Abstract
Objectives
Liver transplantation (LT) can benefit the long-term survival of hepatocellular carcinoma (HCC) patients. We hypothesized that circulating tumor cell (CTC) levels and subtypes are intimately associated with metastasis status of HCC patients. This study was designed to test that compositive hematological indices including CTC can provide a prediction of post-LT metastasis.
Methods
Between 2017 and 2018, 37 HCC patients within Hangzhou criteria receiving LT were included for analysis. The 24-month follow-up was mainly conducted by outpatient and telephone. Blood samples were collected, and hematological indices were examined. The outcomes such as PFS, recurrence, metastasis, location of recurrence/metastasis, and number of metastases were recorded.
Results
The follow-up analysis showed that microvascular invasion (MVI) classification at the baseline is associated with metastasis. Next, α-fetoprotein (AFP) level was another useful indicator of postoperative metastasis, especially at the third or fourth month; the protein induced by vitamin K absence or antagonist-II (PIVKA-II) level three months after LT was significantly higher for those who had later metastasis. The mesenchymal CTC level at the 45th day was increased for in the metastasis group. Using two-ends Logistic regression, the calculated value MP (metastasis predictor, by above factors). Had an AUC of 0.858 in the ROC curve, with a cutoff value of 0.328.
Conclusions
In conclusion, microvascular invasion, AFP level at the third or fourth month, PIVKA-II level at the third month, and mesenchymal CTC level at day 45 were associated with post-LT metastasis. Using Logistic regression based on above variables, the two-year metastasis can be predicted with satisfactory sensitivity and accuracy.
Introduction
Hepatocellular carcinoma (HCC) is a devastating malignant tumor, as one of the top common cancer and a primary cause of cancer-related mortality. Among all viable therapeutic options for HCC, liver transplantation (LT) can benefit the long-term survival of HCC patients, with satisfactory results of 1-, 5-, and 10-year survivals [1], for it addresses both HCC and underlying primary liver diseases (the major cause of HCC) at the same time. However, it is widely noticed that tumor may recur after LT, especially for patients with multinodular and large tumors [2]. Different criteria (e.g., Milan criteria) were established to distinguish who can benefit from LT, but there is insufficiency in metastasis prediction. Metastasis causes more than 90% of cancer mortality. Therefore, post-LT monitoring of metastasis merits attention in clinical practice. Many novel techniques have been proposed to estimate the recurrence and metastasis of HCC [3], and the forefront methods take advantages of the circulating tumor cell (CTC). Generally, presence of CTC implies metastasis to extra-hepatic organs. Several studies have claimed that the quantification of CTCs is an emerging tool for diagnosing, stratifying, and assessing extra-hepatic metastasis in HCC patients [4]. But for post-LT monitoring in HCC patients, the clinical value of CTC, as well as CTC subtypes, remains unclear. We hypothesized that CTC levels and subtypes are intimately associated with metastasis status of HCC patients, and this study was designed to test that available hematological indices including CTC data can provide sensitive and accurate prediction of post-LT metastasis.
Materials and methods
Patients
This study was approved by the Institutional Review Board of Shulan (Hangzhou) Hospital Affiliated to Zhejiang Shuren University Shulan International Medical College. For each patient, the informed consent had been acquired. Between 2017 and 2018, HCC patients who met the Hangzhou criteria and received LT were included for analysis. Immunosuppressive therapies were routinely given after surgery. Besides the base-line demographic and disease information, the data of surgical procedures, immunosuppressive treatments, and pre-LT anti-tumor treatments were prospectively recorded. The exclusion criteria were as follow: (1) combination with other tumors, (2) with possible metastasis before LT, (3) receiving re-transplantation, and (4) receiving combined liver and kidney transplantation. The validation cohort was composed of 37 patients, and their data were analyzed retrospectively. In particular, microvascular invasion (MVI) classification at the baseline was recorded.
Monitoring and follow-up
For discharged individuals, we conducted a 24-month follow-up by telephone, and the patients who received continuous hospital treatment were monitored every day. At 1, 3, 4, 6, 12 and 24 months after LT, patients were asked to receive regular ultrasonography and CT examination. As well, they were informed to receive examinations when they returned a visit to the hospital when necessary. MRI examination and PET-CT were further conducted towards a specific site if regular ultrasonography and CT examination implied some lesion deserving concerns. Using above examination, tumor recurrence or metastasis were confirmed. The follow-up lasted until the patient died or was finished at the 24th month after LT.
Blood samples were collected at appointed time and processed within 24 h. Before surgery and during the first 6 months, following hematological indices were examined. The α-fetoprotein (AFP) level, neutrophil to lymphocyte ratio (NLR), platelet level, and the protein induced by vitamin K absence or antagonist-II (PIVKA-II) level were tested preoperatively, after 1, 3, 4 and 6 months. AFP and PIVKA-II were determined through the routine ELISA methods in our hospital laboratory (using the ELISA kits of human AFP and human PIVKA-II). Additionally, the circulating tumor cell (CTC) counts (per mL), as well as different types of CTCs, were probed before surgery and at postoperative day 45 and 120. For CTC analysis, The Nano-Velcro CTC system was used which could capture total CTCs, epithelial type CTCs, mesenchymal CTCs (mCTC) and mixed type CTCs through the multi-marker capture cocktail. CTCs were immune stained using multi-color cytometric assessment.
For each included case, the outcomes such as PFS (months), metastasis (yes or no within 24 months), location of recurrence/metastasis, and number of metastases were recorded.
Statistical analysis
The SPSS software (25.0) was used for statistical analysis. Categorical variables expressed as frequencies (also as percent) were analyzed using Chi-squared test. Continuous data were expressed in mean ± standard deviation (SD). The t-test or One-way ANOVA was used for comparison of continuous data. A logistic regression was used to predict postoperative metastasis within 24 months based on associated factors. A p-value<0.05 was set as the significance level.
Results
Baseline features of enrolled patients
A total of 37 HCC patients (34 males and three females) were included in this study (with clinical characteristics shown in Table 1), with a medium age of 53 years (IQR 47–57). They had a medium period of 3.3 months (IQR 2–36) from diagnosed to LT. There were seven cases receiving surgery before LT, 13 cases treated by radiofrequency ablation (RFA) before LT, 14 cases with a history of transcatheter arterial chemoembolization (TACE), and four cases receiving targeted therapy before LT. Besides, there were one case in stage I, 15 cases in stage II, 14 cases in stage III, and two cases in stage IV.
Clinical characteristics of included patients.
| Number or mean | % or SD | ||
|---|---|---|---|
| Sex | Male | 34 | 91.9% |
| Female | 3 | 9.1% | |
| Age, year | 51.89 | 9.439 | |
| Diagnosis to LT period, months | 22.36 | 34.61 | |
| Stage | Unknown | 5 | 13.5% |
| I | 1 | 2.7% | |
| II | 15 | 40.5% | |
| III | 14 | 37.8% | |
| IV | 2 | 5.4% | |
| Surgery before LT | No | 30 | 81.1% |
| Yes | 7 | 18.9% | |
| RFA before LT | No | 24 | 64.9% |
| Yes | 13 | 35.1% | |
| TACE before LT | No | 23 | 62.2% |
| Yes | 14 | 37.8% | |
| Targeted therapy before LT | No | 33 | 89.2% |
| Yes | 4 | 10.8% | |
-
LT, liver transplantation; RFA, radiofrequency ablation; TACE, transcatheter arterial chemoembolization.
Factors associated with postoperative metastasis
According to our follow-up observation, a total of eight patients were found to have postoperative metastasis in 24 months; and different factors were associated with postoperative metastasis. No differences in sex distribution and ages were found between two groups. As Table 1 shows, MVI classification at the baseline is associated with metastasis. There are three MVI classifications: MVI-0 (no microvascular invasion), MVI-1 (≤5 MVI counts, and it occurred near the cancer ≤1 cm), MVI-2 (>5 MVI counts, and it occurred far from the cancer >1 cm). As expected, higher grade means more frequent metastasis in the following 24 months, and the whole MVI-2 group were found metastasis (p<0.05). Next, AFP level was another useful indicator of postoperative metastasis (Tables 2 and 3), especially at the third or fourth month (p<0.01), while the postoperative NLR or platelet level was not associated with the metastasis outcome (Tables 4 and 5, p>0.05 at each time point). Interestingly, the PIVKA-Ⅱ level 3 months after LT was significantly higher for those who had later metastasis (Table 6, p<0.05). However, the four-/six-month levels did not show any predictive value. Further, the total CTC, as well as different subtypes, exhibited no correlation with metastasis, except the mesenchymal CTC level at the 45th day (mCTC45d) (Table 7).
Association between microvascular invasion classification and postoperative metastasis.
| No metastasis | Metastasis | |||
|---|---|---|---|---|
| MVI classification | 0 | Number | 8 | 0 |
| % | 27.6% | 0.0% | ||
| 1 | Number | 6 | 0 | |
| % | 20.7% | 0.0% | ||
| 2 | Number | 15 | 8 | |
| % | 51.7% | 100.0% | ||
| Total | Number | 29 | 8 | |
| Chi-square | 6.213 | p-Value | 0.0447 | |
Association between AFP levels (ng/mL) and postoperative metastasis.
| Time points | No metastasis, n=27–29 | Metastasis, n=7–8 | t | p-Value |
|---|---|---|---|---|
| Preoperative | 348.17 ± 1,462.96 | 552.50 ± 888.19 | −0.351 | 0.728 |
| Postoperative 1 M | 159.53 ± 796.77 | 29.462 ± 51.76 | 0.457 | 0.651 |
| Postoperative 3 M | 5.33 ± 7.83 | 74.62 ± 137.59 | −2.802 | 0.008a |
| Postoperative 4 M | 5.27 ± 10.51 | 99.94 ± 180.06 | −2.924 | 0.006a |
| Postoperative 6 M | 5.42 ± 11.20 | 141.43 ± 341.11 | −2.207 | 0.034a |
-
ap-Value <0.05 indicates statistical significance.
Association between NLR levels (%) and postoperative metastasis.
| Time points | No metastasis, n=27–29 | Metastasis, n=7–8 | t | p-Value |
|---|---|---|---|---|
| Preoperative | 3.65 ± 2.94 | 5.39 ± 2.44 | −1.526 | 0.136 |
| Postoperative 1 M | 4.86 ± 3.28 | 4.70 ± 2.89 | 0.112 | 0.912 |
| Postoperative 3 M | 2.30 ± 0.94 | 2.38 ± 1.74 | −0.161 | 0.911 |
| Postoperative 4 M | 2.03 ± 1.20 | 1.98 ± 0.87 | 0.120 | 0.905 |
| Postoperative 6 M | 2.40 ± 1.14 | 2.34 ± 0.97 | 0.135 | 0.893 |
Association between platelet levels and postoperative metastasis.
| Time points | No metastasis, n=27–29 | Metastasis, n=7–8 | t | p-Value |
|---|---|---|---|---|
| Preoperative | 122.79 ± 111.31 | 152.75 ± 99.41 | 0.688 | 0.496 |
| Postoperative 1 M | 185.55 ± 96.18 | 152.75 ± 53.05 | 0.920 | 0.364 |
| Postoperative 3 M | 133.59 ± 66.98 | 119.25 ± 38.01 | 0.576 | 0.568 |
| Postoperative 4 M | 125.38 ± 64.52 | 117.38 ± 46.31 | 0.327 | 0.746 |
| Postoperative 6 M | 129.68 ± 63.64 | 99.14 ± 23.46 | 1.237 | 0.225 |
Association between PIVKA-Ⅱ levels and postoperative metastasis.
| Time points | No metastasis, n=27–29 | Metastasis, n=7–8 | t | p-Value |
|---|---|---|---|---|
| Preoperative | 6,550.28 ± 16,614.56 | 7,468.88 ± 10,681.92 | 0.147 | 0.884 |
| Postoperative 1 M | 60.07 ± 142.97 | 24.13 ± 21.05 | 0.702 | 0.487 |
| Postoperative 3 M | 24.86 ± 7.75 | 47.13 ± 55.89 | 2.149 | 0.039a |
| Postoperative 4 M | 43.45 ± 72.61 | 81.50 ± 133.00 | 1.082 | 0.286 |
| Postoperative 6 M | 42.31 ± 59.54 | 116.00 ± 205.46 | 1.737 | 0.091 |
-
ap-Value <0.05 indicates statistical significance.
Association between mesenchymal CTC levels and postoperative metastasis.
| Time points | No metastasis, n=27–29 | Metastasis, n=7–8 | t | p-Value |
|---|---|---|---|---|
| Preoperative | 1.69 ± 2.35 | 1.38 ± 1.41 | 0.3595 | 0.7213 |
| Postoperative 1.5 M | 0.83 ± 0.97 | 2.88 ± 2.95 | 3.2516 | 0.0025a |
| Postoperative 4 M | 1.14 ± 1.36 | 1.33 ± 1.53 | 0.2356 | 0.8154 |
-
ap-Value <0.05 indicates statistical significance.
Prediction of two-year metastasis
Based on above analysis, three variables had the closest relationship with two-year metastasis: mCTC45d, the AFP level at the fourth month (AFP4M), and the PIVKA-Ⅱ level at the third month (PIVKA-Ⅱ3M). Using the logistic regression (two-ends), we named a variable MP (metastasis predictor), and it was calculated by above three factors using the logistic regression. As the ROC curve shown (Figure 1), the AUC of MP was 0.858, with a cutoff value of 0.328 (sensitivity=0.75, specificity=0.97).
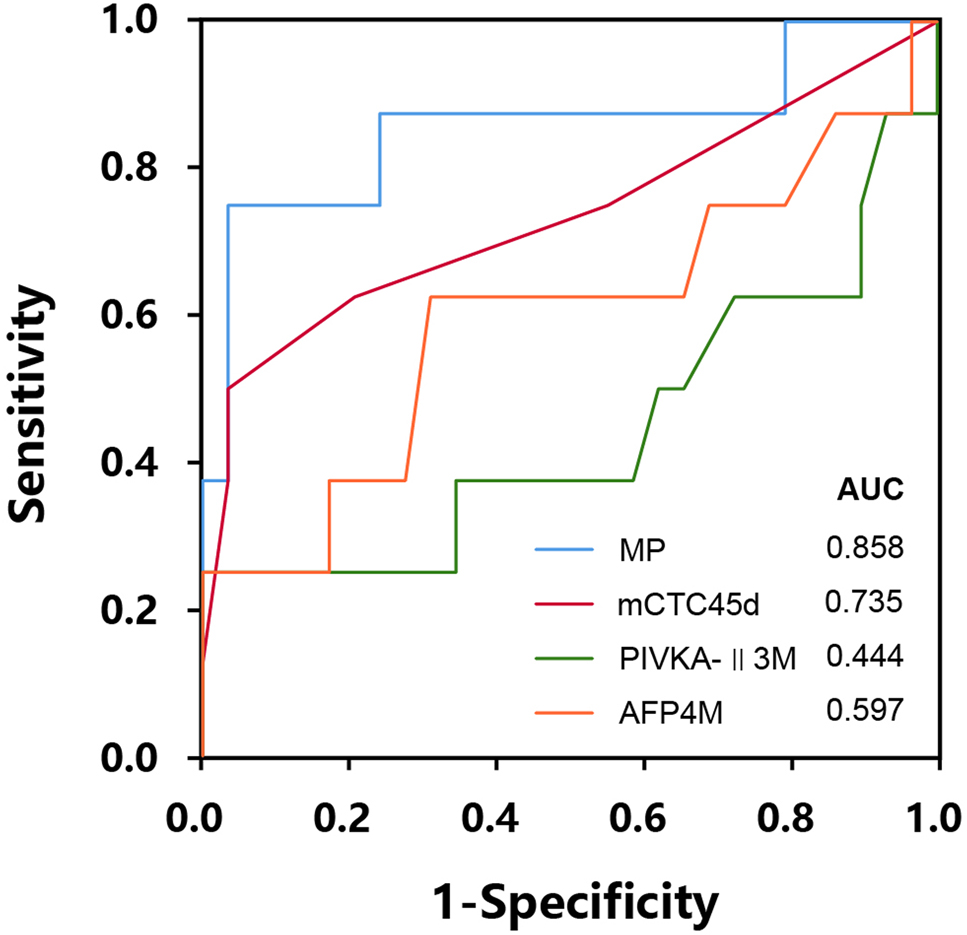
Receiver operating characteristic (ROC) curve for efficacy analysis of risk factors in post-LT metastasis in two years.
The variable MP is calculated by the two-ends logistic regression model, based on variables including mesenchymal CTC level at the 45th day (mCTC45d), the AFP level at the fourth month (AFP4M), and the PIVKA-Ⅱ level at the third month (PIVKA-II3M).
Discussion
There have been a few widely known predictive factors for survival and tumor progression in HCC patients with LT, such as cumulative tumor size [5], histological grade of differentiation [6], vascular invasion [7], lymph node involvement [8], higher TNM stages [9], and high dosage of cyclosporine [10]. In particular, microvascular invasion, macrovascular invasion, tumor number, and total tumor diameter were the strongest prognostic factors in Chinese patients [11]. In the aspect of postoperative monitoring, different hematological indices have also been investigated (e.g., platelet counts [12], CD133 and CD90 [3]). Moreover, some prophylactic treatments (e.g., Licartin administration, Sorafenib treatment) are helpful for blocking HCC recurrence or metastasis [13]. Theoretically, CTCs may serve as a liquid biopsy for metastatic tumors, with adequate and unique advantages, that the collection of peripheral blood is easy, rapid, lowly invasive, cost-effective, and feasible for serial real-time monitoring. Currently, the index CTC (especially mCTC) has seldom used in monitoring metastasis or recurrence after LT. We here for the first time found that combination of CTC/AFP/PIVKA-Ⅱ can effectively indicate the later metastasis. In particular, hematological indexes from day 45 to 4 months are useful references in prediction of LT metastasis.
However, we failed to find clear links between post-LT metastasis and traditional factors, such as Milan criteria, Child-Pugh-Turcotte classification, lymph node involvement, cirrhosis background or age [14]. Also, prophylactic treatments after surgery did not show significant benefits in our work. This may be due to a limited sample size. Overall, the positive results we discovered are consistent with most published research. Similar to the study of Zavaglia [6], we also noticed microvascular invasion classification influenced the postoperative metastasis. Nevertheless, this variable has a relatively lower association (p=0.045) in comparison with postoperative hematological indices. AFP above 100 or 200 ng/mL indicates poorer outcomes, as reported by other independent studies [15, 16]. We also noticed that AFP monitoring helps reflect the postoperative progression of HCC. Especially, the value at the time point of the fourth month is more valuable. PIVKA-II is an important biomarker for HCC surveillance in conjunction with AFP [17]. AFP and PIVKA-II are associate with giant lymph node metastasis in hepatoid gastric cancer [18]. Some study even claimed that the AFP level, PIVKA-II level, portal vein invasion were the most important prognostic factors for HCC patients [19]. Ultra-high PIVKA-II level is correlated with hepatic portal lymph node metastasis [20]. Our work firstly demonstrated that the PIVKA-II level at postoperative three month is elevated in the metastasis group after LT, which emphasizes its value of metastasis monitoring.
Still, there are some limitations in this study. For the scarcity of HCC patients with LT treatment, the sample size was small, and no significant results were found regarding the progression free survival and overall survival. Besides, also for the limited sample size, we did not observe a regular trend of changes in hematological indices. Some indices were significantly changed at one time point (e.g., the third month) but turned negative at another (e.g., the fourth month) [21], [22], [23], [24], [25]. Although the significantly changed time point may be a key stage of post-LT metastasis (e.g., at round day 45), the clear trend, changing curves, and burst-out time point of metastasis are still to be explored. Moreover, we found there was a relationship between the mesenchymal CTC level at postoperative 1.5 M and postoperative metastasis; however, no difference was found at later period (4 M). This may be due to that the level at 1.5 M is more sensitive before a fully developed metastasis. But the sample size in the metastasis group was small, so the mesenchymal CTC level at 1.5 M was even higher than 4 M. This association (at 1.5 M) needs more data to confirm.
In conclusion, microvascular invasion, AFP level at the third or fourth month, PIVKA-Ⅱ level at the third month, and mesenchymal CTC level at day 45 were associated with post-LT metastasis. Using the logistic regression based on above variables, the two-year metastasis can be predicted with satisfactory sensitivity and accuracy. We recommend that in the clinical practice of preventing/monitoring metastasis, AFP level at the third or fourth month, PIVKA-Ⅱ level at the third month, and mesenchymal CTC level at day 45 can be paid special attention.
Funding source: Health Commission of Zhejiang Province
Award Identifier / Grant number: 2016KYA073
Funding source: The National Major Science and Technology Projects of China
Award Identifier / Grant number: 2017ZX10203201
-
Research funding: The study was approved by the National Major Science and Technology Projects of China (No. 2017ZX10203201) and Health Commission of Zhejiang Province (2016KYA073).
-
Author contributions: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Writing – original draft: Li Zhuang; Conceptualization, Supervision, Writing – review & editing: Shu-Sen Zheng; Data curation, Methodology, Investigation: Xiang-Yan Liu; Data curation, Formal analysis, Investigation: Heng-Kai Zhu; Data curation, Investigation: Zhuo-Yi Wang, Wu Zhang; Writing – review & editing: Guo-Ping Jiang. All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Competing interests: Authors state no conflict of interest.
-
Informed consent: For each patient, the informed consent had been acquired.
-
Ethical approval: This study was approved by the Institutional Review Board of Shulan (Hangzhou) Hospital Affiliated to Zhejiang Shuren University Shulan International Medical College.
References
1. Vitale, A, Gringeri, E, Valmasoni, M, D’Amico, F, Carraro, A, Pauletto, A, et al.. Long-term results of liver transplantation for hepatocellular carcinoma: an update of the University of Padova experience. Transplant Proc 2007;39:1892–4. https://doi.org/10.1016/j.transproceed.2007.05.031.Search in Google Scholar
2. Herrero, JI, Sangro, B, Quiroga, J, Pardo, F, Herraiz, M, Cienfuegos, JA, et al.. Influence of tumor characteristics on the outcome of liver transplantation among patients with liver cirrhosis and hepatocellular carcinoma. Liver Transplant 2001;7:631–6. https://doi.org/10.1053/jlts.2001.25458.Search in Google Scholar
3. Yao, Y, Li, Y, Liu, Q, Zhou, K, Zhao, W, Liu, S, et al.. Rapid detection of hepatocellular carcinoma metastasis using reverse transcription loop-mediated isothermal amplification. Talanta 2020;208:120402. https://doi.org/10.1016/j.talanta.2019.120402.Search in Google Scholar
4. Min, AL, Choi, JY, Woo, HY, Kim, JD, Kwon, JH, Bae, SH, et al.. High expression of snail mRNA in blood from hepatocellular carcinoma patients with extra-hepatic metastasis. Clin Exp Metastasis 2009;26:759–67. https://doi.org/10.1007/s10585-009-9275-6.Search in Google Scholar
5. Fan, ZC, Yan, J, Liu, GD, Tan, XY, Weng, XF, Wu, WZ, et al.. Real-time monitoring of rare circulating hepatocellular carcinoma cells in an orthotopic model by in vivo flow cytometry assesses resection on metastasis. Cancer Res 2012;72:2683–91. https://doi.org/10.1158/0008-5472.can-11-3733.Search in Google Scholar
6. Yin, LC, Luo, ZC, Gao, YX, Li, Y, Peng, Q, Gao, Y. Twist expression in circulating hepatocellular carcinoma cells predicts metastasis and prognoses. Biomed Res Int 2018;2018:3789613. https://doi.org/10.1155/2018/3789613.Search in Google Scholar
7. Merli, M, Nicolini, G, Gentili, F, Novelli, G, Iappelli, M, Casciaro, G, et al.. Predictive factors of outcome after liver transplantation in patients with cirrhosis and hepatocellular carcinoma. Transplant Proc 2005;37:2535–40. https://doi.org/10.1016/j.transproceed.2005.06.031.Search in Google Scholar
8. Reyes, JD, Carr, B, Dvorchik, I, Kocoshis, S, Jaffe, R, Gerber, D, et al.. Liver transplantation and chemotherapy for hepatoblastoma and hepatocellular cancer in childhood and adolescence. J Pediatr 2000;136:795–804. https://doi.org/10.1016/s0022-3476(00)44469-0.Search in Google Scholar
9. Zavaglia, C, De Carlis, L, Alberti, AB, Minola, E, Belli, LS, Slim, AO, et al.. Predictors of long-term survival after liver transplantation for hepatocellular carcinoma. Am J Gastroenterol 2005;100:2708–16. https://doi.org/10.1111/j.1572-0241.2005.00289.x.Search in Google Scholar PubMed
10. Fan, J, Yang, GS, Fu, ZR, Peng, ZH, Xia, Q, Peng, CH, et al.. Liver transplantation outcomes in 1,078 hepatocellular carcinoma patients: a multi-center experience in Shanghai, China. J Cancer Res Clin Oncol 2009;135:1403–12. https://doi.org/10.1007/s00432-009-0584-6.Search in Google Scholar PubMed
11. Neuhaus, P, Jonas, S. Liver transplantation in hepatocellular carcinoma: new developments. Praxis (Bern 1994) 2002;91:1396–400. https://doi.org/10.1024/0369-8394.91.35.1396.Search in Google Scholar PubMed
12. Vivarelli, M, Bellusci, R, Cucchetti, A, Cavrini, G, De Ruvo, N, Aden, AA, et al.. Low recurrence rate of hepatocellular carcinoma after liver transplantation: better patient selection or lower immunosuppression? Transplantation 2002;74:1746–51. https://doi.org/10.1097/00007890-200212270-00017.Search in Google Scholar PubMed
13. Li, J, Yan, LN, Yang, J, Chen, ZY, Li, B, Zeng, Y, et al.. Indicators of prognosis after liver transplantation in Chinese hepatocellular carcinoma patients. World J Gastroenterol 2009;15:4170–6. https://doi.org/10.3748/wjg.15.4170.Search in Google Scholar PubMed PubMed Central
14. Han, S, Lee, S, Yang, JD, Leise, MD, Ahn, JH, Kim, S, et al.. Risk of posttransplant hepatocellular carcinoma recurrence is greater in recipients with higher platelet counts in living donor liver transplantation. Liver Transplant 2018;24:44–55. https://doi.org/10.1002/lt.24961.Search in Google Scholar PubMed
15. Xu, J, Shen, ZY, Chen, XG, Zhang, Q, Bian, HJ, Zhu, P, et al.. A randomized controlled trial of Licartin for preventing hepatoma recurrence after liver transplantation. Hepatology 2007;45:269–76. https://doi.org/10.1002/hep.21465.Search in Google Scholar PubMed
16. Yan, J, Tan, C, Gu, F, Jiang, J, Xu, M, Huang, X, et al.. Sorafenib delays recurrence and metastasis after liver transplantation in a rat model of hepatocellular carcinoma with high expression of phosphorylated extracellular signal-regulated kinase. Liver Transplant 2013;19:507–20. https://doi.org/10.1002/lt.23619.Search in Google Scholar PubMed
17. Zheng, SS, Xu, X, Liang, TB, Wang, WL, Shen, Y, Zhang, M, et al.. Liver transplantation for hepatocellular carcinoma: prognostic analysis of 89 cases. Zhonghua Wai Ke Za Zhi 2005;43:450–4.Search in Google Scholar
18. Adler, M, De Pauw, F, Vereerstraeten, P, Fancello, A, Lerut, J, Starkel, P, et al.. Outcome of patients with hepatocellular carcinoma listed for liver transplantation within the Eurotransplant allocation system. Liver Transplant 2008;14:526–33. https://doi.org/10.1002/lt.21399.Search in Google Scholar PubMed
19. Varona, MA, Soriano, A, Aguirre-Jaime, A, Garrido, S, Oton, E, Diaz, D, et al.. Risk factors of hepatocellular carcinoma recurrence after liver transplantation: accuracy of the alpha-fetoprotein model in a single-center experience. Transplant Proc 2015;47:84–9. https://doi.org/10.1016/j.transproceed.2014.12.013.Search in Google Scholar PubMed
20. Lee, JH, Cho, Y, Kim, HY, Cho, EJ, Lee, DH, Yu, SJ, et al.. Serum tumor markers provide refined prognostication in selecting liver transplantation candidate for hepatocellular carcinoma patients beyond the milan criteria. Ann Surg 2016;263:842–50. https://doi.org/10.1097/sla.0000000000001578.Search in Google Scholar
21. Orita, K, Sakamoto, A, Okamoto, T, Matsuda, S. Solitary muscle metastasis of hepatocellular carcinoma to the biceps femoris muscle with only elevated serum PIVKA-II: a case report. Am J Case Rep 2019;20:306–9. https://doi.org/10.12659/ajcr.913730.Search in Google Scholar
22. Iso, Y, Sawada, T, Shimoda, M, Rokkaku, K, Ohkura, Y, Kubota, K. Solitary AFP- and PIVKA-II-producing hepatoid gastric cancer with giant lymph node metastasis. Hepatogastroenterology 2005;52:1930–2.Search in Google Scholar
23. Kim, JM, Kwon, CH, Joh, JW, Park, JB, Lee, JH, Kim, SJ, et al.. Intrahepatic metastasis is more risky than multiple occurrence in hepatocellular carcinoma patients after curative liver resection. Hepatogastroenterology 2015;62:399–404.Search in Google Scholar
24. Ishiyama, K, Onoe, T, Kubota, H, Kojima, M, Hadano, N, Tazawa, H, et al.. Surgical resection of hepatic portal lymph node metastasis after repeated treatment for hepatocellular carcinoma recurrence. Gan To Kagaku Ryoho 2018;45:2003–5.Search in Google Scholar
25. Matsuda, Y, Ichida, T, Fukumoto, M. Hepatocellular carcinoma and liver transplantation: clinical perspective on molecular targeted strategies. Med Mol Morphol 2011;44:117–24. https://doi.org/10.1007/s00795-011-0547-2.Search in Google Scholar PubMed
© 2021 Li Zhuang et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Obituary
- In Memoriam of Beniam Ghebremedhin
- Review
- New and emerging technologies for the diagnosis of urinary tract infections
- Original Articles
- Blood indices and circulating tumor cells for predicting metastasis of hepatocellular carcinoma after liver transplantation
- Comparison of APACHE II scores and mortality with CRP/albumin, neutrophil/lymphocyte and thrombocyte/lymphocyte ratios in patients admitted to internal medicine and anesthesia reanimation intensive care unit
- The correlation between the prevalence of gestational diabetes mellitus and maternal age in Southern China
- The investigation of serum protein profiles in anal fistula for potential biomarkers
- Evaluation of antioxidant and antiinflammatory activity of ethanolic extracts of Polygonum senticosum in lipopolysaccharide-induced RAW 264.7 macrophages
- Prevalence of SARS-CoV-2 antibodies in hospital employees, Central Germany
- Fast forward evolution in real time: the rapid spread of SARS-CoV-2 variant of concern lineage B.1.1.7 in Saxony-Anhalt over a period of 5 months
- Letters to the Editor
- It did not stop there: rapid substitution of circulating SARS-CoV-2 variant of concern B.1.1.7 (Alpha) by variant of concern B.1.617.2 (Delta) and further evolution of different Delta sublineages in Southern Saxony-Anhalt in late summer 2021
- In reply to a review article “Review of potentials and limitations of indirect approaches for estimating reference limits/intervals of quantitative procedures in laboratory medicine”
- Reply to the letter of Katayev and Fleming
Articles in the same Issue
- Frontmatter
- Obituary
- In Memoriam of Beniam Ghebremedhin
- Review
- New and emerging technologies for the diagnosis of urinary tract infections
- Original Articles
- Blood indices and circulating tumor cells for predicting metastasis of hepatocellular carcinoma after liver transplantation
- Comparison of APACHE II scores and mortality with CRP/albumin, neutrophil/lymphocyte and thrombocyte/lymphocyte ratios in patients admitted to internal medicine and anesthesia reanimation intensive care unit
- The correlation between the prevalence of gestational diabetes mellitus and maternal age in Southern China
- The investigation of serum protein profiles in anal fistula for potential biomarkers
- Evaluation of antioxidant and antiinflammatory activity of ethanolic extracts of Polygonum senticosum in lipopolysaccharide-induced RAW 264.7 macrophages
- Prevalence of SARS-CoV-2 antibodies in hospital employees, Central Germany
- Fast forward evolution in real time: the rapid spread of SARS-CoV-2 variant of concern lineage B.1.1.7 in Saxony-Anhalt over a period of 5 months
- Letters to the Editor
- It did not stop there: rapid substitution of circulating SARS-CoV-2 variant of concern B.1.1.7 (Alpha) by variant of concern B.1.617.2 (Delta) and further evolution of different Delta sublineages in Southern Saxony-Anhalt in late summer 2021
- In reply to a review article “Review of potentials and limitations of indirect approaches for estimating reference limits/intervals of quantitative procedures in laboratory medicine”
- Reply to the letter of Katayev and Fleming

