Elevated plasma interleukin 21 is associated with higher probability and severity of idiopathic membranous nephropathy
-
Miao Liu
und Min He
Abstract
Objectives
Interleukin 21 (IL-21) is a receptor participating in innate immunity and correlates with the activation of innate immune cells. We sought to investigate the role of plasma IL-21 in patients with idiopathic membranous nephropathy (IMN).
Methods
This was a cross-sectional and case-control study. We analyzed plasma IL-21 in patients with IMN, with other kidney diseases as the diseased controls (DCs), and the healthy controls (HCs), regarding their associations with the risk of having IMN and IMN severity.
Results
We enrolled 132, 22, and 38 patients with IMN, DC, and HC, respectively. Plasma IL-21 was significantly higher in those with IMN [9.42 (6.93, 12.60)] and DC [7.84 (2.90, 7.95)] compared with HC [5.60 (2.90, 7.10)] (p<0.05). Plasma IL-21 was significantly higher in those with IMN stage III [10.36 (6.94, 20.88)] and II [9.75 (7.60, 14.27)] than those with IMN stage I [6.99 (3.91, 9.08)] (p<0.05). Plasma IL-21 was significantly higher in those with a positive anti-phospholipase A2 receptor antibody (PLA2R) [9.60 (8.27, 12.93)] than those with a negative anti-PLA2R antibody [4.84 (2.90, 11.28)] (p<0.05). Receiver operator characteristic curve analysis showed that a cutoff value of 7.665 pg/mL distinguished patients with IMN from HC and DC with a sensitivity and specificity of 68.94 and 89.47 %, respectively, and the area under the curve was 0.8184. A cutoff value of 7.830 pg/mL identified those with IMN stage II with a sensitivity and specificity of 74.03 and 89.47 %, respectively, with an area under the curve of 0.8718 (p<0.001). Multivariate regression showed that plasma IL-21 was positively correlated with anti-PLA2R and 24 h urine protein, and negatively correlated with total protein and serum albumin.
Conclusions
Plasma IL-21 levels increased significantly in patients with IMN. IL-21 may therefore serve as a biomarker for IMN.
Introduction
Idiopathic membranous nephropathy (IMN) is an important origin of nephrotic syndrome (NS) and accounts for about 25 % of NS in adults, and its prevalence among the spectrum of primary glomerular diseases is rising [1]. IMN exhibits a male predominance [2].
The pathological features of IMN include diffuse immune complex deposition underneath glomerular epithelia’s basement membrane, followed by the diffuse basement membrane thickening [3]. Target antigens have been identified from podocyte membranes, and when such antigens are bound by autoantibodies, immune complexes will form a “spike” under the epithelium processes and damage the glomerular filtration barrier, causing proteinuria [4]. IMN has a slow disease course with one-third of IMN patients having spontaneous remission, another one-third having persistent NS and the remaining one-third having renal function decline leading to end-stage kidney disease [5]. IMN constitutes huge burden to patients, their family and society as a whole. Recent studies showed that the proportion of IMN among all primary glomerulopathy is on the rise [6]. Currently, renal biopsy remains the golden standard for diagnosing IMN [7]. However, renal biopsy can be invasive and entails complications and this disadvantage renders serum and urine biomarkers more attractive, as the latter are more convenient, relatively non-invasive and of lower cost. There is a paucity of effective biomarkers capable of predicting IMN disease course, especially during the early period of IMN. Consequently, uncovering such biomarkers before the development of irreversible renal damage becomes an important research priority.
Interleukin-21 (IL-21) is an important cytokine among the interleukin family with many functions, and it is mainly produced by follicular helper T cells and T helper 17 cells [8]. IL-21 participates in class transfer and in the production of antibody-secreting cells [9], and is widely involved in immunologic actions and the propagation of inflammatory responses. These actions are mainly mediated by JAK/STAT, MAPK and PI3K pathways [10]. Several targets of IL-21 such as B lymphocyte-induced maturation protein 1, suppressor of cytokine signaling, C-X-C chemokine receptor type 5, and B cell lymphoma 6 contribute to immunological responses [11]. IL-21 may play a role in the development of autoimmune diseases. Furthermore, IL-21 has been recognized for affecting glomerulopathy pathogenesis [12]. Our study therefore aimed to analyze IL-21 levels in IMN patients and their relationship with patients’ clinical features. We will also evaluate clinical implications of IL-21 in the progression, pathological staging, and the diagnosis of IMN, trying to provide potential diagnostic strategies.
Materials and methods
Ethical approval
This study complied with the Helsinki Declaration and was approved by the Ethics Committee of the Second Affiliated Hospital of Guangzhou University of Chinese Medicine (BF2020-094-01). All participants signed informed consents before study participation.
Study population
This was a cross-sectional and case-control study, consisting of patients consecutively enrolled with IMN or other kidney diseases, all of whom underwent routine follow-up at the Second Affiliated Hospital of Guangzhou University of Chinese Medicine. Inclusion criteria comprised of the following 2 conditions: (1) IMN was diagnosed based on renal biopsy; and (2) participants should have complete clinical and laboratory data. Exclusion criteria included the following 2 conditions: (1) those with secondary membranous nephropathy associated with hepatitis B or C virus, malignancy, lupus, or drug-related membranous nephropathy; and (2) the receipt of any immunosuppressants or cytotoxic agents within the past 6 months [13].
As disease controls for IMN, we included patients with non-immunologic chronic glomerular disease (type II diabetes nephropathy, n=6; hypertensive nephropathy, n=3; nephrotic syndrome of non-membranous nephropathy, n=6; chronic nephritis syndrome, n=2; and chronic kidney disease, n=5) [14]. We also included sex-and age-matched healthy subjects with no known autoimmune or inflammatory diseases.
Sample collection and processing
Participants’ blood was collected using vacuum collection tubes (Becton Dickinson Medical Devices Co. Ltd., NJ, USA). All participants were required to fast for at least 8–10 h before their blood was collected. The procedure of blood drawing, sample transportation and centrifugation were performed in accordance with the Clinical & Laboratory Standards Institute guideline EP28-A3c [15]. The recruitment of reference populations was strictly in accordance with the Clinical & Laboratory Standards Institute guideline EP28-A3c. Samples were stored at −80 °C until analysis, and none were thawed prior to analysis.
Laboratory testing
All laboratory parameters were assayed routinely in the laboratories of the Second Affiliated Hospital of Guangzhou University of Chinese Medicine during the participants’ most recent clinic visit.
IL-21 levels were assayed with an enzyme-linked immunosorbent assay (ELISA) assay (DY8879-05, R&D, USA) [16]. The polystyrene microwells were precoated with mouse anti-human IL-21 capture antibody and biotinylated goat anti-human IL-21 detection antibody, with the concentrations of IL-21 calculated using a four-parameter logistic regression. Serum anti-phospholipase A2 receptor (PLA2R) IgG was tested using another ELISA kit (E210606AF, EUROIMMUN Biotech, Beijing, China) [17]. The polystyrene microwells were precoated with PLA2R antigen and peroxidase labeled anti human IgG (rabbit origin), with the concentrations of PLA2R IgG calculated using a four-parameter logistic regression. A value equal to or greater than 20 RU/ml was regarded positive. An Mk3 enzyme label analyzer was purchased from the Shanghai Somerset Fisher Instruments Co. Ltd.
Twenty-four-hour urine protein (24hUpro), urine creatinine (Ucr) , serum urea nitrogen (urea), serum creatinine (Scr) , total cholesterol (TC), triglyceride(TG), total protein (TP) and albumin (ALB) were analyzed with the Cobas 8,000 analyzer (Roche Diagnostics, Shanghai, China). Serum IgG, Urine IgG (IgGU), urine microalbumin(mAlb) and urine transferrin (Trf) were measured with the BN ProSec analyzer (Siemens, Shanghai, China). The estimated glomerular filtration rate (eGFR) was calculated.
Statistical analysis
Statistical analyses were conducted using the SPSS Software version 20.0 and the Prime Prism Software version 5.0. We described continuous variables in each group using the interquartile median (25 and 75 quartiles). We compared between two groups using the t tests for parametric variables and the Wilcoxon rank sum test for the non-parametric variables. We used the Spearman linear correlation analysis to analyze correlations between non-parametric variables, and calculated the Pearson’s correlation coefficient (Correlations (r)) between parametric variables. Finally, we used logistic regression analyses for receiver operating characteristic (ROC) curve analysis. Two-sided p-values less than 0.05 were considered statistically significant [18].
Results
Clinical characteristics and laboratory profiles
In total, 192 subjects participated in this study, with their plasma samples collected and analyzed for IL-21 and anti-PLA2R IgG levels. Among these subjects, 132, 22, and 38 patients had IMN, diseased controls (DC) and healthy controls (HC), respectively. IMN patients had a mean age of 57 years (range 21–84), with 46 % being women. DC patients had a mean age of 56 years (range 19–75), with 55 % being women. HC patients had a mean age of 46 years (range 22–73), with 50 % being women. We found significantly higher IL-21 levels in IMN patients [9.42 (6.93, 12.60) pg/mL] compared with those in the HC group [5.60 (2.90, 7.10) pg/mL] (p<0.001) and those in the DC group [7.84 (2.90, 7.95) pg/mL] (p=0.020). The demographic and clinical features of each group are summarized in Table 1.
Clinical characteristics and laboratory profiles of the 3 groups of participants.
| Variables | HC (n=38) | IMN (n=132) | DC (n=22) | HC-IMN p-Value | IMN-DC p-Value |
|---|---|---|---|---|---|
| Age, years | 46 (22, 73) | 57 (21, 84) | 56 (19, 75) | <0.0001 | 0.910 |
| Sex (male/female) | 19/19 | 71/61 | 12/10 | 0.683 | 0.947 |
| IL-21, pg/mL | 5.60 (2.90, 7.10) | 9.42 (6.93, 12.60) | 6.22 (2.90, 9.24) | 0.000 | 0.020 |
| anti-PLA2R, RU/mL | 6.37 (5.91, 7.18) | 64.72 (26.41, 183.73) | 8.85 (4.75, 25.88) | 0.000 | 0.000 |
| 24hUpro, mg/L | 55.00 (45.00, 62.00) | 3936.45 (1451.00, 6967.00) | 1989.65(817.70, 3484.80) | 0.000 | 0.597 |
| eGFR (mL/min/1.73m2) | 105.09 (97.97, 114.19) | 81.43 (57.76, 99.85) | 51.58 (13.85, 70.00) | 0.000 | 0.000 |
| TP, g/L | 74.25 (72.20, 76.50) | 55.50 (43.50, 64.55) | 6653.50(4515.00, 9620.00) | 0.000 | 0.804 |
| UCr, μmol/L | 8,059.50 (6789.00, 9238.00) | 9253.00 (6071.00, 13236.00) | 56.50 (51.60, 62.90) | 0.020 | 0.094 |
| ALB, g/L | 48.35 (46.80, 49.70) | 31.50 (22.30, 38.80) | 30.50 (27.50, 40.00) | 0.000 | 0.507 |
| SCr, μmol/L | 64.00 (57.00, 73.00) | 80.80 (64.50, 108.00) | 111.50 (95.00, 363.00) | 0.000 | 0.013 |
| Urea, mmol/L | 4.55 (4.01, 5.18) | 6.12 (4.61, 8.25) | 8.20 (5.80, 13.68) | 0.000 | 0.254 |
| TC, mmol/L | 4.52 (4.04, 4.89) | 6.22 (4.97, 7.71) | 5.64 (4.00, 7.08) | 0.000 | 0.948 |
| TG, mmol/L | 0.89 (0.71, 1.13) | 1.83 (1.30, 2.56) | 1.40 (0.95, 2.59) | 0.000 | 0.128 |
| mAlb, mg/L | 11.00 (10.30, 11.40) | 1671.50 (630.00, 3435.00) | 1031.00 (313.00, 2820.00) | 0.000 | 0.433 |
| IgG, g/L | 13.75 (11.50, 14.80) | 6.45 (4.60, 9.04) | 8.59 (5.45, 10.90) | 0.000 | 0.977 |
-
Values are presented as [median (P25, P75)], unless otherwise stated. IL-21 and anti-PLA2R (assayed by ELISA), eGFR, estimated glomerular filtration rate. 24hUpro, UCr, mAlb indicators were from urine, others were from plasma.
Correlations between IL-21 levels and other laboratory parameters
Using multiple regression analysis, we identified that IL-21 levels were positively correlated with anti-PLA2R and 24hUpro levels (correlation coefficients, 0.436 and 0.393, respectively, both p<0.05), and negatively correlated with TP and ALB (correlation coefficients, −0.279 and −0.329, respectively, both p<0.05). Correlation coefficients between plasma IL-21 levels and laboratory parameters are summarized in Table 2 and Figure 1.
Correlations between IL-21 and other laboratory parameters.
| Variables | Correlation coefficient | p-Value |
|---|---|---|
| anti-PLA2R, RU/mL | 0.436 | 0.000 |
| 24hUpro, mg/L | 0.393 | 0.000 |
| UCr, μmol/L | 0.176 | 0.022 |
| TP, g/L | −0.279 | 0.000 |
| ALB, g/L | −0.329 | 0.000 |
| Urea, mmol/L | 0.157 | 0.041 |
| TC, mmol/L | 0.325 | 0.000 |
| TG, mmol/L | 0.287 | 0.000 |
| IgGU, mg/L | 0.350 | 0.000 |
| Trf, mg/L | 0.355 | 0.000 |
| eGFR, mL/min/1.73m2 | −0.118 | 0.124 |
| SCr, μmol/L | 0.117 | 0.127 |
-
24hUpro, UCr, IgGU, Trf indicators were from urine, others were from plasma.
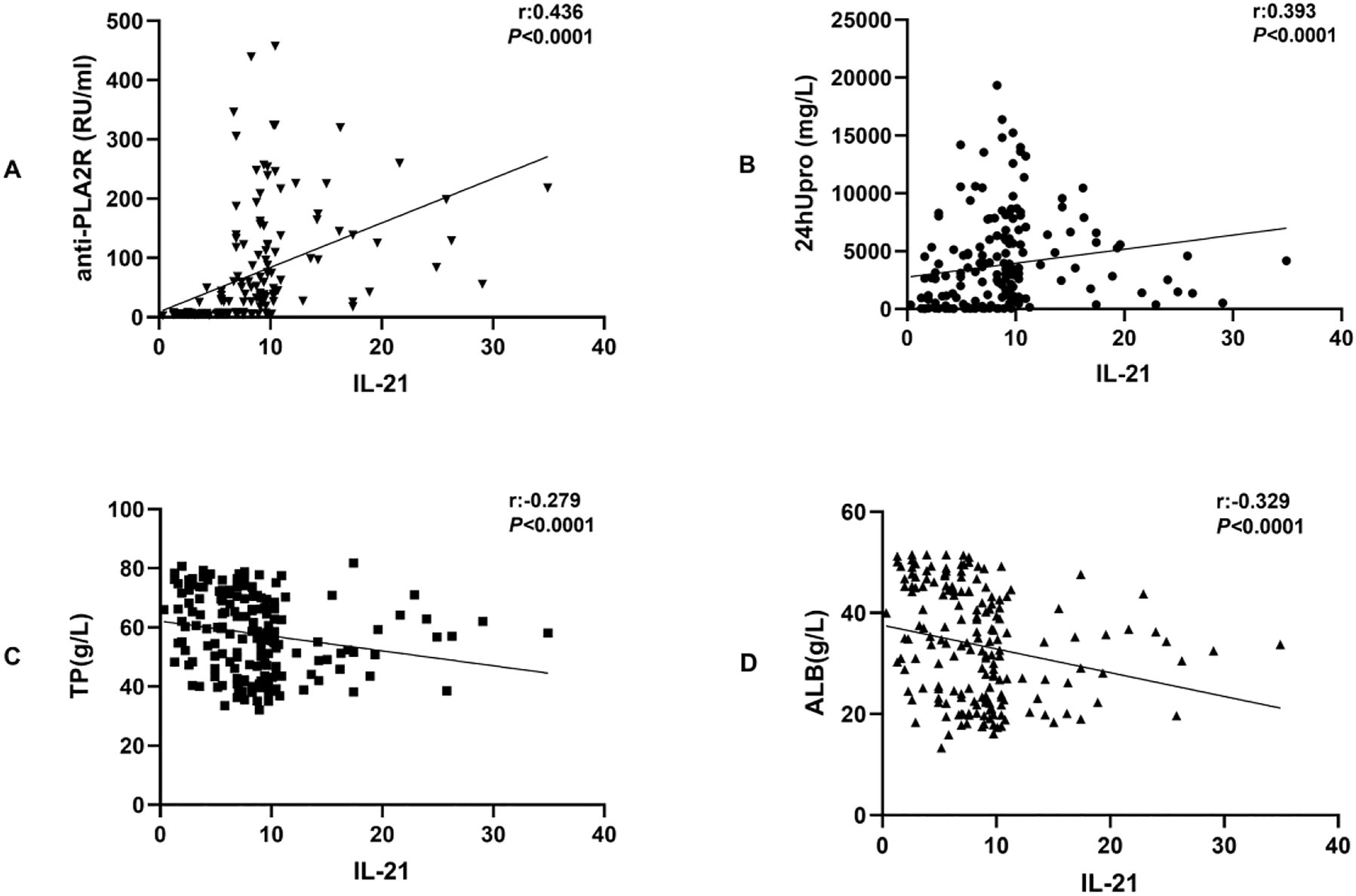
Correlation analysis between IL-21 and anti-PLA2R IgG, 24hUpro, TP, and ALB.
Laboratory parameters between IMN patients with negative and positive anti-PLA2R antibody
We subsequently discovered that IL-21 did not differ significantly between the IMN and the DC groups [19]. We then divided the IMN group into those with negative and positive anti-PLA2R IgG (Table 3).
Comparing laboratory parameters between IMN patients with negative and positive anti-PLA2R antibody.
| Variables | A2+ (n=106) | A2− (n=26) | p-Value |
|---|---|---|---|
| IL-21, pg/mL | 9.60 (8.27,12.93) | 4.84 (2.90,11.28) | 0.001 |
| 24hUpro, mg/L | 4666.40 (2324.40,7873.00) | 1177.50 (432.00,3542.00) | 0.000 |
| TP, g/L | 53.40 (42.00,63.70) | 60.45 (50.80,69.70) | 0.004 |
| ALB, g/L | 28.50 (20.40,36.60) | 37.40 (31.00,42.70) | 0.000 |
| TC, mmol/L | 6.73 (5.12,8.13) | 5.07 (4.81,5.74) | 0.000 |
| mAlb, mg/L | 2075.00 (923.00,4150.00) | 488.00 (62.00,2389.00) | 0.000 |
| Trf, mg/L | 139.00 (63.70,330.00) | 31.65 (9.00,172.00) | 0.001 |
| eGFR, mL/min/1.73m2 | 82.48 (58.09,99.89) | 73.08 (54.32,99.11) | 0.714 |
| SCr, μmol/L | 81.80 (64.00,118.00) | 76.50 (67.00,102.00) | 0.714 |
| Urea, mmol/L | 6.07 (4.54,8.08) | 6.35 (4.74,9.40) | 0.563 |
| TG, mmol/L | 1.83 (1.34,2.62) | 1.86 (1.01,2.32) | 0.248 |
-
Values are presented as [median (P25, P75)], unless otherwise stated. A2+, anti-PLA2R Positive; A2−, anti-PLA2R negative. 24hUpro, mAlb, Trf indicators were from urine, others were from plasma.
Plasma IL-21 levels were significantly higher in those with positive anti-PLA2R [9.60 (8.27, 12.93) pg/mL] than those (9.60 (8.27, 12.93) pg/mL) than those with negative anti-PLA2R (4.84 (2.90, 11.28) pg/mL) (p=0.001). eGFR, SCr, Urea and TG levels did not differ between the two populations (all p>0.05). The comparisons of other laboratory parameters between those with negative and positive PLA2R IgG are summarized in Table 3.
IL-21 levels in IMN patients according to pathological stage
It is plausible that IL-21 can not only help distinguish IMN from DC, but also correlates with disease course [20]. Therefore, we examined the relationship between IL-21 and the pathological stages of IMN. We showed that plasma IL-21 levels in those with IMN stage I were significantly lower than those in patients with IMN stages II and III (p<0.05) (Figure 2).
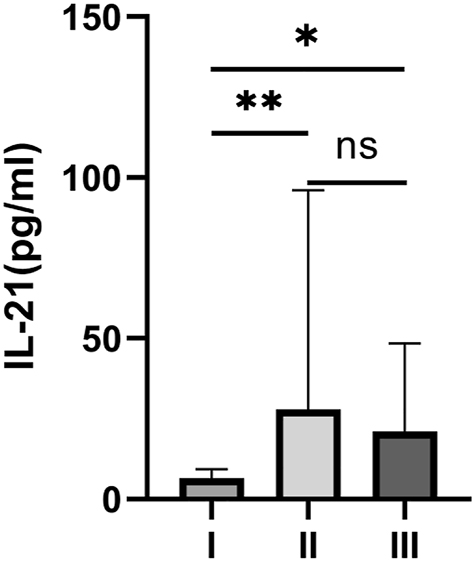
IL-21 levels in IMN patients according to their pathological stage. *p<0.05, **p<0.01, p >0.05 as ns.
We performed a ROC analysis to evaluate the role of plasma IL-21 in distinguishing patients with IMN from DC ones and in distinguishing between different IMN pathological stages [21]. We found that a cutoff level of 7.665 pg/mL had a sensitivity and specificity of 68.94 and 89.47 %, respectively, for distinguishing between patients with IMN and other kidney diseases, with an area under the curve of 0.8184 (95 % confidence interval 0.756–0.881, p<0.001) (Figure 3). We also found that a cutoff level of 7.830 pg/mL had a sensitivity and specificity of 74.03 and 89.47 %, respectively, for IMN stage II, with an area under the curve of 0.8718 (p<0.001) (Figure 4).
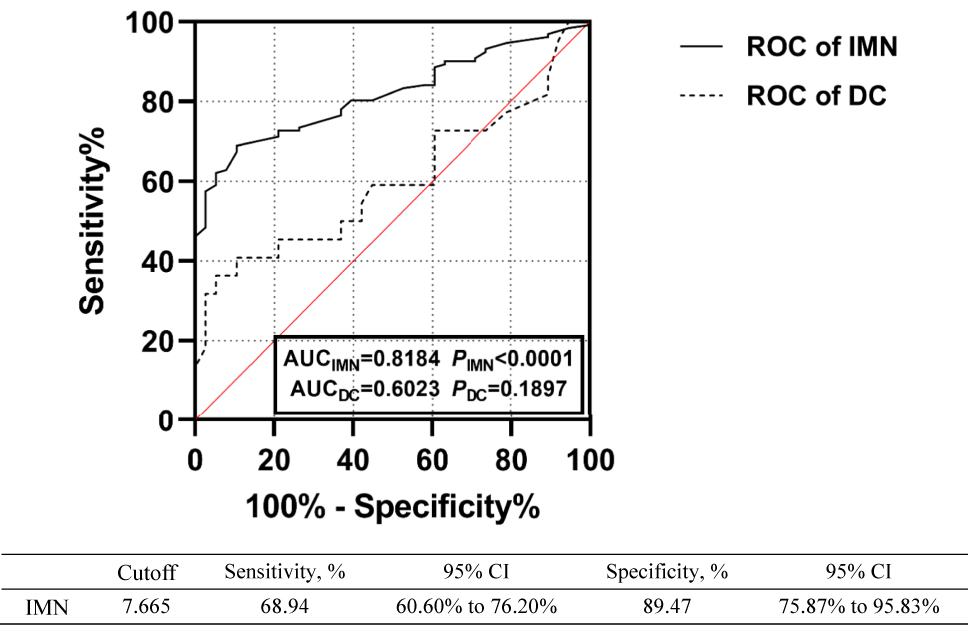
ROC curves of using plasma IL-21 to distinguish between patients with IMN and DC.
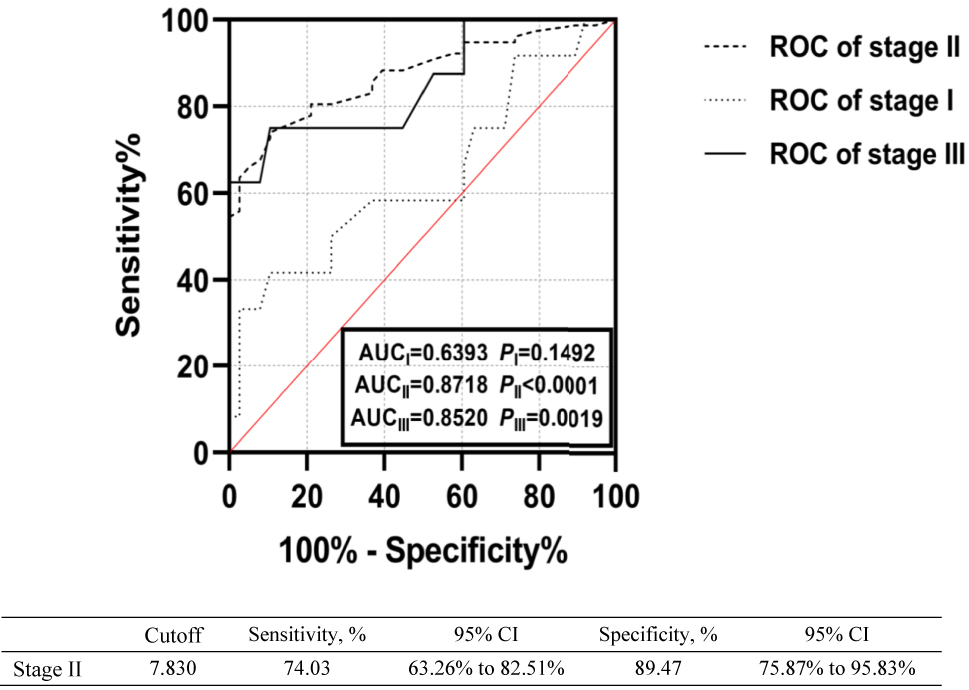
ROC curves of using plasma IL-21 levels to distinguish between patients with IMN stages I, II, and III.
Discussion
In this study, we found that there was no statistical difference regarding age between the IMN patients, DCs and HCs. There were, however, significant differences regarding gender between IMN patients and HCs.
Previously, 24hUpro and serum anti-PLA2R were the mainstay for predicting disease progression among IMN patients [22]. However, these two parameters potentially lag behind the occurrence of kidney damage [23]. In this study, through examining the associations between plasma IL-21 and key clinical parameters, we aimed to elucidate whether IL-21 has a potential role in assisting the diagnosis of IMN. Indeed, we found that IL-21 levels were higher in IMN patients compared to those in the DC and the HC groups [24]. Although IMN patients’ anti-PLA2R IgG and 24hUpro levels were higher than those of the HC group, no statistical difference was observed between those of the IMN and the DC groups. These findings are consistent with those reported by Liu W et al. [25].
We then found that plasma IL-21 and 24hUPo levels in those with positive anti-PLA2R were significantly higher than those in the negative anti-PLA2R group. In addition, TP and ALB levels in those with positive anti-PLA2R were lower than those in the negative anti-PLA2R group. Prior studies reported that IL-21 exhibited a close correlation with anti-PLA2R [26], and these findings in combination further support the fact that IL-21 could serve as a new biomarker for IMN.
Anti-PLA2R antibody has been established as a diagnostic indicator for IMN [27], but we did not find significant differences regarding IL-21 levels between IMN patients and DCs. In addition, there was a study suggesting that the correlation between IL-21 and anti-PLA2R was high [28]. We therefore divided IMN patients into those with negative and positive anti-PLA2R for further analyses, and found that IMN patients with positive anti-PLA2R had more severe kidney damage than those with negative anti-PLA2R. This finding may help explain why we did not identify differences in IL-21 levels between IMN patients and DCs [29]. We presumed that IMN patients with negative anti-PLA2R had lower plasma IL-21 levels, and indeed those with positive anti-PLA2R had 2-fold higher IL-21 levels than those with negative anti-PLA2R. This phenomenon may indirectly support the correlation between IL-21 and anti-PLA2R, as reported by others [30]. Therefore, we believe that plasma IL-21 level may be an indicator of IMN disease course.
We further revealed that IL-21 could not only distinguish IMN from DC, but also predict IMN severity [31]. Plasma IL-21 levels differed significantly between those with stage I and those with stages II/III, but they did not differ between those with stage II and stage III. It is suggested that IL-21 is more valuable for the early diagnosis of IMN [32], and the detection of IL-21 helps assess IMN severity at the very early stage of disease [33]. From this perspective, IL-21 may be used to detect early IMN progression.
Our ROC curves showed that IL-21 worked fairly during the differential diagnosis of IMN from other kidney diseases, and also assisted in identifying the pathological staging of IMN. IMN is a primary glomerulopathy whose severity depends on the extent of glomerular damage [34], and podocytes are the main targets injured immunologically by the anti-PLA2R antibody [35]. Depending on the injury extent, affected podocytes may undergo apoptosis or assume serial adaptive changes, leading to foot process effacement and the detachment of podocytic proteins [36]. This finding explains why IMN patients have higher IL-21 levels than other patients. Therefore, plasma IL-21 may serve as an early biomarker in patients with IMN. Identifying IL-21 as a potential IMN marker and for disease progression assists critically in IMN management.
During the past decade, the mechanism by which IL-21 affected our immune system has been extensively studied. More and more studies have identified a critical role of IL-21 in the pathogenesis, initiation, and progression of multiple autoimmune diseases [37]. More studies are needed to elucidate the underlying mechanisms of IMN and even other kidney diseases.
Funding source: The Guangzhou Basic and Applied Basic Research Project
Award Identifier / Grant number: 202102021139
Funding source: Science and Technology Planning Project of Guangzhou
Award Identifier / Grant number: 202201020556, 202201020487
Funding source: Science and Technology Research Project of Traditional Chinese Medicine of Guangdong Provincial Hospital of Chinese Medicine
Award Identifier / Grant number: YN2019QL06
Funding source: State Key Laboratory of Dampness Syndrome of Chinese Medicine
Award Identifier / Grant number: SZ2021ZZ24, SZ2021ZZ3003, SZ2021ZZ09
-
Research funding: The study was supported by project grants from the National Natural Science Foundation of China (81503317, 81974565), State Key Laboratory of Dampness Syndrome of Chinese Medicine (SZ2021ZZ24, SZ2021ZZ3003, SZ2021ZZ09), Science and Technology Planning Project of Guangzhou (202201020556, 202201020487), Science and Technology Research Project of Traditional Chinese Medicine of Guangdong Provincial Hospital of Chinese Medicine (YN2019QL06), and the Guangzhou Basic and Applied Basic Research Project (202102021139).
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Competing interests: The authors declared no potential conflict of interest with respect to the research, authorship and publication of this article.
-
Informed consent: Informed consent was obtained from all individuals included in this study.
-
Ethical approval: This study was in compliance with the Helsinki Declaration and was approved by the Ethics Committee of the Second Affiliated Hospital of Guangzhou University of Chinese Medicine. All participants had signed an informed consent before the participation of this study. Ethics number: 2020-094-01.
References
1. Couser, WG. Primary membranous nephropathy. Clin J Am Soc Nephrol 2017;12:983–97. https://doi.org/10.2215/cjn.11761116.Suche in Google Scholar
2. Liu, WB, Gao, C, Liu, ZY, Dai, H, Feng, Z, Dong, Z, et al.. Idiopathic membranous nephropathy: glomerular pathological pattern caused by extrarenal immunity activity. Front Immunol 2020;11:1846. https://doi.org/10.3389/fimmu.2020.01846.Suche in Google Scholar PubMed PubMed Central
3. Zhao, QH, Dai, H, Liu, XL, Jiang, H, Liu, W, Feng, Z, et al.. Helper T cells in idiopathic membranous nephropathy. Front Immunol 2021;12:665629. https://doi.org/10.3389/fimmu.2021.665629.Suche in Google Scholar PubMed PubMed Central
4. Molina Andujar, A, Castrejon de Anta, N, Rodriguez-Espinosa, D, Hermida, E, Larque, AB, Esforzado, N, et al.. Antiphospholipase A2 receptor antibody-positive membranous nephropathy in the kidney donor: lessons from a serendipitous transplantation. Am J Transplant 2022;22:299–303. https://doi.org/10.1111/ajt.16813.Suche in Google Scholar PubMed
5. Cai, Q, Hendricks, AR. Membranous nephropathy: a ten-year journey of discoveries. Semin Diagn Pathol 2020;37:116–20. https://doi.org/10.1053/j.semdp.2020.01.001.Suche in Google Scholar PubMed
6. Han, WW, Tang, LJ, Kong, XL, Yang, H, Xu, DM. Clinical significance of autoantibodies in the assessment and treatment of idiopathic membranous nephropathy. Exp Ther Med 2019;17:1825–30. https://doi.org/10.3892/etm.2018.7108.Suche in Google Scholar PubMed PubMed Central
7. Ren, S, Wu, C, Zhang, Y, Wang, AY, Li, G, Wang, L, et al.. An update on clinical significance of use of THSD7A in diagnosing idiopathic membranous nephropathy: a systematic review and meta-analysis of THSD7A in IMN. Ren Fail 2018;40:306–13. https://doi.org/10.1080/0886022x.2018.1456457.Suche in Google Scholar
8. Long, D, Chen, Y, Wu, H, Zhao, M, Lu, Q. Clinical significance and immunobiology of IL-21 in autoimmunity. J Autoimmun 2019;99:1–14. https://doi.org/10.1016/j.jaut.2019.01.013.Suche in Google Scholar PubMed
9. Solaymani-Mohammadi, S, Eckmann, L, Singer, SM. Interleukin (IL)-21 in inflammation and immunity during parasitic diseases. Front Cell Infect Microbiol 2019;9:401. https://doi.org/10.3389/fcimb.2019.00401.Suche in Google Scholar PubMed PubMed Central
10. Loureiro-Amigo, J, Franco-Jarava, C, Perurena-Prieto, J, Palacio, C, Martínez-Valle, F, Solans-Laque, R. Serum CXCL13, BAFF, IL-21 and IL-22 levels are related to disease activity and lymphocyte profile in primary Sjögren’s syndrome. Clin Exp Rheumatol 2021;39:131–9. https://doi.org/10.55563/clinexprheumatol/fp741f.Suche in Google Scholar PubMed
11. Motavalli, R, Etemadi, J, Soltani-Zangbar, MS, Ardalan, MR, Kahroba, H, Roshangar, L, et al.. Altered Th17/Treg ratio as a possible mechanism in pathogenesis of idiopathic membranous nephropathy. Cytokine 2021;141:155452. https://doi.org/10.1016/j.cyto.2021.155452.Suche in Google Scholar PubMed
12. Zhang, Z, Shi, Y, Yang, K, Crew, R, Wang, H, Jiang, Y. Higher frequencies of circulating ICOS+, IL-21+ T follicular helper cells and plasma cells in patients with new-onset membranous nephropathy. Autoimmunity 2017;50:458–67.Suche in Google Scholar
13. Dai, P, Xie, W, Yu, X, Sun, J, Wang, S, Kawuki, J. Efficacy and cost of different treatment in patients with idiopathic membranous nephropathy: a network meta-analysis and cost-effectiveness analysis. Int Immunopharm 2021;94:107376. https://doi.org/10.1016/j.intimp.2021.107376.Suche in Google Scholar PubMed
14. Cantarelli, C, Jarque, M, Angeletti, A, Manrique, J, Hartzell, S, O’Donnell, T, et al.. A comprehensive phenotypic and functional immune analysis unravels circulating anti-phospholipase A2 receptor antibody secreting cells in membranous nephropathy patients. Kidney Int Rep 2020;5:1764–76.10.1016/j.ekir.2020.07.028Suche in Google Scholar PubMed PubMed Central
15. Wei, B, Guo, Y, Zhang, L, Zhong, H, Miao, Q, Yan, L, et al.. Reference ranges of T lymphocyte subsets by single-platform among healthy population in southwest China. BMC Immunol 2021;22:80. https://doi.org/10.1186/s12865-021-00474-0.Suche in Google Scholar PubMed PubMed Central
16. Ren, HM, Lukacher, AE, Rahman, ZSM, Olsen, NJ. New developments implicating IL-21 in autoimmune disease. J Autoimmun 2021;122:102689. https://doi.org/10.1016/j.jaut.2021.102689.Suche in Google Scholar PubMed PubMed Central
17. van de Logt, AE, Fresquet, M, Wetzels, JF, Brenchley, P. The anti-PLA2R antibody in membranous nephropathy: what we know and what remains a decade after its discovery. Kidney Int 2019;96:1292–302. https://doi.org/10.1016/j.kint.2019.07.014.Suche in Google Scholar PubMed
18. Cui, HY, Li, C, Li, H, Wen, YB, Duan, L, Li, Y, et al.. Analysis of glomerular IgG subclasses switch in idiopathic membranous nephropathy classified by glomerular phospholipase A2 receptor antigen and serum antibody. Dis Markers 2021;2021:9965343. https://doi.org/10.1155/2021/9965343.Suche in Google Scholar PubMed PubMed Central
19. Xing, R, Sun, L, Wu, D, Jin, Y, Li, C, Liu, X, et al.. Autoantibodies against interleukin-21 correlate with disease activity in patients with rheumatoid arthritis. Clin Rheumatol 2018;37:75–80. https://doi.org/10.1007/s10067-017-3862-8.Suche in Google Scholar PubMed
20. Xing, R, Jin, Y, Sun, L, Yang, L, Li, C, Li, Z, et al.. Interleukin-21 induces migration and invasion of fibroblast-like synoviocytes from patients with rheumatoid arthritis. Clin Exp Immunol 2016;184:147–58. https://doi.org/10.1111/cei.12751.Suche in Google Scholar PubMed PubMed Central
21. Safar-Boueri, Piya, AA, Beck, LHJr, Ayalon, R. Membranous nephropathy: diagnosis, treatment, and monitoring in the post-PLA2R era. Pediatr Nephrol 2021;36:19–30. https://doi.org/10.1007/s00467-019-04425-1.Suche in Google Scholar PubMed
22. Xu, J, Shen, C, Lin, W, Meng, T, Ooi, JD, Eggenhuizen, PJ, et al.. Single-cell profiling reveals transcriptional signatures and cell-cell crosstalk in anti-PLA2R positive idiopathic membranous nephropathy patients. Front Immunol 2021;12:683330. https://doi.org/10.3389/fimmu.2021.683330.Suche in Google Scholar PubMed PubMed Central
23. Wan, N, Li, D, Zhou, Z, Shao, Y, Zheng, S, Wang, S. Comprehensive RNA-sequencing analysis in peripheral blood cells reveals differential expression signatures with biomarker potential for idiopathic membranous nephropathy. DNA Cell Biol 2019;38:1223–32. https://doi.org/10.1089/dna.2019.4701.Suche in Google Scholar PubMed
24. You, L, Ye, P, Xiao, G, Liang, J, Kong, Y. Rituximab for the treatment of idiopathic membranous nephropathy with nephrotic syndrome: a systematic review and meta-analysis. Turk J Med Sci 2021;51:2870–80. https://doi.org/10.3906/sag-2104-177.Suche in Google Scholar PubMed
25. Liu, W, Gao, C, Dai, H, Zheng, Y, Dong, Z, Gao, Y, et al.. Immunological pathogenesis of membranous nephropathy: focus on PLA2R1 and its role. Front Immunol 2019;10:1809. https://doi.org/10.3389/fimmu.2019.01809.Suche in Google Scholar PubMed PubMed Central
26. Zhang, QH, Wu, M, Hu, ZG, Liu, XB, Huang, B, Zhang, Y, et al.. Serum antibody and glomerular antigen of antiphospholipase A2 receptor in Chinese patients with idiopathic membranous nephropathy. BioMed Res Int 2020;2020:1693710. https://doi.org/10.1155/2020/1693710.Suche in Google Scholar PubMed PubMed Central
27. Guo, N, Cao, Y, Dai, H, Yuan, L, Shi, L, Zhang, Y. Anti-phospholipase A2 receptor (Anti-PLA2R) antibody in diagnosis and treatment of idiopathic membranous nephropathy: a single-center observational study in China. Med Sci Mon Int Med J Exp Clin Res 2019;25:9364–8. https://doi.org/10.12659/msm.917732.Suche in Google Scholar
28. Zhang, Z, Shi, Y, Yang, K, Crew, R, Wang, H, Jiang, Y. Higher frequencies of circulating ICOS(+), IL-21(+) T follicular helper cells and plasma cells in patients with new-onset membranous nephropathy. Autoimmunity 2017;50:458–67. https://doi.org/10.1080/08916934.2017.1385775.Suche in Google Scholar PubMed
29. Tavakolpour, S. Interleukin 21 as a new possible player in pemphigus: is it a suitable target? Int Immunopharm 2016;34:139–45. https://doi.org/10.1016/j.intimp.2016.02.020.Suche in Google Scholar PubMed
30. Guo, W, Zhang, Y, Gao, C, Huang, J, Li, J, Wang, R, et al.. Retrospective study: clinicopathological features and prognosis of idiopathic membranous nephropathy with seronegative anti-phospholipase A2 receptor antibody. PeerJ 2020;8:e8650. https://doi.org/10.7717/peerj.8650.Suche in Google Scholar PubMed PubMed Central
31. Shi, X, Qu, Z, Zhang, L, Zhang, N, Liu, Y, Li, M, et al.. Increased ratio of ICOS(+)/PD-1(+) follicular helper T cells positively correlates with the development of human idiopathic membranous nephropathy. Clin Exp Pharmacol Physiol 2016;43:410–6. https://doi.org/10.1111/1440-1681.12555.Suche in Google Scholar PubMed
32. Wu, L, Lai, J, Ling, Y, Weng, Y, Zhou, S, Wu, S, et al.. A review of the current practice of diagnosis and treatment of idiopathic membranous nephropathy in China. Med Sci Mon Int Med J Exp Clin Res 2021;27:e930097. https://doi.org/10.12659/msm.930097.Suche in Google Scholar PubMed PubMed Central
33. Wang, R, Wu, Y, Zheng, B, Zhang, X, An, D, Guo, N, et al.. Clinicopathological characteristics and prognosis of hepatitis B associated membranous nephropathy and idiopathic membranous nephropathy complicated with hepatitis B virus infection. Sci Rep 2021;11:18407. https://doi.org/10.1038/s41598-021-98010-y.Suche in Google Scholar PubMed PubMed Central
34. Nagahama, K, Isomura, A, Shimoyamada, H, Masuko, S, Shimoda, S, Karube, M, et al.. Membranous nephropathy with masked polyclonal IgG deposits associated with primary Sjögren’s syndrome. CEN Case Rep 2021;10:53–8. https://doi.org/10.1007/s13730-020-00516-3.Suche in Google Scholar PubMed PubMed Central
35. Guo, Y, Wu, X, Liu, L, Zhang, H, Yang, L, Chen, W. Efficacy of leflunomide combined with prednisone for the treatment of PLA2R-associated primary membranous nephropathy. Ren Fail 2020;42:122–30. https://doi.org/10.1080/0886022x.2020.1713806.Suche in Google Scholar PubMed PubMed Central
36. Gilbert, A, Changjuan, A, Guixue, C, Jianhua, L, Xiaosong, Q. Urinary matrix metalloproteinase-9 and nephrin in idiopathic membranous nephropathy: a cross-sectional study. Dis Markers 2021;2021:1620545. https://doi.org/10.1155/2021/1620545.Suche in Google Scholar PubMed PubMed Central
37. Edel, Y, Kliminski, V, Pokroy-Shapira, E, Oren, S, Dortort Lazar, A, Pri-Paz Basson, Y, et al.. Elevated plasma level of soluble triggering receptor expressed on myeloid cells-1 is associated with inflammation activity and is a potential biomarker of thrombosis in primary antiphospholipid syndrome. Arthritis Res Ther 2019;21:10. https://doi.org/10.1186/s13075-018-1779-5.Suche in Google Scholar PubMed PubMed Central
© 2023 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- Original Articles
- Differences in venous, capillary and interstitial glucose concentrations in individuals without diabetes after glucose load
- EDTA-associated pseudothrombocytopenia: definition and real-world occurrence
- Predictive value of C-reactive protein-to-albumin ratio for risk of 28-day mortality in patients with severe pneumonia
- Elevated plasma interleukin 21 is associated with higher probability and severity of idiopathic membranous nephropathy
- Performance evaluation of cobas pure integrated solutions at multiple sites in Europe and Asia
Artikel in diesem Heft
- Frontmatter
- Original Articles
- Differences in venous, capillary and interstitial glucose concentrations in individuals without diabetes after glucose load
- EDTA-associated pseudothrombocytopenia: definition and real-world occurrence
- Predictive value of C-reactive protein-to-albumin ratio for risk of 28-day mortality in patients with severe pneumonia
- Elevated plasma interleukin 21 is associated with higher probability and severity of idiopathic membranous nephropathy
- Performance evaluation of cobas pure integrated solutions at multiple sites in Europe and Asia

