderate or severe liver fibrosis (LF), 73 patients with non-cirrhotic chronic HBV infection (CHB) and 66 patients with benign liver disease (BLD) such as hepatic cysts or hepatic hemangioma.
In the HCC group, there were 39 patients with liver cirrhosis (LC), 45 patients with moderate or severe liver fibrosis (LF).
Early stage HCC was defined at 0 and A stage of Barcelona Clinic Liver Cancer (BCLC), and late-stage HCC was B, C and D stage of BCLC. Fibrosis degree was defined by APRI index or Fibroscan with moderate or severe fibrosis (F2 to F3 by Metavir) and cirrhosis (F4 by Metavir).
Key exclusion criteria were the presence of any other cancer, recurrent HCC, current or previous treatment for HCC and using vitamin K antagonists, renal failure, and acute inflammation diseases. Eligible LC, CHB, and BLD cases were confirmed to be free of HCC by ultrasound or computed tomography imaging after 06 months (+0–8 weeks) of sampling (in the next routine examination). This study was approved by the Ethics Committee of the National Hospital of Tropical Diseases (NHTD) allowing for analysis of anonymized, retrospective data, and the requirement for written informed consent.
Measurements of AFP and PIVKA-II; GAAD score calculation
Serum samples were collected in routine examination of CHB patients every 6 months, before surgery or tumor dissection if HCC was detected. AFP and PIVKA-II levels were detected by the electrochemiluminescence immunoassay according to the manufacturer’s manual (Roche®Diagnostics, Germany). The GAAD score was calculated by the in vitro diagnostics algorithm incorporating the patient’s age, gender, AFP, PIVKA-II via the Roche’s website. The recommended cut-offs for HCC detection were 2.57 for the GAAD score (on a scale from 0 to 10), 20 ng/mL for AFP, and 28.4 ng/mL for PIVKA-II.
Statistical analysis
All statistical analyses were performed using SPSS version 22 software (IBM, Armonk, USA). Receiver operating characteristic (ROC) curves and area under the curve (AUC) with 95 % CIs were conducted to elucidate the diagnostic values of AFP, PIVKA-II, and the GAAD. Diagnostic efficiency-related parameters including sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated. Descriptive statistics were generated for this patient dataset, with association testing performed with Chi-square and Fisher’s exact test for proportions of categorical variables, respectively.
Results
Patient characteristics
The baseline characteristics of 337 Vietnamese participants enrolled in this study are presented in Table 1. There were 142 first detected HCC patients and 195 non-HCC disease controls. The non-HCC group included 74 patients with CHB, 56 patients with LC and 66 patients with BLD such as hepatic cysts or hepatic hemangioma. All participants had a current HBV infection background and only 38.1 % of patients were on anti-viral treatment. The mean age of these 337 patients was 55.6 ± 12.7 years, of which 73.6 % were aged 41–70 and the mean age of the HCC group was higher than non-HCC disease controls (p<0.001). There was a male predominance in both HCC and non-HCC disease control groups (p<0.001). In the HCC group, 45.8 % of patients were in the early stage (BCLC 0 or A stage), while 54.2 % of patients were in the late stage (BCLC C or D stage). Tumors with a diameter of less than 5 cm accounted for 66.2 %, of which 28.7 % were lower than 2 cm; and most HCC tumors (80.3 %) were localized or non-invasive to portal vein or extrahepatic tissue.
Clinical Characteristics of study cohorts.
| HCC | Non-HCC disease control | |||||||
|---|---|---|---|---|---|---|---|---|
| Total n=142 | Early n=65 | Late n=77 | Total n=195 | CHB n=73 | LC n=56 | BLD n=66 | p-Value | |
| Age, years, mean ± SD (min-max) | ||||||||
| Total | 60.4 ± 11.0 (28–84) | 62.1 ± 11.4 (31–84) | 59.1 ± 10.8 (28–79) | 52.2 ± 12.7 (18–86) | 50.4 ± 12.2 (29–76) | 55.8 ± 11.1 (36–83) | 51.1 ± 13.9 (18–86) | <0.001 |
| ≤40 | 11 (21 %) | 5 (7.7 %) | 6 (7.8 %) | 41 (78.8 %) | 19 (26 %) | 5 (8.9 %) | 17 (25.8 %) | <0.001 |
| 41–70 | 109 (44 %) | 45 (69.2 %) | 58 (75.3 %) | 139 (56 %) | 49 (67.1 %) | 45 (80.4 %) | 43 (65.2 %) | <0.001 |
| >70 | 22 (59.5 %) | 15 (23.1 %) | 13 (16.9 %) | 15 (40.5 %) | 5 (6.8 %) | 6 (10.7 %) | 6 (9.1 %) | <0.001 |
| Gender, n (%) | ||||||||
| Female | 30 (21.1) | 15 (23.1) | 15 (19.5) | 52 (26.6) | 16 (21.9) | 17 (30.4) | 19 (28.8) | N/Sa |
| Male | 112 (78.9) | 50 (76.9) | 62 (80.5) | 143 (73.4) | 57 (78.1) | 39 (69.6) | 47 (71.2) | <0.001b |
| <0.001c | ||||||||
| Ongoing anti-viral therapy, n (%) | ||||||||
| Yes | 54 (38.1) | 27 (41.6) | 27 (35.1) | N/A | N/A | N/A | N/A | |
| No | 53 (37.3) | 22 (33.8) | 31 (40.3) | N/A | N/A | N/A | N/A | |
| Missing | 35 (24.6) | 16 (24.6) | 19 (24.6) | |||||
| Tumor number, n (%) | ||||||||
| 1 | 92 (64.8) | 58 (89.2) | 34 (44.2) | |||||
| 2 | 9 (6.3) | 7 (10.8) | 2 (2.6) | |||||
| ≥3 | 31 (21.8) | 0 | 31 (40.3) | |||||
| Diffuse infiltration | 10 (7) | 0 | 10 (13) | |||||
| Tumor characteristics, n (%) | ||||||||
| < 2 cm | 27 (19) | 25 (38.5) | 2 (2.6) | |||||
| 2–5 cm | 67 (47.2) | 40 (61.5) | 27 (35.1) | |||||
| >5 cm | 48 (33.8) | 0 | 48 (62.3) | |||||
| Portal invasion or extrahepatic spread | ||||||||
| Yes | 28 (19.7) | 0 | 28 (36.4) | |||||
| No | 114 (80.3) | 65 (100) | 49 (63.6) | |||||
| BCLC stage, n (%) | ||||||||
| 0 | 19 (13.4) | 19 (29.2) | 0 | |||||
| A | 46 (32.4) | 46 (70.8) | 0 | |||||
| B | 34 (23.9) | 0 | 34 (44.2) | |||||
| C And D | 43 (30.3) | 0 | 43 (55.8) | |||||
| AFP, ng/μL | ||||||||
| Mean ± SD | 249 ± 440 | 90.2 ± 226 | 386 ± 528.5 | 123.8 ± 265.6 | 122.1 ± 252.3 | 199 ± 352.7 | 53.1 ± 134.3 | <0.001d |
| Median | 25.86 | 17.49 | 48.60 | 7.30 | 7.70 | 13.99 | 4.10 | <0.001e |
| N/Sf | ||||||||
| N/Sg | ||||||||
| <0.05h | ||||||||
| N/Si | ||||||||
| PIVKA-II, ng/μL | ||||||||
| Mean ± SD | 2,156 ± 3,874 | 282.9 ± 946 | 3,738 ± 4,643 | 181 ± 972 | 234 ± 1,409 | 157.6 ± 657 | 142 ± 502 | <0.001d |
| Median | 151.10 | 47.50 | 1,282.00 | 17.80 | 17.20 | 18.20 | 18.80 | <0.001e |
| N/Sf | ||||||||
| N/Sg | ||||||||
| N/Sh | ||||||||
| N/Si | ||||||||
| GAAD score | 6.9 ± 3.4 | 5.4 ± 3.4 | 8.2 ± 2.8 | 3.7 ± 3.8 | 3.6 ± 3.9 | 4.6 ± 3.8 | 2.9 ± 3.5 | <0.01d |
| N/Se | ||||||||
| N/Sf | ||||||||
| N/Sg | ||||||||
| N/Sh | ||||||||
| N/Si | ||||||||
-
p-Value of Chi-square statistics when comparing the male and female ratio abetween HCC vs. non-HCC groups; bin the HCC group, and cin the non-HCC group. p-Value of Chi-square statistics when comparing AFP, PIVKA-II, GAAD value between dHCC vs. non-HCC groups, ethe early-stage HCC vs. late-stage HCC groups, fthe early-stage HCC vs. non HCC groups, gthe early-stage HCC and CHB groups, hthe early-stage HCC and LC groups, ithe early-stage HCC and BLD groups. N/S, not significant.
In general, the GAAD score and single markers all presented significant higher in the HCC group compared to the non-HCC group at all-stage (p<0.01), but not at early stage. PIVKA-II and AFP, but not GAAD, showed a significantly greater increase in the late stage than in the early stage (Table 1).
The general performance of GAAD for HCC diagnosis in chronic hepatitis B patients
To evaluate the diagnostic accuracy of GAAD in overall HBV-related HCC patients, LC and BLD with HBV-infected cases as non-HCC controls were pooled for analysis. The accuracy and predictive values for the GAAD and AFP, PIVKA-II are shown in Table 2.1 and Figure 1.
Diagnostic value of AFP, PIVKA-II and GAAD in HCC surveillance in chronic hepatitis B patients.
| AUC (95%CI) | Sensitivity, % | Specificity, % | PPV, % | NPV, % | |
|---|---|---|---|---|---|
| 2.1. The general performance of GAAD for detecting HCC in chronic hepatitis B patients | |||||
|
|
|||||
| 2.1.1. All-stage | |||||
| AFP | 0.662 (0.602–0.722) | 57.4 | 64.9 | 56.1 | 66.47 |
| PIVKA-II | 0.778 (0.726–0.830) | 73.5 | 69 | 65.82 | 78.77 |
| GAAD | 0.767 (0.716–0.818) | 80.1 | 55.7 | 58.82 | 78.86 |
| 2.1.2. Early-stage | |||||
| AFP | 0.591 (0.518–0.664) | 47.6 | 64.9 | 32.96 | 77.39 |
| PIVKA-II | 0.677 (0.603–0.752) | 58.7 | 69.0 | 41.30 | 83.93 |
| GAAD | 0.673 (0.606–0.741) | 68.3 | 55.7 | 36.36 | 83.62 |
| 2.1.3. Tumor size ≤2 cm | |||||
| AFP | 0.614 (0.527–0.700) | 44.0 | 64.9 | 15.27 | 88.97 |
| PIVKA-II | 0.612 (0.507–0.717) | 44.0 | 69.0 | 18.18 | 90.38 |
| GAAD | 0.660 (0.577–0.743) | 64.0 | 55.7 | 18.08 | 92.38 |
|
|
|||||
| 2.2. The performance of GAAD for detecting HCC in chronic hepatitis B patients with cirrhosis or liver fibrosis | |||||
|
|
|||||
| 2.2.1. Cirrhosis | |||||
| AFP | 0.687 (0.585–0.789) | 70.3 | 53.0 | 76.08 | 45.61 |
| PIVKA-II | 0.827 (0.733–0.921) | 81.1 | 57.6 | 82.97 | 52.54 |
| GAAD | 0.803 (0.714–0.891) | 89.2 | 40.9 | 86.67 | 45.20 |
| 2.2.2. Moderate or severe fibrosis | |||||
| AFP | 0.599 (0.478–0.721) | 58.1 | 56.1 | 56.09 | 58.14 |
| PIVKA-II | 0.803 (0.709–0.898) | 83.7 | 63.4 | 82.05 | 71.69 |
| GAAD | 0.772 (0.673–871) | 90.7 | 48.8 | 83.33 | 65.00 |
| 2.2.3. Moderate, severe fibrosis and cirrhosis | |||||
| AFP | 0.635 (0.556–0.715) | 63.8 | 54.2 | 66.67 | 51.00 |
| PIVKA-II | 0.812 (0.748–0.876) | 82.5 | 59.8 | 82.55 | 61.60 |
| GAAD | 0.781 (0.715–0.847) | 90.0 | 43.0 | 85.18 | 54.13 |
|
|
|||||
| 2.3. The performance of GAAD for detecting HCC in chronic hepatitis B patients with benign liver tumors (hepatic cysts or hemangioma) | |||||
|
|
|||||
| 2.3.1. All-stage | |||||
| AFP | 0.755 (0.675–0.835) | 57.4 | 77.2 | 85.71 | 43.13 |
| PIVKA-II | 0.780 (0.711–0.849) | 74.3 | 73.7 | 87.39 | 57.30 |
| GAAD | 0.822 (0.756–0.887) | 80.1 | 64.9 | 84.61 | 58.73 |
| 2.3.2. Early-stage | |||||
| AFP | 0.697 (0.600–0.795) | 47.6 | 77.2 | 69.76 | 57.14 |
| PIVKA-II | 0.682 (0.585–0.779) | 60.3 | 73.7 | 71.69 | 65.38 |
| GAAD | 0.746 (0.655–0.838) | 68.3 | 64.9 | 68.75 | 66.07 |
-
AFP, alpha-fetoprotein; HCC, hepatocellular carcinoma; GAAD, gender (biological sex), age, AFP, DCP; NPV, negative predictive value; PIVKA-II, protein induced by vitamin K Absence-II; PPV, positive predictive value. Bold values emphasize the GAAD score.
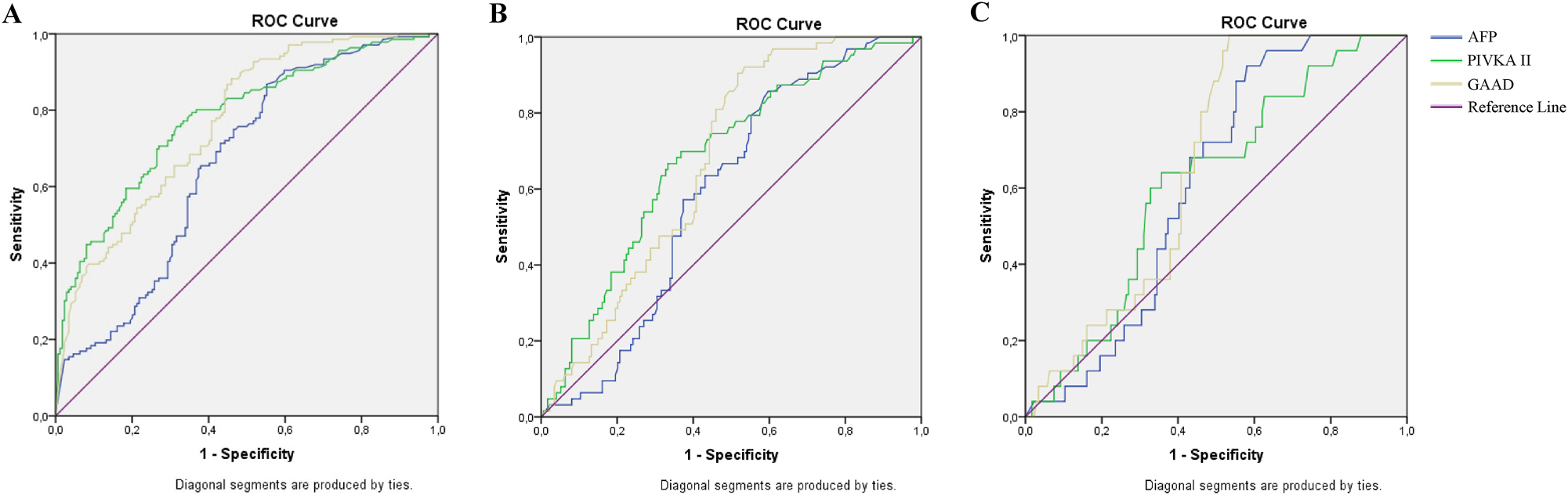
The general performance of GAAD for HCC diagnosis in chronic hepatitis B patients. ROC curve of AFP, PIVKA-II and GAAD in HCC diagnosis of chronic hepatitis B patients at (A) all stages; (B) early stage; and (C) tumor size ≤2 cm.
The AUCs of GAAD were 0.767 (95 % CI 0.716–0.818) at all-stage and 0.673 (95 % CI 0.606–0.741) at early-stage which were higher than the AUCs of AFP, but lower than the AUCs of PIVKA-II, corresponding. GAAD showed greater sensitivity and NPV but lower specificity and PPV than single AFP, PIVKA-II at all- and early-stages (Table 2.1, Figure 1A and B). However, in patients with tumor size ≤2 cm undetected by ultrasound, the diagnostic performance of the GAAD was shown to be more effective than single serum AFP, PIVKA-II (Table 2.1, Figure 1C)
The performance of GAAD for detecting HCC in chronic hepatitis B patients with cirrhosis (LC) and liver fibrosis (LF)
Diagnostic values of GAAD to discriminate HCC from LC in HBV infected patients had an AUC of 0.803 (95 % CI 0.714–0.891) with sensitivity 89.2 % which were higher than the AUCs of AFP, but lower than the AUCs of PIVKA-II (Figure 2A and Table 2.2.1). In addition, the performance of both GAAD and PIVKA-II were not more effective in detecting HCC in moderate and severe fibrosis (LF) than in LC patients. In LF patients, GAAD presented an AUC of 0.772 (95 % CI 0.673–0.871), sensitivity of 90.7 %, which was not more beneficial than PIVKA-II with AUC of 0.803 (95 % CI 0.709–0.898), a sensitivity of 83.7 % for detecting HCC at all stages (Figure 2B and Table 2.2.2). Although GAAD was only presented as more effective than AFP and PIVKA-II in detecting HCC, the sensitivity of the GAAD was shown to be better than either of these single markers at the recommended cut-off in all stages of moderate, severe liver fibrosis, and cirrhosis (Table 2.2.3 and Figure 2C).
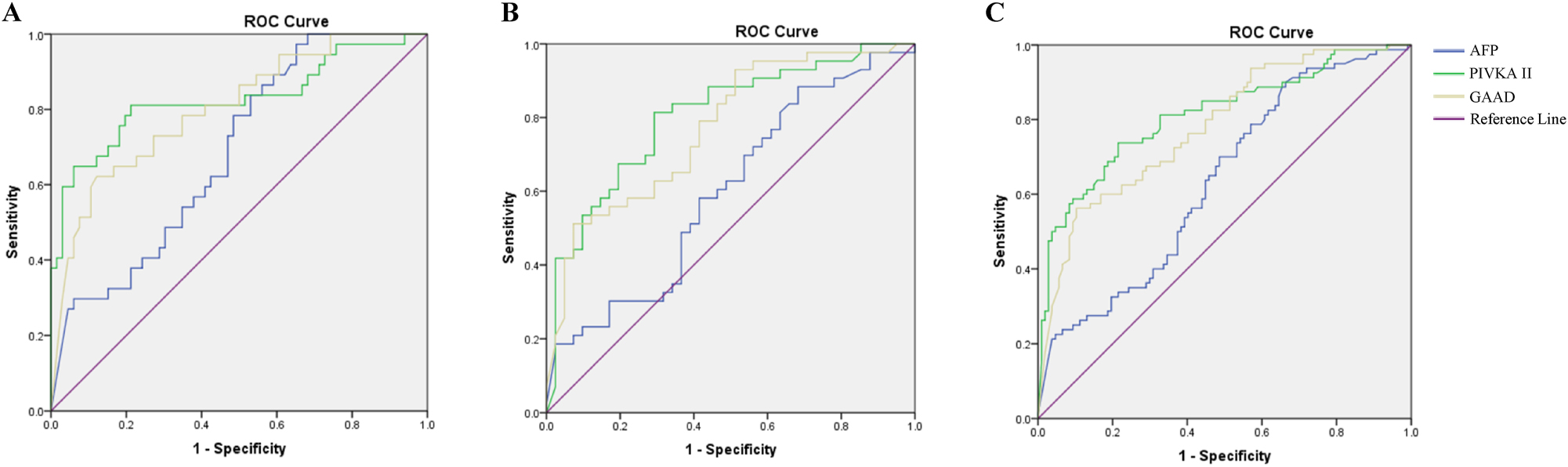
The performance of GAAD for detecting HCC in chronic hepatitis B patients with cirrhosis and liver fibrosis. ROC curve of AFP, PIVKA-II and GAAD in HCC diagnosis of chronic hepatitis B patients with (A) cirrhosis (LC); (B) moderate/severe liver fibrosis (LF); and early stage (C) liver fibrosis and cirrhosis (LF&LC).
Performance of GAAD for detecting HCC in chronic hepatitis B patients with benign liver tumors
The diagnostic performance of GAAD was exhibited more effectively compared to AFP and PIVKA-II for distinguishing HCC from benign liver tumors such as hepatic cysts or hemangioma. in HBV-infected patients at all- and early-stage. The AUCs of GAAD were at good levels with 0.822 (95 % CI 0.756–0.887) and 0.746 (95 % CI 0.655–0.838) at all- and early-stage, respectively (Table 2.3, Figure 3).
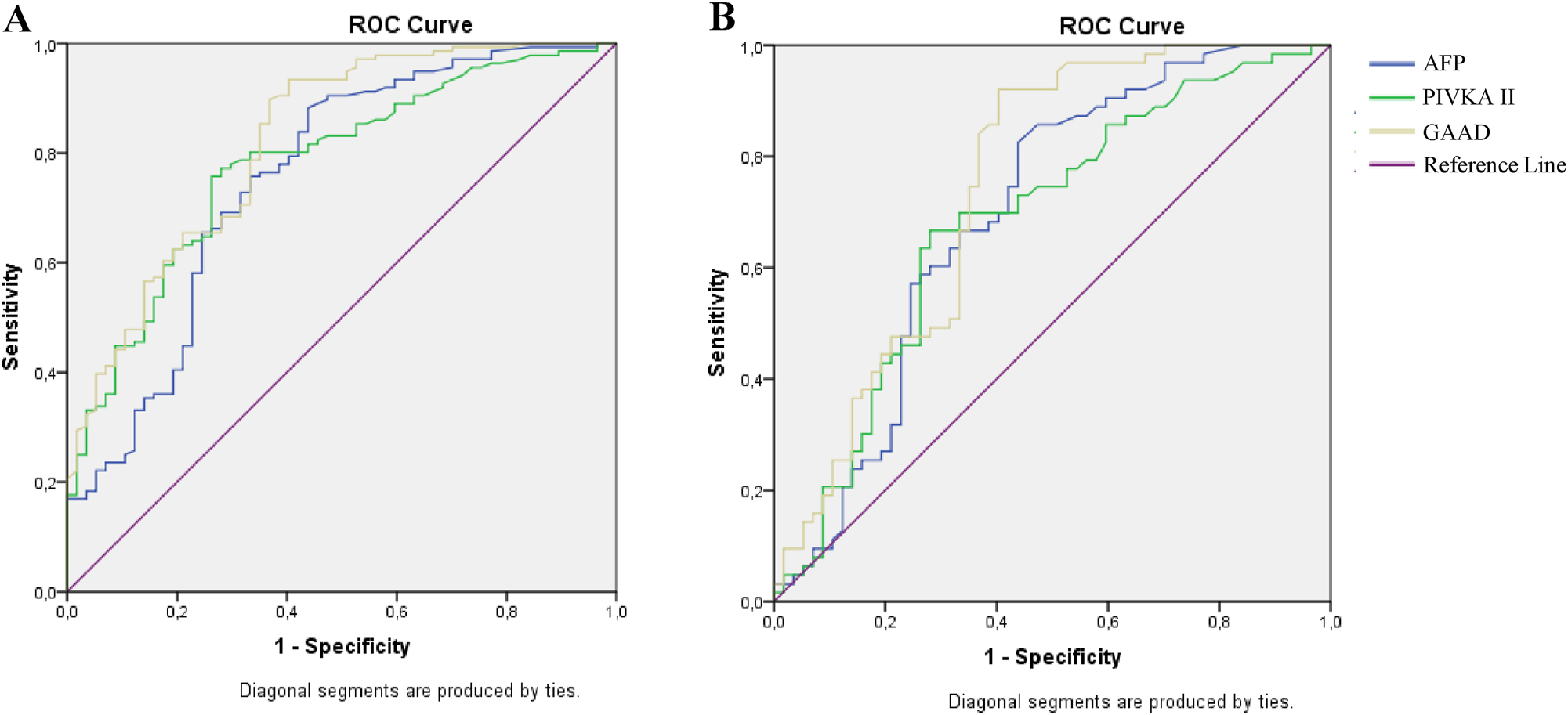
The performance of GAAD for detecting HCC in chronic hepatitis B patients with benign liver tumors. ROC curve of AFP, PIVKA-II and GAAD in HCC diagnosis of chronic hepatitis B patients with benign liver tumor at (A) all stages; (B) early stage.
Correlation between GAAD score and clinical characteristics of HCC
To evaluate the relationship between GAAD score and clinical features of HCC, the HCC group was divided into the lower and upper recommended cut-off of the GAAD (Table 3). The GAAD score was significantly correlated with tumor number (p<0.05), portal invasion or extrahepatic metastasis (p<0.05), and BCLC stage (p<0.05) but not significant differences in tumor sizes (p>0.05). This result suggested that the GAAD score may correlate prognosis migration, or metastasis, and dynamically monitoring HCC after treatment.
Correlation between the GAAD score and clinical characteristics of HCC.
| Characteristics | n | Mean ± SD Median |
≤2.57, n | >2.57, n | p-Value |
|---|---|---|---|---|---|
| Tumor size, cm |
N/S |
||||
| ≤ 2 | 25 | 4.9 ± 3.3 3.80 |
8 | 17 | |
| 2–5 | 66 | 6.58 ± 3.3 7.78 |
13 | 53 | |
| >5 | 45 | 8.65 ± 2.9 9.95 |
5 | 40 | |
| Tumor number |
<0.05 |
||||
| 1 | 89 | 6.18 ± 3.5 7.36 |
22 | 67 | |
| 2 | 9 | 6.06 ± 3.9 7.42 |
3 | 6 | |
| ≥3 | 30 | 8.75 ± 2.1 9.91 |
1 | 29 | |
| Diffuse infiltration | 8 | 9.83 ± 0.2 9.95 |
0 | 8 | |
| No | 110 | 6.61 ± 3.5 8.18 |
25 | 85 | <0.05 |
| Yes | 26 | 8.81 ± 2.3 9.91 |
1 | 25 | |
| BCLC stages |
<0.05 |
||||
| 0 | 17 | 5.19 ± 3.3 4.24 |
5 | 12 | |
| A | 46 | 5.41 ± 3.4 66.65 |
14 | 32 | |
| B | 33 | 8.23 ± 2.9 9.75 |
3 | 30 | |
| C+D | 40 | 8.53 ± 2.5 9.91 |
3 | 37 |
-
Using Chi-square (n≤5) and Fisher’s exact tests (n≥5) for different statistics. N/S, not signifcant.
Discussion
Chronic infection with hepatitis B virus (HBV) is the most common risk factor for hepatocellular carcinoma (HCC) [4], one of the most common causes of cancer-related death worldwide and in Vietnam [5], [6], [7]. In Vietnam, the prevalence of hepatitis B infection is approximately 10 % of the population (HBsAg+) [1], [2], [3]. Therefore, HCC surveillance for early detection is essential for the management of HBV-infected patients. The commonly used methods for HCC surveillance in Vietnam are a combination of ultrasound and AFP. However, the majority of HCC patients, including HBV-related HCC in Vietnam, are diagnosed at an advanced stage and the prognosis remains poor, with a median survival of less than 6 months [7]. The development of novel, affordable, and noninvasive biomarkers to increase the effectiveness of liver cancer screening in hepatitis B patients is essential.
Our study validated the clinical performance of the GAAD algorithm for HCC surveillance in chronic hepatitis B patients in Vietnam. The GAAD showed moderate performance with AUC of 0.767 (95 % CI 0.716–0.818) at all-stage and 0.673 (95 % CI 0.606–0.741) at early-stage. The GAAD showed greater sensitivity and NPV but lower specificity and PPV than single tumor markers AFP and PIVKA-II at the recommended cut-off of HCC diagnosis. In particularly, when compared with AFP, the results revealed that GAAD provided greater benefits in detecting HCC regardless of the presence or absence of a cirrhotic background or in distinguishing HCC from benign liver tumors such as hepatic cysts or hemangioma; and when compared with PIVKA-II, the GAAD was also more effective in detecting early-stage HCC with tumor ≤2 cm and distinguishing HCC from benign liver tumors, but not better than PIVKA-II in detecting HCC at all stage or in patients with cirrhosis.
These findings support previous studies that the GAAD algorithm has superior diagnostic value compared to single tumor markers like AFP and PIVKA-II in HCC surveillance [20]. Indeed, AFP and PIVKA-II tended to change in age, and sex, similar to our observation, GAAD improves upon this by integrating these biomarkers with age and gender into a single diagnostic algorithm, enhancing performance over its individual components. However, our results showed that although GAAD was more sensitive than single markers in detecting early-stage and all-stage HCC, the AUC of GAAD was significantly lower than that of PIVKA-II, which is consistent with a recent report of Chung-Feng Huang et al. [21]. In addition, in Vietnamese cohorts with chronic hepatitis B, its effectiveness was limited when detecting tumors against a background of fibrosis, and the GAAD algorithm’s clinical performance showed an AUC below 80 %, consistent with the Taiwanese patient group [21] but lower than other regions where the AUC exceeds 90 % [20]. The reliance of GAAD on AFP and PIVKA-II – both with lower AUC values in our results – likely contributes to this reduced performance. We suppose that there are intrinsic and environmental factors such as genetics, living habits, cultures…that can influence the expression of each marker and thus may influence the diagnostic performance of these markers. Indeed, previous studies also reported that the baseline PIVKA-II and AFP values were also significantly lower in women and blacks [17], or AFP was less sensitive in HCC detection in African Americans with hepatitis C related cirrhosis compared to non-African Americans [22]. This indicates that patient demographics influenced both PIVKA-II and AFP values; and they may not perform equally across different populations with varying etiologies. Therefore, it is necessary to validate the clinical performance of the GAAD and other cancer markers for specific patient populations with diverse risk factors.
Besides the patient demographic factors, the presence of cirrhosis may impact the performance of HCC biomarkers. Many previous studies reported that the baseline or value of AFP and PIVKA-II increased in patients with cirrhosis [10], 17], 22], 23]. In our results, GAAD was effective for HCC detection with the higher sensitivity than both single markers at the recommended cut-off in moderate and severe fibrosis (LF) and cirrhosis patients (LC). This suggests that GAAD is a useful adjunct marker to detect HCC together with PIVKA-II in LF and LC patients in whom AFP does not appear to be beneficial. This observation provided further evidence that GAAD was an effective marker for HCC surveillance in CHB patients with liver fibrosis.
Furthermore, the GAAD was shown to be significantly associated with tumor aggressive behavior, including tumor number, vascular invasion and BCLC stage. Since GAAD incorporates both PIVKA-II and AFP, it likely benefits from the properties of these components. Previous studies have identified PIVKA-II as a novel vascular endothelial growth factor with potent migration activities [24], [25], [26]. Therefore, GAAD may be valuable for assessing BCLC stage and predicting tumor migration and metastasis. In addition, we found 7/142 HCC (∼5 %) cases positive for GAAD but negative for other single biomarkers. Therefore, GAAD may be an adjunct to single biomarkers for HCC surveillance. We recommend the use of GAAD as an additional reference marker in the management program of patients with chronic hepatitis B every 6 months to improve the rate of HCC detection.
The advantage of this study is its careful design and execution at a national center, focusing on Vietnamese patients to evaluate the clinical diagnostic value of the novel GAAD in HCC surveillance. All participants, both in the HCC and non-HCC groups, had a background of HBV infection to ensure homogenous conditions. Disease controls were confirmed to be free of HCC through ultrasound or computed tomography imaging after at least 6 months of sample collection, ensuring the reliability of the data. However, to further elucidate the clinical value of the GAAD algorithm, it is essential to analyze larger sample sizes with case-control design in longer follow-up periods, and to investigate the dynamic changes in GAAD from chronic hepatitis B (CHB) or liver cirrhosis (LC) to HCC.
In conclusion, we verified that the novel GAAD algorithm had moderate performance in HCC detection for HBV-infected patients. It seems to be more effective than single AFP in HCC surveillance, and it may be superior to PIVKA-II for detecting early-stage HCC with tumors ≤2 cm, and distinguishing HCC from benign liver tumors, in chronic hepatitis B patients. In addition, the GAAD may be valuable in assessing stage according to BCLC and predicting tumor migration and metastasis.
Acknowledgments
The authors thank the patients for their contribution to the study. We also thank Dr. Irma Wang, Dr. Truong Thu Nguyet, and Dr. Pham Thi Thuy Trang (Roche Diagnostics) for their contributions to the support for the pilot application of the trial version of the GAAD algorithm in NHTD.
-
Research ethics: The study design was reviewed and approved by the Institutional Ethical Review Board of National Hospital for Tropical Diseases (NHTD). The study complies with the Declaration of Helsinki regarding the use of human samples and identifiable information. The patients have given consent regarding the use of the information including clinical and blood test results for research and publication purposes. No identifiable information was disclosed in any form.
-
Informed consent: Informed consent was obtained from all individuals included in this study.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Use of Large Language Models, AI and Machine Learning Tools: This manuscript used Language Tools (https://languagetool.org/) to improve grammar and spelling mistake.
-
Conflict of interest: The authors state no conflict of interest.
-
Research funding: This study was funded by Roche® Diagnostics.
-
Data availability: The authors confirm that the data supporting the findings of this study are available within the article.
References
1. Flower, B, Du Hong, D, Vu, TKH, Pham Minh, K, Geskus, RB, Day, J, et al.. Seroprevalence of hepatitis B, C and D in Vietnam: a systematic review and meta-analysis. Lancet Reg Health West Pac 2022;24:100468. https://doi.org/10.1016/j.lanwpc.2022.100468.Search in Google Scholar PubMed PubMed Central
2. Cam Huong, NT, Van Luu, N, Nam, NH, Ghula, S, Atieh Qarawi, AT, Mai Truc, PT, et al.. Prevalence of hepatitis B virus infection in health checkup participants: a cross-sectional study at university medical center, Ho Chi Minh City, Vietnam. Hosp Pract (1995)2023;51:163–7. https://doi.org/10.1080/21548331.2023.2221132.Search in Google Scholar PubMed
3. Nguyen, VT. Hepatitis B infection in Vietnam: current issues and future challenges. Asia Pac J Publ Health 2012;24:361–73. https://doi.org/10.1177/1010539510385220.Search in Google Scholar PubMed
4. Bruix, J, Sherman, M. Practice guidelines committee AeAftSoLD. Management of hepatocellular carcinoma. Hepatology 2005;42:1208–36. https://doi.org/10.1002/hep.20933.Search in Google Scholar PubMed
5. Siegel, RL, Miller, KD, Wagle, NS, Jemal, A. Cancer statistics, 2023. CA Cancer J Clin 2023;73:17–48. https://doi.org/10.3322/caac.21763.Search in Google Scholar PubMed
6. Sung, H, Ferlay, J, Siegel, RL, Laversanne, M, Soerjomataram, I, Jemal, A, et al.. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209–49. https://doi.org/10.3322/caac.21660.Search in Google Scholar PubMed
7. Le, DC, Nguyen, TM, Nguyen, DH, Nguyen, DT, Nguyen, LTM. Survival outcome and prognostic factors among patients with hepatocellular carcinoma: a hospital-based study. Clin Med Insights Oncol 2023;17:11795549231178171. https://doi.org/10.1177/11795549231178171.Search in Google Scholar PubMed PubMed Central
8. Padhya, KT, Marrero, JA, Singal, AG. Recent advances in the treatment of hepatocellular carcinoma. Curr Opin Gastroenterol 2013;29:285–92. https://doi.org/10.1097/mog.0b013e32835ff1cf.Search in Google Scholar PubMed
9. Liu, R, Li, H, Qiu, Y, Liu, H, Cheng, Z. Recent advances in hepatocellular carcinoma treatment with radionuclides. Pharmaceuticals 2022;15. https://doi.org/10.3390/ph15111339.Search in Google Scholar PubMed PubMed Central
10. Tzartzeva, K, Obi, J, Rich, NE, Parikh, ND, Marrero, JA, Yopp, A, et al.. Surveillance imaging and alpha fetoprotein for early detection of hepatocellular carcinoma in patients with cirrhosis: a meta-analysis. Gastroenterology 2018;154:1706–18.e1. https://doi.org/10.1053/j.gastro.2018.01.064.Search in Google Scholar PubMed PubMed Central
11. Ahmed Mohammed, HF, Roberts, LR. Should AFP (or any biomarkers) be used for HCC surveillance? Curr Hepat Rep 2017;16:137–45. https://doi.org/10.1007/s11901-017-0349-7.Search in Google Scholar PubMed PubMed Central
12. Hoshida, Y. Hepatocellular carcinoma: translational precision medicine approaches 2019.10.1007/978-3-030-21540-8Search in Google Scholar PubMed
13. Ikoma, J, Kaito, M, Ishihara, T, Nakagawa, N, Kamei, A, Fujita, N, et al.. Early diagnosis of hepatocellular carcinoma using a sensitive assay for serum des-gamma-carboxy prothrombin: a prospective study. Hepatogastroenterology 2002;49:235–8.Search in Google Scholar
14. Izuno, K, Fujiyama, S, Yamasaki, K, Sato, M, Sato, T. Early detection of hepatocellular carcinoma associated with cirrhosis by combined assay of des-gamma-carboxy prothrombin and alpha-fetoprotein: a prospective study. Hepatogastroenterology 1995;42:387–93.Search in Google Scholar
15. Shimauchi, Y, Tanaka, M, Kuromatsu, R, Ogata, R, Tateishi, Y, Itano, S, et al.. A simultaneous monitoring of lens culinaris agglutinin A-reactive alpha-fetoprotein and des-gamma-carboxy prothrombin as an early diagnosis of hepatocellular carcinoma in the follow-up of cirrhotic patients. Oncol Rep 2000;7:249–56. https://doi.org/10.3892/or.7.2.249.Search in Google Scholar PubMed
16. Amit, S, Jorge, AM. Screening for hepatocellular carcinoma. Gastroenterol Hepatol 2008;4:201–8.Search in Google Scholar
17. Lok, AS, Sterling, RK, Everhart, JE, Wright, EC, Hoefs, JC, Di Bisceglie, AM, et al.. Des-gamma-carboxy prothrombin and alpha-fetoprotein as biomarkers for the early detection of hepatocellular carcinoma. Gastroenterology 2010;138:493–502. https://doi.org/10.1053/j.gastro.2009.10.031.Search in Google Scholar PubMed PubMed Central
18. McMahon, BJ, Bulkow, L, Harpster, A, Snowball, M, Lanier, A, Sacco, F, et al.. Screening for hepatocellular carcinoma in Alaska natives infected with chronic hepatitis B: a 16-year population-based study. Hepatology 2000;32:842–6. https://doi.org/10.1053/jhep.2000.17914.Search in Google Scholar PubMed
19. Piratvisuth, T, Tanwandee, T, Thongsawat, S, Sukeepaisarnjaroen, W, Esteban, JI, Bes, M, et al.. Multimarker panels for detection of early stage hepatocellular carcinoma: a prospective, multicenter, case-control study. Hepatol Commun 2022;6:679–91. https://doi.org/10.1002/hep4.1847.Search in Google Scholar PubMed PubMed Central
20. Piratvisuth, T, Hou, J, Tanwandee, T, Berg, T, Vogel, A, Trojan, J, et al.. Development and clinical validation of a novel algorithmic score (GAAD) for detecting HCC in prospective cohort studies. Hepatol Commun 2023;7. https://doi.org/10.1097/hc9.0000000000000317.Search in Google Scholar PubMed PubMed Central
21. Huang, CF, Kroeniger, K, Wang, CW, Jang, TY, Yeh, ML, Liang, PC, et al.. Surveillance imaging and GAAD/GALAD scores for detection of hepatocellular carcinoma in patients with chronic hepatitis. J Clin Transl Hepatol 2024;12:907–16. https://doi.org/10.14218/JCTH.2024.00172.Search in Google Scholar PubMed PubMed Central
22. Nguyen, MH, Garcia, RT, Simpson, PW, Wright, TL, Keeffe, EB. Racial differences in effectiveness of α-fetoprotein for diagnosis of hepatocellular carcinoma in hepatitis C virus cirrhosis. Hepatology 2002;36:410–7. https://doi.org/10.1053/jhep.2002.34744.Search in Google Scholar PubMed
23. Di Bisceglie, AM, Sterling, RK, Chung, RT, Everhart, JE, Dienstag, JL, Bonkovsky, HL, et al.. Serum alpha-fetoprotein levels in patients with advanced hepatitis C: results from the HALT-C trial. J Hepatol 2005;43:434–41. https://doi.org/10.1016/j.jhep.2005.03.019.Search in Google Scholar PubMed
24. Fujikawa, T, Shiraha, H, Ueda, N, Takaoka, N, Nakanishi, Y, Matsuo, N, et al.. Des-gamma-carboxyl prothrombin-promoted vascular endothelial cell proliferation and migration. J Biol Chem 2007;282:8741–8. https://doi.org/10.1074/jbc.m609358200.Search in Google Scholar
25. Park, H, Kim, SU, Park, JY, Kim, DY, Ahn, SH, Chon, CY, et al.. Clinical usefulness of double biomarkers AFP and PIVKA-II for subdividing prognostic groups in locally advanced hepatocellular carcinoma. Liver Int 2014;34:313–21. https://doi.org/10.1111/liv.12274.Search in Google Scholar PubMed
26. Fujikawa, T, Shiraha, H, Yamamoto, K. Significance of des-gamma-carboxy prothrombin production in hepatocellular carcinoma. Acta Med Okayama 2009;63:299–304. https://doi.org/10.18926/AMO/31826.Search in Google Scholar PubMed
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Mini Review
- Current status and future perspectives on standardized training for laboratory medicine resident physicians in China
- Original Articles
- Comprehensive utilization of NMR spectra–evaluation of NMR-based quantification of amino acids for research and patient care
- Establishing serum zinc reference intervals with two different photometric assays and evaluating their impact on zinc deficiency prevalence
- Diagnostic validation of the GAAD algorithm for hepatitis B virus-related hepatocellular carcinoma surveillance in Vietnam
- Effect of DNA input on analytical and clinical parameters of a circulating tumor DNA assay for comprehensive genomic profiling
- Application value of ddPCR in rapid detection of pathogens in abdominal sepsis
- Congress Abstracts
- German Congress of Laboratory Medicine: 20th Annual Congress of the DGKL and 7th Symposium of the Biomedical Analytics of the DVTA e. V.
Articles in the same Issue
- Frontmatter
- Mini Review
- Current status and future perspectives on standardized training for laboratory medicine resident physicians in China
- Original Articles
- Comprehensive utilization of NMR spectra–evaluation of NMR-based quantification of amino acids for research and patient care
- Establishing serum zinc reference intervals with two different photometric assays and evaluating their impact on zinc deficiency prevalence
- Diagnostic validation of the GAAD algorithm for hepatitis B virus-related hepatocellular carcinoma surveillance in Vietnam
- Effect of DNA input on analytical and clinical parameters of a circulating tumor DNA assay for comprehensive genomic profiling
- Application value of ddPCR in rapid detection of pathogens in abdominal sepsis
- Congress Abstracts
- German Congress of Laboratory Medicine: 20th Annual Congress of the DGKL and 7th Symposium of the Biomedical Analytics of the DVTA e. V.

