Abstract
Objectives
To explore the predictive value of C-reactive protein (CRP)-to-albumin (ALB) ratio (CAR) for the risk of 28-day mortality in patients with severe pneumonia.
Methods
A total of 152 patients with severe pneumonia treated from January 2020 to January 2022 were enrolled and assigned into survival group (n=107) and death group (n=45) according to their survival status after treatment for 28 d. Their clinical data were compared, and the influencing factors for 28-day mortality were explored by multiple logistic regression analysis. The receiver operating characteristic (ROC) curve was plotted to assess the value of CAR for predicting 28-day mortality risk. A risk prediction model was constructed, and its prediction efficiency was evaluated.
Results
The death group had significantly older age, Acute Physiology and Chronic Health Evaluation (APACHE) II score, Murray Lung Injury Score, Sequential Organ Failure Assessment score, white blood cell count, neutrophil count, red cell volume distribution width, neutrophil-to-lymphocyte ratio (NLR), fibrinogen, procalcitonin, blood lactic acid (Lac), CRP and CAR and significantly lower oxygenation index and ALB than those of the survival group (p<0.05). APACHE II score, NLR, Lac and CAR were independent risk factors for 28-day mortality (p<0.05). AUC of the established prediction model was 0.826, with sensitivity of 88.45 % and specificity of 87.32 %, indicating high discrimination. The nomogram model had clinical value when the risk threshold probability was 11–93 %.
Conclusions
CAR is an independent risk factor that shows a high predictive value for the 28-day mortality risk in patients with severe pneumonia.
Introduction
Pneumonia refers to lung inflammation induced by bacteria, viruses, etc. [1], whose severity is decided by the degree of local inflammation, the dispersal of lung inflammation and the severity of systemic inflammatory response [2]. Patients showing symptoms of severe hypoxemia and acute respiratory failure, signs of circulatory failure (such as shock) or dysfunction of other organs can be diagnosed with severe pneumonia [3]. Characterized by fast onset, rapid progression and multiple complications [4], severe pneumonia contributes to 40 % of the total number of deaths in the intensive care unit (ICU) [5], becoming one of the primary causes of death in the ICU [6], severely threatening the lives and health of patients. Hence, accurately assessing the prognosis of patients with severe pneumonia and taking intervention measures to cut the mortality rate is of important significance. C-reactive protein (CRP)-to-albumin (ALB) ratio (CAR), a new index to assess inflammation [7], [8], [9], can be adopted to evaluate the severity and prognosis of multiple diseases such as respiratory tract infection, acute coronary syndrome and cerebral infarction. Nevertheless, whether CAR can serve as a prognostic index for severe pneumonia remains unclarified. In this study, therefore, 152 patients with severe pneumonia were enrolled to evaluate the predictive value of CAR for the 28-day mortality risk in patients with severe pneumonia, aiming to provide references for prognosis evaluation in clinical practice.
Materials and methods
A total of 152 patients with severe pneumonia treated in Affiliated Danzhou People’s Hospital of Hainan Medical University from January 2020 to January 2022 were enrolled. The subjects consisted of 74 males and 78 females aged (68.69 ± 3.16) averagely. Patients conforming to the diagnostic criteria for severe pneumonia formulated by the Respiratory Society of the Chinese Medical Association [10] and having complete clinical data were included. Those complicated with malignancies, severe blood diseases, pulmonary tuberculosis or asthma were excluded. Based on the survival status of patients after 28 days of treatment, the subjects were divided into survival group (n=107) and death group (n=45). All subjects and their families were informed of the study and signed the informed consent.
The Acute Physiology and Chronic Health Evaluation (APACHE) II score of the patients was recorded on the first day of admission. The total score was 71 points, and the higher the score, the severer the condition. Besides, the Sequential Organ Failure Assessment (SOFA) was conducted, which consisted of 6 items, with a total score of 0–24 points, and the higher the score, the severer the condition. In addition, the Murray Lung Injury Score (MLIS) was recorded, which included chest X-ray findings, hypoxemia score, positive end-expiratory pressure and compliance, and the higher the score, the severer the lung injury.
Within 24 h after admission, 5 mL of peripheral blood sample was collected from each subject for detection of white blood cell count (WBC), neutrophil count (NEU), neutrophil granulocyte percentage (GR), lymphocyte count (LYM), red cell volume distribution width (RDW), blood platelet count (PLT), neutrophil-to-lymphocyte ratio (NLR), alanine aminotransferase (ALT), aspartate aminotransferase (AST), fibrinogen (FIB), procalcitonin (PCT), blood lactic acid (Lac), oxygenation index (OI), CRP, ALB and CAR.
SPSS 23.0 software was utilized for statistical analysis. The measurement data were expressed as mean ± standard deviation (
Results
Age, APACHE II score, MLIS, SOFA score, WBC, NEU, RDW, NLR, FIB, PCT, Lac, CRP and CAR were significantly higher, while OI and ALB were significantly lower in death group than those in survival group (p<0.05). No significant differences were observed in other relevant data between the two groups (p>0.05) (Table 1).
Clinical data.
| Item | Death group (n=45) | Survival group (n=107) | χ2/t | p-Value |
|---|---|---|---|---|
| Age, year | 69.15 ± 2.76 | 67.38 ± 2.94 | 3.449 | 0.001 |
| Male | 22 (48.87 %) | 52 (48.60 %) | 0.001 | 0.974 |
| BMI, kg/m2 | 23.76 ± 3.15 | 23.74 ± 3.08 | 0.036 | 0.971 |
| Hypertension | 13 (28.89 %) | 31 (28.97 %) | 0.000 | 0.992 |
| Diabetes | 15 (33.33 %) | 35 (32.71 %) | 0.006 | 0.941 |
| Cerebrovascular disease | 9 (20.00 %) | 20 (18.69 %) | 0.035 | 0.851 |
| COPD | 11 (24.44 %) | 26 (24.30 %) | 0.000 | 0.985 |
| CHD | 10 (22.22 %) | 24 (22.43 %) | 0.001 | 0.978 |
| APACHE II score | 26.75 ± 5.32 | 21.14 ± 4.28 | 6.850 | <0.001 |
| MLIS | 2.62 ± 0.68 | 2.19 ± 0.37 | 5.020 | <0.001 |
| SOFA score | 12.98 ± 4.56 | 11.23 ± 3.84 | 2.423 | 0.017 |
| WBC, ×109 L | 22.16 ± 5.23 | 15.42 ± 4.62 | 7.892 | <0.001 |
| NEU, ×109 L | 10.84 ± 6.70 | 7.71 ± 5.69 | 2.934 | 0.004 |
| GR, % | 78.27 ± 9.89 | 77.46 ± 8.73 | 0.502 | 0.617 |
| LYM, ×109 L | 1.52 ± 0.39 | 1.61 ± 0.42 | 1.231 | 0.220 |
| RDW | 18.89 ± 2.36 | 14.03 ± 1.44 | 15.538 | <0.001 |
| PLT, ×109 L | 125.96 ± 30.12 | 133.28 ± 33.21 | 1.274 | 0.205 |
| NLR | 7.33 ± 2.45 | 4.28 ± 2.09 | 7.797 | <0.001 |
| ALT, U/L | 52.12 ± 16.97 | 50.98 ± 16.84 | 0.380 | 0.704 |
| AST, U/L | 52.28 ± 16.37 | 49.79 ± 17.21 | 0.826 | 0.410 |
| FIB, g/L | 6.86 ± 0.67 | 6.03 ± 0.56 | 7.859 | <0.001 |
| PCT, μg/L | 9.21 ± 3.08 | 4.24 ± 2.49 | 10.451 | <0.001 |
| Lac, mmol/L | 4.94 ± 0.11 | 4.28 ± 0.09 | 38.575 | <0.001 |
| OI, mmHg | 142.72 ± 13.76 | 197.62 ± 17.39 | 18.831 | <0.001 |
| CRP, mg/L | 33.66 ± 36.17 | 17.28 ± 10.38 | 4.299 | <0.001 |
| ALB, g/L | 28.10 ± 3.42 | 34.20 ± 2.04 | 13.601 | <0.001 |
| CAR | 2.06 ± 1.51 | 0.53 ± 0.47 | 9.481 | <0.001 |
Multiple logistic regression analysis was performed with the indices with significant differences between the two groups as independent variables and the 28-day mortality in patients with severe pneumonia (No=0, Yes=1) as dependent variables. It was found that APACHE II score, NLR, Lac and CAR were independent risk factors for 28-day mortality in patients with severe pneumonia (p<0.05) (Figure 1).
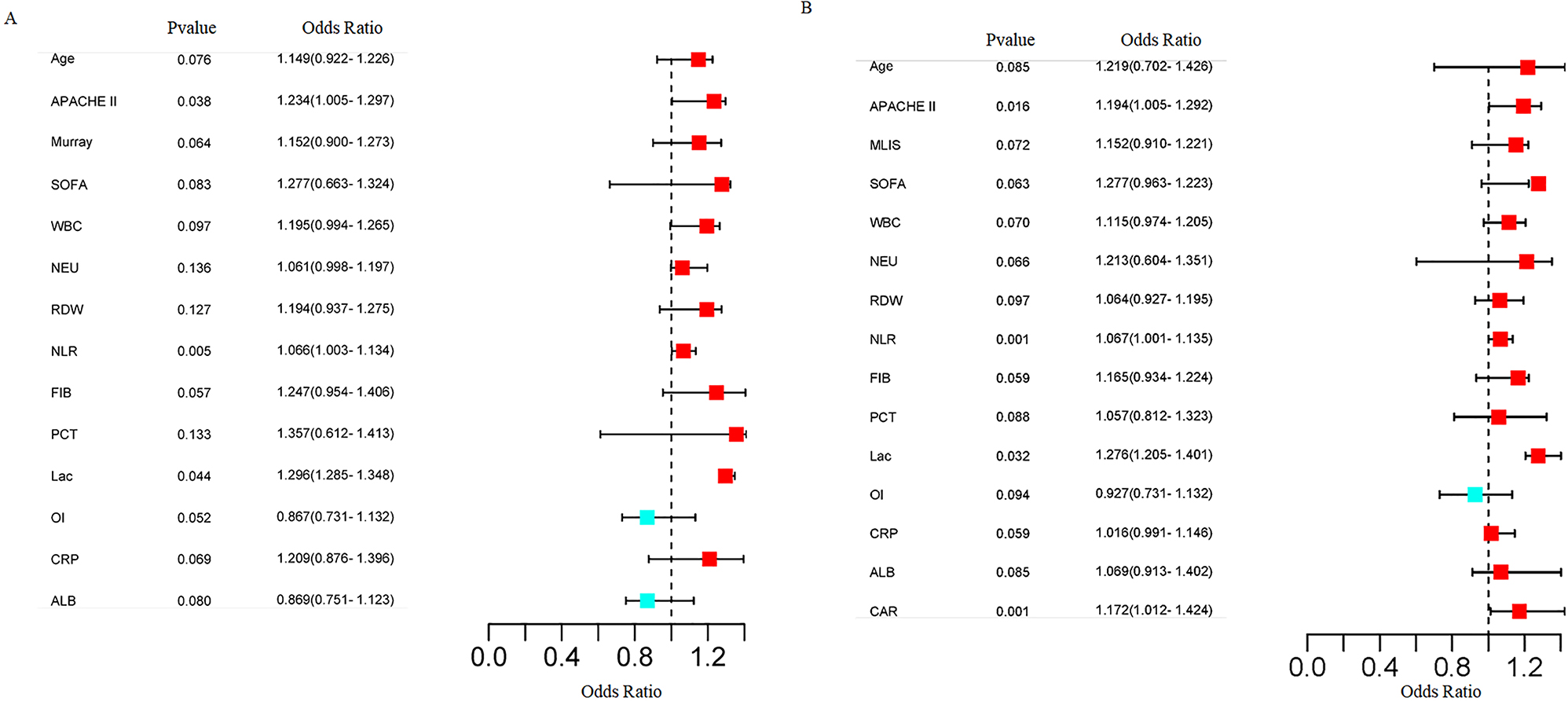
Forest map of multiple logistic regression analysis of independent influencing factors for 28-day mortality in patients with severe pneumonia. (A) Forest map without incorporating CAR; (B) Forest map incorporating CAR. Blue dots denote significant, independent risk factors; ranges denote confidence intervals.
Pearson’s correlation analysis indicated significant positive correlations among CAR, NLR, Lac and APACHE II score (p<0.05) (Figure 2).

Correlation analysis results of influencing factors.
The AUC of CAR for predicting the 28-day mortality risk in patients with severe pneumonia was 0.768 [95 % confidence interval (CI): 0.721–0.794], and the optimal cutoff value was 0.475, with sensitivity of 78.26 % and specificity of 70.75 % (Table 2 and Figure 3). These indicate that CAR has a significantly higher predictive value than other independent risk factors.
Predictive value for mortality risk in patients.
| Index | AUC | 95 % CI | Optimal cutoff value | Sensitivity | Specificity |
|---|---|---|---|---|---|
| APACHE II score | 0.693 | 0.597–0.713 | 0.296 | 52.54 % | 72.86 % |
| NLR | 0.702 | 0.684–0.732 | 0.453 | 71.16 % | 70.94 % |
| Lac | 0.642 | 0.607–0.695 | 0.278 | 78.95 % | 49.83 % |
| CAR | 0.768 | 0.721–0.794 | 0.475 | 78.36 % | 70.75 % |

Predictive value of each independent risk factor for mortality risk in patients with severe pneumonia.
A prediction model for 28-day mortality risk in patients with severe pneumonia was constructed, which included four independent risk factors (Figure 4). Each factor had a different weight in the model. and the corresponding scores for APACHE II, NLR, Lac and CAR were 48.52, 57.93, 55.23 and 65.24 points, respectively, with a total score of 226.92 points, indicating a 28-day mortality risk value of 0.73 in patients with severe pneumonia, and a mortality probability of 73 %.

Nomogram prediction model for 28-day mortality risk in patients with severe pneumonia.
The discrimination of the risk model was evaluated by ROC curve (Figure 5). AUC of the model was 0.826 (95 % CI: 0.803–0.847, p<0.001), with sensitivity and specificity of 88.45% and 87.32 %, respectively. Internal validation was conducted with the self-sampling method (Bootstrap). The C-index of the prediction model was calculated as 0.848 (95 % CI: 0.823–0.876). The results suggested a good discrimination (Figure 6).

ROC curve of prediction model.

Internal validation calibration curve of nomogram model.
Clinical decision curves demonstrated that when the risk threshold probability was 11–93 %, the nomogram model for predicting the 28-day mortality risk in patients with severe pneumonia could create net clinical benefits (Figure 7).

Clinical decision curves of nomogram model for predicting 28-day mortality risk in patients with severe pneumonia.
Discussion
At present, infectious diseases have emerged as one of the major contributors to death, and the mortality rate of severe pneumonia ranks No.1 among all types of pneumonia [11]. Although the guidelines for the treatment of severe pneumonia have been constantly updated in recent years, its morbidity and mortality rates remain high, seriously threatening people’s lives [12]. Hence, probing into the factors affecting the prognosis of patients with severe pneumonia is of great importance for evaluating the prognosis of patients. APACHE II score serves as an important scoring index to assess the prognosis of patients with inflammation and sepsis [13]. Nevertheless, due to cumbersome calculation, its clinical application is inconvenient. According to a study [14], many indices such as WBC, PCT, OI and MLIS can be adopted to assess the severity and prognosis of severe pneumonia. However, there are still controversies over these indices and they possess low value for predicting the prognosis of the disease [15]. Hence, it is of great importance to probe into ideal biomarkers with superior sensitivity and specificity for prognostic evaluation of severe pneumonia.
Inflammatory response occurs throughout the development of severe pneumonia. CAR, as a new marker of systemic inflammation, exhibits prognostic value for many diseases such as gastric cancer, acute coronary syndrome and respiratory failure. CAR is known as the combination of CRP and ALB, and can reflect the balance between the two. The value of CAR relies on the variation of CRP and ALB levels [16]. CRP, an acute phase reactive protein synthesized by hepatocytes, is largely present in the serum of patients with acute inflammation. A previous study has revealed that [17] CRP levels will rise to varying degrees during the progression of severe pneumonia, so dynamic monitoring of serum CRP levels is significantly important for judging the prognosis of severe pneumonia. ALB, as the most essential protein in human plasma, exerts important physiological functions in human bodies [18]. It has been uncovered that the joint use of ALB with treatment in patients with severe pneumonia can cut the fatality rate, suggesting a close relationship between serum ALB level and the prognosis of patients with severe pneumonia [19]. Considering the correlations of CRP and ALB with severe pneumonia, it was speculated that CRP is also associated with the prognosis of severe pneumonia. In this study, death group had a significantly higher CRP level and a significantly lower ALB level than survival group. Meanwhile, CAR was significantly higher in death group than that in survival group. Multiple logistic regression analysis demonstrated that CAR was an independent risk factor for 28-day mortality in patients with severe pneumonia, whereas CRP and ALB were not independent risk factors in the analysis. The above findings suggest that CAR (the combination of CRP and ALB) has a higher value for predicting the prognosis of severe pneumonia. The AUC of CAR for predicting the 28-day mortality in patients with severe pneumonia was 0.768, indicating a high predictive value. In addition, the calculation of CAR was relatively simple and costs less, so CAR can serve as an ideal marker for predicting the prognosis of severe pneumonia.
In addition, multiple logistic regression analysis results indicated that APACHE II score, Lac, and NLR were independent risk factors for 28-day mortality in patients with severe pneumonia. The level of Lac is mainly decided by the synthesis rate and metabolic rate of the liver and kidney. In some pathological conditions, lactic acid in vivo can rise due to tissue hypoxia [20]. Hence, determination of Lac level can reflect the severity of underlying diseases as well as the prognosis. NLR refers to NEU-to-LYM ratio, which can indicate the severity of inflammatory response in patients promptly [21]. Correlation analysis results suggested that Lac, NLR and APACHE II score of patients with severe pneumonia were significantly positively correlated with CAR, further illustrating the close association between CAR and the prognosis of severe pneumonia. Moreover, a certain synergistic effect was observed among Lac level, NLR and CAR in patients with severe pneumonia. To figure out the mechanism, it was speculated that the CRP level was increased in patients with severe pneumonia due to inflammatory response during the disease progression [22], leading to insufficient ALB synthesis and elevated CAR. As a result, lung tissue hypoxia is induced, which leads to lung injury and an increased Lac level. As the disease deteriorates, the inflammatory response is aggravated, activating neutrophils, and thereby raising NLR. At this time, all clinical indices of the patients are changed, eventually influencing the APACHE II score. In addition, on the basis of the above risk factors, a nomogram prediction model was constructed in this study, which displayed good discrimination and accuracy, and could provide references for clinical evaluation of the prognosis of patients with severe pneumonia.
Regardless, this study still has limitations. This is a single-center study with a small sample size, and we did not perform pathogen analysis, so some results may be biased. In the future, multicenter studies with larger sample sizes will be carried out to verify the results of this study.
Conclusions
In summary, we demonstrate CAR to be an independent prediction factor for 28-day mortality in patients with severe pneumonia. We hope our data helps to develop improved risk scoring systems for patients with severe respiratory infections in the future.
-
Research funding: None declared.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Competing interests: Authors state no conflict of interest.
-
Informed consent: Informed consent was obtained from all individuals included in this study.
-
Ethical approval: Research involving human subjects complied with all relevant national regulations, institutional policies and is in accordance with the tenets of the Helsinki Declaration (as revised in 2013), and has been approved by the authors’ Institutional Review Board.
References
1. Musher, DM, Abers, MS, Bartlett, JG. Evolving understanding of the causes of pneumonia in adults, with special attention to the role of pneumococcus. Clin Infect Dis 2017;65:1736–44. https://doi.org/10.1093/cid/cix549.Search in Google Scholar PubMed PubMed Central
2. Mothes, A, Léotard, S, Nicolle, I, Smets, A, Chirio, D, Rotomondo, C, et al.. Community-acquired pneumonia and positive urinary antigen tests: factors associated with targeted antibiotic therapy. Med Maladies Infect 2016;46:365–71. https://doi.org/10.1016/j.medmal.2016.05.009.Search in Google Scholar PubMed
3. Mizgerd, JP. Pathogenesis of severe pneumonia: advances and knowledge gaps. Curr Opin Pulm Med 2017;23:193–7. https://doi.org/10.1097/mcp.0000000000000365.Search in Google Scholar PubMed PubMed Central
4. Quinton, LJ, Walkey, AJ, Mizgerd, JP. Integrative Physiology of pneumonia. Physiol Rev 2018;98:1417–64. https://doi.org/10.1152/physrev.00032.2017.Search in Google Scholar PubMed PubMed Central
5. Liu, J, Han, P, Wu, J, Gong, J, Tian, D. Prevalence and predictive value of hypocalce mia in severe COVID-19 patients. J Infect Public Health 2020;13:1224–8. https://doi.org/10.1016/j.jiph.2020.05.029.Search in Google Scholar PubMed PubMed Central
6. Nair, GB, Niederman, MS. Updates on community acquired pneumonia management in the ICU. Pharmacol Ther 2021;217:107663. https://doi.org/10.1016/j.pharmthera.2020.107663.Search in Google Scholar PubMed PubMed Central
7. Rowland, MJ, Garry, P, Westbrook, J, Corkill, R, Antoniades, CA, Pattinson, KTS. Acute impairment of saccadic eye movements is associated with delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. J Neurosurg 2017;127:754–60. https://doi.org/10.3171/2016.8.jns16408.Search in Google Scholar PubMed
8. Lublinsky, S, Major, S, Kola, V, Horst, V, Santos, E, Platz, J, et al.. Early blood-brain barrier dysfunction predicts neurological outcome following aneurysmal subarachnoid hemorrhage. EBioMedicine 2019;43:460–72. https://doi.org/10.1016/j.ebiom.2019.04.054.Search in Google Scholar PubMed PubMed Central
9. Liu, G, Zhang, B, Zhang, S, Hu, H, Liu, T. LDH, CRP and ALB predict nucleic acid turn negative within 14 days in symptomatic patients with COVID-19. Scot Med J 2021;66:108–14. https://doi.org/10.1177/0036933021994243.Search in Google Scholar PubMed PubMed Central
10. Cao, B, Huang, Y, She, DY, Cheng, QJ, Fan, H, Tian, XL, et al.. Diagnosis and treatment of community-acquired pneumonia in adults: 2016 clinical practice guidelines by the Chinese Thoracic Society, Chinese Medical Association. Clin Res J 2018;12:1320–60. https://doi.org/10.1111/crj.12674.Search in Google Scholar PubMed PubMed Central
11. Cillóniz, C, Torres, A, Niederman, MS. Management of pneumonia in critically ill patients. Brit Med J 2021;375:e065871. https://doi.org/10.1136/bmj-2021-065871.Search in Google Scholar PubMed
12. Wongsurakiat, P, Chitwarakorn, N. Severe community-acquired pneumonia in general medical wards: outcomes and impact of initial antibiotic selection. BMC Pulm Med 2019;19:179. https://doi.org/10.1186/s12890-019-0944-1.Search in Google Scholar PubMed PubMed Central
13. Zhang, HF, Zhang, X, Sha, YX, Zhou, HQ, Pan, JH, Xun, X, et al.. Value of sTREM-1 in serum and bronchoalveolar lavage fluid, Apache II score, and SOFA score in evaluating the conditions and prognosis of children with severe pneumonia. Zhong Guo Dang Dai Er Ke Za Zhi 2020;22:626–31. https://doi.org/10.7499/j.issn.1008-8830.1912134.Search in Google Scholar PubMed PubMed Central
14. Ghahramani, S, Tabrizi, R, Lankarani, KB, Kashani, SMA, Rezaei, S, Zeidi, N, et al.. Laboratory features of severe vs. non-severe COVID-19 patients in Asian populations: a systematic review and meta-analysis. Eur J Med Res 2020;25:30. https://doi.org/10.1186/s40001-020-00432-3.Search in Google Scholar PubMed PubMed Central
15. Pan, F, Yang, L, Li, Y, Liang, B, Li, L, Ye, T, et al.. Factors associated with death outcome in patients with severe coronavirus disease-19 (COVID-19): a case-control study. Int J Med Sci 2020;17:1281–92. https://doi.org/10.7150/ijms.46614.Search in Google Scholar PubMed PubMed Central
16. Shen, Y, Wang, H, Li, W, Chen, J. Prognostic significance of the CRP/Alb and neutrophil to lymphocyte ratios in hepatocellular carcinoma patients undergoing TACE and RFA. J Clin Lab Anal 2019;33:e22999. https://doi.org/10.1002/jcla.22999.Search in Google Scholar PubMed PubMed Central
17. Raymond, F, Rola, KF, Nicola, M. Consecutive measuresof CRP correlate with length of hospital stay in patients with community-acquired pneumonia. Isr Med Assoc J 2018;20:345–8.Search in Google Scholar
18. Xia, X, Wen, M, Zhan, S, He, J, Chen, W. An increased neutrophil/lymphocyte ratio is an early warning signal of severe COVID-19. Nan Fang Yi Ke Da Xue Xue Bao 2020;40:333–6. https://doi.org/10.12122/j.issn.1673-4254.2020.03.06.Search in Google Scholar PubMed PubMed Central
19. Hsieh, WC, Aboud, A, Henry, BM, Omara, M, Lindner, J, Pirk, J. Serum albumin in patients undergoing transcatheter aortic valve replacement: a meta-analysis. Rev Cardiovasc Med 2019;20:161–9. https://doi.org/10.31083/j.rcm.2019.03.524.Search in Google Scholar PubMed
20. Liu, CG, Meng, S, Chu, YM, Lu, Y, Wang, PC. Severely high lactic acid in severe pneumonia patient: a case report. Clin Lab 2021;67:3. https://doi.org/10.7754/clin.lab.2020.200720.Search in Google Scholar PubMed
21. Stanski, NL, Wong, HR. Prognostic and predictive enrichment in sepsis. Nat Rev Nephrol 2020;16:20–31. https://doi.org/10.1038/s41581-019-0199-3.Search in Google Scholar PubMed PubMed Central
22. Soda, H, Ogawara, D, Fukuda, Y, Tomono, H, Okuno, D, Koga, S, et al.. Dynamics of blood neutrophil-related indices during nivolumab treatment may be associated with response to salvage chemotherapy for non-small cell lung cancer: a hypothesis-generating study. Thorac Cancer 2019;10:341–6. https://doi.org/10.1111/1759-7714.12952.Search in Google Scholar PubMed PubMed Central
© 2023 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Original Articles
- Differences in venous, capillary and interstitial glucose concentrations in individuals without diabetes after glucose load
- EDTA-associated pseudothrombocytopenia: definition and real-world occurrence
- Predictive value of C-reactive protein-to-albumin ratio for risk of 28-day mortality in patients with severe pneumonia
- Elevated plasma interleukin 21 is associated with higher probability and severity of idiopathic membranous nephropathy
- Performance evaluation of cobas pure integrated solutions at multiple sites in Europe and Asia
Articles in the same Issue
- Frontmatter
- Original Articles
- Differences in venous, capillary and interstitial glucose concentrations in individuals without diabetes after glucose load
- EDTA-associated pseudothrombocytopenia: definition and real-world occurrence
- Predictive value of C-reactive protein-to-albumin ratio for risk of 28-day mortality in patients with severe pneumonia
- Elevated plasma interleukin 21 is associated with higher probability and severity of idiopathic membranous nephropathy
- Performance evaluation of cobas pure integrated solutions at multiple sites in Europe and Asia

