Abstract
With big data algorithms and artificial intelligence (AI) at stake the optimal assembly of the most appropriate lab assays selected to diagnose, treat and follow up patients suffering from well-delineated disease may get lost. The physician ordering a lab test, instead of asking for a good composition of screening tests is tempted to order a large number of assays, including genome sequencing hoping to find the diagnostic evidence for his/her patient at once. Four major specialities of medical laboratory assays, i.e. clinical chemistry, hematology, immunology and microbiology are embraced by genome sequencing techniques and have attained the degree of robotics, facilitating assays to such a degree, that the prescriber is free of concern as to how costly/complicated an investigation might become. Diagnostics with autoimmune diseases is not an exemption and autoantibody screening using multiplex assays or therapeutic drug monitoring to adjust treatments of inflammatory/autoimmune diseases is bound to become more and more informative even more so as the pharmacodynamics of modern pharmaceutical agents are explored. As the most appropriate therapeutical agents to monitor in the lab, biological response modifiers, immunosuppressants and monoclonal antibodies are at the forefront and we need to explore their efficacy and side effect profiles not only using phase III clinical studies but also by using postmarketing surveillance. Behind the profiles provided by big data and artificial intelligence, the therapeutically-induced regained immune balance can thus be traced to the single best lab assay. The next decade promises a series of new assays, e.g. inflammasome profiles, lymphocyte markers by fluorescence activated cell sorters as well as single cell secretome analysis.
Zusammenfassung
Bei aller Technikaffinität für die richtige Diagnose bleiben vom Arzt erhobene Anamnese und klinischen Befunde wichtige Pfeiler zur Einleitung der Behandlung einer Krankheit. Dazu kommt aber heute unabdingbar die Computer-Unterstützung – anfänglich mit der Anhäufung von grossen Datenmengen nicht nur aus dem medizinischen Labor sondern aus Bildgebung, Histopathologie, und Ergebnissen aus Gen- und FACS- Analysen peripherer Blutzellen. Das reichliche Angebot von Laboranalysen ist derzeit so verlockend gross, dass der Auftrag-gebende Arzt der Versuchung oft nicht widerstehen kann, eine exzessive Anzahl Marker-Analysen aus einem Tropfen Blut zu verlangen. Die generierten Datenmengen bergen jedoch die Gefahr, dass das Bestellmuster von Analysen vom Leidensmuster des Patienten abweichen wird. Hier setzt die künstliche Intelligenz (AI: artificial intelligence) den Griffel an: dem geduldigen Computer werden Anamnese- und Untersuchungs-relevante Daten eingespeist und der Algorhythmus berechnet Wahrscheinlichkeiten mit integrierten Grundannahmen, in welche Richtung die Laboranalysen der klinischen Diagnostik und Therapie hilfreich sein würden. Die vorliegende Arbeit versucht diese Entscheidungswege für Arzt und Patient am Beispiel der Autoimmun-Krankheiten zu konkretisieren. In einem solchen Fach wie auch in der Krebsbehandlung mit der Immune Checkpoint – Identifizierung entsteht dank der derzeit unaufhörlichen Entwicklung monoklonaler Antikörper für Diagnostik und Therapie eine zusätzliche Ebene mit grossen Datenmengen – ohne Bioinformatik ist die moderne Medizin undenkbar geworden.
Reviewed Publication:
Sack U. Redaktion Conrad K.
Brief summary
To understand the complexity of autoimmunity, big data processing by artificial intelligence (AI) is on the rise. AI algorithms serve physicians and the lab test validation community for improved diagnosis. The potential drawbacks which AI might bring to the diagnosis of autoimmune diseases, with their overlap into inflammatory conditions, are exposed here in the light of recent progress in lab techniques.
Introduction
Autoimmunity is pre-eminently a dialectical form of immunity. The reason is in no way obscure: each moment in life, innate and, on its pathway, acquired and synonymous: adaptive, immune functions must decide between self and foreign – practically a creative effort. Conditional acceptance of autoimmunity in global health by autoimmunity networking (www.autoimmunity-network.com) is no more justified the more so as autoimmune diseases, in the chapter of non-communicable diseases have become a global health issue attracting worldwide attention. At least, assertive analysis of this field lets discussants go into the depths of the science designed to improve the care of patients.
The components taking part in the overdrive of the immune system, with the now well-recognized excess of T helper 17 (TH17) cells in autoimmune diseases including the cytokine signaling molecules they produce, have become a sizeable workload for medical laboratories – more so for diagnostic reasons than for therapeutic drug monitoring (TDM) and toxicity prediction.
With over 100 nosological entities, most of them non-communicable rare diseases, the autoimmune diseases constitute a chapter of disorders now accessible for efficient treatment. Biomarker and genomic identifications have improved our insight in immunopathological disease triggers and efficient immuno-suppressive or -modulating drugs, including polyclonal antibodies from intravenous immunoglobulin (IVIG) hyperimmunoglobulins and highly specific monoclonal antibodies produced by cell clones which revolutionize the approach on how we treat some of the more frequent autoimmune diseases (Figure 1). Before instigating treatment schedules, one strives for optimal definition/ascertainment of the diagnosis and this includes a series of more or less reliable laboratory assays. Understanding from lab validation helps to delineate the hypochondriac’s uncertainty.
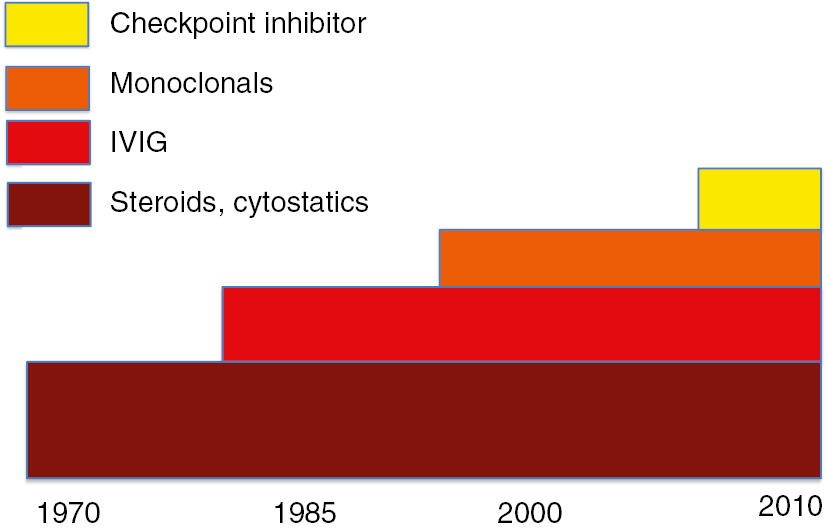
Tempestuous development of treatment possibilities for autoimmune disorders.
Within the last 50 years first line treatments with immunsuppressive agents have been completed with IVIG, monoclonal antibodies, biosimilars, calcineurin inhibitors and checkup inhibitors.
Systems biology includes systems immunology and a plethora of data provides information on the interaction and balance of immune reactions. Single immune cell phenotyping and genotyping (“proteogenomics”) now can use marker antibodies on cells that are run through microfluidic droplet-based systems: the marker intensity on a single cell provides for deep phenotyping and is used for functional immune cell genomics [1]. With code bars and cell tags inserted by retroviruses, mosaic patterns emerge which allow tracing of cell lineages: germinal centers can then be depicted by multicolor mosaics. Antigen-specific T cell profiling can be strengthened using major histocompatibility complex (MHC) tetramers and in this context, one is now able to isolate IgG molecules and resolve single molecule peptide sequences using mass spectrometry (MS)/laser microprobe mass spectrometry (LM-MS). Bioinformatics thus resolves the serum IgG repertoire by machine learning which is programmable to deploy antibodies, including autoantibodies using AI.
Autoantibody formation in many patients often comes as a surprise (Table 1). The isotypes are found in all classes and subclasses, IgM, IgD, IgG 1–4, IgA 1–2 and IgE whereby the heavy-chain constant regions of all antibody molecules of one isotype or subtype have the same amino acid sequence. Antigens that constitute different inherited alleles of Ig molecules are called allotopes; antibodies sharing the same allotopes belong to the same allotype. Finally, the unique antigenic determinants of individual antibody clones that arise both from inherited germ line diversity and from somatic events are called idiotypes. Unlike allotopes, idiotopes are functionally significant because they are involved in the regulation of B-cell function and are targets of anti-idiotypic antibodies in IVIG preparations [2]. Activation-induced deaminase causes DNA deamination which in autoantibody formation may underpin immunoglobulin variable gene conversion. Researchers at the Masaryk University in Brno (Czech Republic) are now working on full-length immunoglobulin profiling which allows sequencing of variable regions and we hope that special software will help us to track the provenience of abnormal autoantibodies [3].
Current autoantibody exploration and future trends.
| Specificity | Main disease | LOINC code |
|---|---|---|
| Current, established | ||
| Anti-nuclear Ab (ANA) | Screening autoimmity | 51153-2 |
| Anti-cardiolipin IgM, IgG | Anti-phospholipid syndrome, Behçet disease | 20424-8 |
| Anti-MPO (pANCA) | Vascular inflammation, polyangiitis | 6969-0 |
| Anti-thyroxin | Autoimmune thyroiditis | 9580-2 |
| Anti SSP | Primary Sjögren snydrome | Not found on search.loinc.org |
| Anti-extractable nuclear antigens | SLE | Dito |
| Anti-double-stranded DNA | SLE | |
| Anti-ribosomal P | Highly specific for SLE | 13636-6 |
| Anti-β2 glycoprotein I | ||
| Coombs test | Haemolytic anemia | 1003-3 |
| Future trends, awaiting confirmation | ||
| Inflammasome | Cancer diagnostics | Not found |
| PD-L1 | Cancer diagnostics, immune checkpoint | 83056-2 |
| Multiplex design | Several diseases in one step | |
| CD19+ cells in FACS | Therapeutic drug monitoring | 60434-8 |
| Single cell genomics | Genome wide association studies | |
| Anti-pentraxin 3 | Undifferentiated connective tissue disease | |
Table assembled in part by https://labtestsonline.org/understanding/conditions/autoimmune/start/1.
Prediction, therapeutic approaches and other aspects of humoral immunity to avoid autoimmune disease are being studied. Toll-like receptor 4 (TLR4) has been found to promote the activation of CD4+ and γδ T cells, which contributes to the initiation of autoimmune inflammation. Targeting TLR pathways directly on T lymphocytes is promising to be effective in treating autoimmune-inflammatory disorders such as multiple sclerosis (MS) [4].
Immunopathological update to better understand the diagnostic lab
Currently, as the complexity of the immune system becomes exploitable using tools of systems biology we are witnessing a better view on the derangements which are at the origin of autoimmune disease. Four main advances are in focus:
Malfunctioning regulation
For a long time determining specificity for self-antigens has been at the forefront of research: how, all of a sudden, can a self-antigen turn into a foreign structure provoking immune defence? A typical example that researchers pursue has recently been exposed in patients suffering from pulmonary alveolar proteinosis, a severe autoimmune disease, with autoantibodies that neutralize the granulocyte monocyte colony stimulating factor (GM-CSF); dissociation of GM-CSF-containing immune complexes takes place in an Fc-dependent manner thus liberating the antibody portion initiating further damage [5]. Antibody repertoire diversity, when well balanced is crucial for broad protective immunity, and is susceptible for disequilibrium and the methods used by such innovators as the Laboratory for Systems and Synthetic Immunology at the ETH Zürich are now able to explore homoeostasis of the antibody spectrum at high resolution using immune repertoire sequencing [6].
The IL17 and T CD17+constellation overexpression is a mainstay of active autoimmune diseases. Soluble B-cell activating factor (BAFF), B lymphocytes and immunoglobulins are upregulated in autoimmunity [7]. Decoding the antigen specificity of T-cell receptor (TCR) repertoires stands as a future approach to distinguish cross-reactive TCRs directed, at least in part, towards autoantigens [8]. For an autoimmune disease to break out, several prerequisites may play a role: human leukocyte antigen (HLA) disponibility, T cell/cytokine derangement and microbial aggression. At least two primarily unrelated mishaps must occur to trigger autoimmunity. This may be because one single incident has been sidetracked during evolution and the individual can handle such a situation. However, the train derails when a second mishap becomes apparent, in many cases a microbial aggression, such as Campylobacter jejuni in Guillain-Barré syndrome, or human papilloma virus (HPV) which is now suspected to align in the list of triggers for systemic lupus erythematosus (SLE) [9] (Table 2).
Microbial – induced or microbial – associated autoimmunity.
| Germ | Disease | Autoantibody specificity | LOINC codea | Reference |
|---|---|---|---|---|
| Campylobacter jejuni | Guillain-Barré syndrome | Anti-ganglioside GM | 31496-3 | [10] |
| Simulium black fly | Pemphigus foliaceus | Anti-desmoglein (Dsg) 3 | 43311-0 | [11] |
| Epstein barr virus | Systemic lupus erythematosus (SLE) | Anti-dsDNA | 37993-3 | [12] |
| Human papilloma virus | Onset or exacerbation of SLE | Anti-HPV | 13321-5 | [13] |
| Various vaccines | ASIA syndrome | Anti-phospholipid Anti-dsDNA | 55395-8 (Ig class panel) | [14] |
ahttps://search.loinc.org → one of several code possibilities is selected, according to the bodily fluid the analyte is taken from and according to the lab assay in use.
Infection
It is difficult to establish a direct role for a virus responsible for autoimmunity in humans when there is no biological material to study long before the onset of the autoimmune disease. If such a biosample was available, one could witness the emergence of an immune response against the virus (or the microbe) in the form of a mounting IgM immune response subsequently switching to high-affinity IgG with or without inflammation in the affected tissue. Soon after or years later, one may get an autoimmune response with autoantibodies, organ or non-organ specific, without an apparent reason other than a viral infection. One would need then to prove that the infection is the cause of the induction of autoantibodies with the establishment of molecular mimicry. The microbiome, i.e. the bacterial colonization of intestines with ongoing scanning by the gut-associated lymphoid tissue (GALT), has recently received much attention, not least as a possible player in autoimmunity. This infectome and autoinfectome, due to recirculation of immune cells throughout the organism with a major stop-over in abundant GALT has been suspected to favor autoimmunity through mechanisms still poorly known but certainly also through molecular mimicry [15]. Under current active research a gut-brain axis operative in patients suffering from MS is postulated based on solid experimental evidence using the animal model of autoimmune experimental encephalomyelitis [16].
The microbial trigger for autoimmune diseases outbreak has been under scrutiny ever since
Guillain-Barré syndrome linked to C. jejuni infection became notorious for autoimmune degeneration: molecular mimicry by C. jejuni with ganglioside epitopes on the human nerves that generates cross-reactive immune responses results in autoimmune-driven nerve damage [10, 17] by antibodies against GM1 ganglioside.
Less established, but under intense investigation, we suspect that the central nervous system and autoimmunity are related under the influence of other infectious agents [18]: rather than initiating MS, some parasites may in turn avoid its manifestation; from an epidemiological observational standpoint it is well known that there is no MS in regions where malaria has been eliminated (Yehuda Shoenfeld, oral communication). Interest is directed to helminth-associated immune regulation which may ameliorate autoimmunity [19]. One the one hand, helmintic infections exert downregulation of the immune system but on the other worms may protect the host from damaging immunopathological reactions; further studies are needed to make this clear.
Pemphigus foliaceus (PF) can be induced by auto-anti-desmoglein antibodies which provokes cutaneous blistering [11]. The initial insult is sustained by the autoantibodies to cell membrane receptor antigens which activate phospholipase C, mTOR and other tyrosine kinases and calmodulin responsible for basal skin cell shrinking. The tributary area of the rivers in Mato Grosso (Brazil) are infested with blackflies (Simuliidae) their saliva inducing “Fogo Selvagem” (Portuguese; English: wildfire). Insect antigens are held to damage skin and trigger endemic PF [20]; most people living in Western Brazil have low levels of IgG1 anti-Dsg1 antibodies: upon turning to an autoimmune attack, a switch to IgG4, the pathogenic, albeit poorly complement activating subclass found in sporadic PF appears in the serum.
The so far flimsy association of gut microbiome composition and autoimmunity is gaining momentum: the lymphoid tissues of the gut are eminently specialized for the programming of regulatory cells (Treg) which are responsible for the suppression of autoreactive T cells [16].
Side effects of drugs
The use of checkpoint inhibitors to treat cancer was markedly expanded when the anti-CTLA-4 agents ipilimumab or nivolumab began to be used in melanoma, and subsequently in non-small cell lung cancer, bladder cancer, Hodgkin lymphoma, renal cell carcinoma, and squamous cell head and neck carcinoma [21]. Early on programmed death-ligand 1 (PD-L1) was identified as a biomarker for these conditions and is currently enrolling clinical trials for advanced bladder cancer which marks this new era of approach; other discoveries with biomarker status are emerging [22]. Indeed laboratory medicine will direct attention to PD-1/PD-L1 interaction as this process is implicated in autoimmunity which animal models support: NOD mice develop diabetes type I and other autoimmune diseases – in the animals it can be observed that autoimmunity might result from the blockade of PD-1 of PD-L2 [23]. In humans, PD-L1 was found to have altered expression in pediatric patients suffering from SLE. Studying isolated PBMC from healthy children, immature myeloid dendritic cells and monocytes expressed little PD-L1 on initial isolation but sponateoulsy up-regulated PD-L1 within 24 h. In contrast, monocytes from patients with active SLE failed to upregulate PD-L1 over a 5-day time course expressing this protein only during disease remissions. This may be one way to lose peripheral tolerance in SLE [24].
Immune checkpoint inhibitor therapy associated with inflammatory, autoimmune side effects are fastidiously termed “immune related adverse events” (irAE): the most common irAEs are cutaneous rash, colitis, hepatitis and endocrinopathies, which all remain rare and are published only in the form of case reports with the underlying mechanisms about to be identified; patients with a pre-existing autoimmune tendency experience a flare-up. Autoantibody screening in irAE is not yet ready for textbooks not least because irAE is relatively easy to treat using short term and low dose steroid therapy.
Vaccines
Recently Shoenfeld and Agmon-Levin proposed a new clinical entity called autoimmune/inflammatory syndrome induced by adjuvants (ASIA). Both, live and attenuated vaccines may provoke this syndrome as an undesired secondary event, preferentially directed at the endocrine organs. The ASIA syndrome is so rare an event, that it goes unnoticed in field studies designed for health agency approval of vaccines. Therefore, postmarketing surveillance on vaccination involves ASIA detection prominently being observed with (1) siliconosis, (2) Gulf War syndrome, (3) macrophage myofasciitis and (4) general post-vaccination events. The emergence of ASIA syndrome is facilitated by individual genetic predisposition, for instance, when HLA-DRB1*01 or HLA-DRB4 are present in the vaccinee [14]. A recent case-control study investigated exposure to adjuvants prior to disease onset in patients suspected of being affected by ASIA. A group of patients affected with undifferentiated chronic tissue disease (UCTD) syndrome was compared to healthy controls. A questionnaire exploring the exposure to vaccinations, foreign materials and environmental and occupational exposures were part of a questionnaire for both patients and controls. Autoantibodies were screened (anti-nuclear, anti-extractable nuclear antigens, anti-double-stranded DNA, anti-cardiolipin, anti-β2 glycoprotein I). Indeed, patients surmised on undifferentiated connective tissue disease (UCTD) displayed a greater exposure to hepatitis B virus, tetanus toxoid vaccinations, metal implants and cigarette smoking as compared to controls. ASIA and UCTD can be considered as related entities in the “mosaic of autoimmunity”: the genetic predisposition and the environmental exposure to adjuvants elicit a common clinical phenotype characterized by signs and symptoms of systemic autoimmunity [25]. The role of HLA B27 in spondylarthritis and its possible autoimmune background has been updated recently [26] such that the MHC sets the stage for any one of the autoimmune diseases, as exemplified by a recent report on scleroderma (SSc) [27]. Responding CD4 T cells are found infiltrating salivary gland lesions of Sjögren’s syndrome and the risk haplotype HLA DR3/DQ2 is found in the majority of patients. Clonal expansion in glandular cell populations correlates with impaired saliva production [8].
Going to the medical laboratory
Suspicion of the presence of an autoimmune background for a patients’ presentation lets physicians search for lab assays bearing the potential for it to turn out to be pathological. The shortest link between clinical signs and symptoms and selection of the right assay places a bet which tries to avoid ordering from the lab an oversized array of possible assays and thus inadvertently contributing to the health costs; mediocre algorithms initiated with global assays within the all too detailed/disease-specific abnormality by AI might obtrude the approach. As a bad example, to start the algorithm with a search for single gene defect resulting in unusual susceptibility to autoimmunity would put the cart before the horse, although gene wide association studies of autoimmune diseases so far have mapped hundreds of susceptibility regions in the genome. Interpretation and clinical translation of cloning assays remain fraught with difficulty and ethical questions.
A holistic approach comprising lab assays classified as clinical chemistry, hematology, microbiology and immunology surrounded by genome wide screening using PCR technology is mandatory [28]. Thereafter, laboratory tests ordered to diagnose autoimmunity depend on the particular disorder the health practioner suspects a patient suffers from and usually includes blood tests of one or more autoantibodies as well as tests for inflammation, i.e. C-reactive protein, erythrocyte sedimentation rate and circulating immune complexes with or without complement sytem exploration (www.labtestsonline.org). Autoimmunity-specific assays are under development. A dabbler in science is seduced to directly jump to a large number of testable autoantibodies (Table 1). In view of the long list of identified autoantibody specificities currently available for the clinician, the search for so far unknown specificities and the search for new ones should not be neglected; the diagnostic value of antibodies in RA is completed by predictive, prognostic and follow-up value – follow-up reductions of their serum concentration are a good sign of treatment response for the patient. This is true for anti-citrullinated protein antibodies (ACPA) recognizing cyclic citrullinated peptides (CCP), mutated citrullinated vimentin and viral citrullinated peptides. It also applies for anti-cytosolic autoantibodies. Thus, posttranslational protein modifications as with citrullinated rheumatoid arthritis (RhFs) can be identified to enhance the meaning of RhF detection [29]. The European Autoimmunity Standardisation Initiative (EASI) or the forthcoming Dresden Symposium on Autoantibodies (Germany) (www.gfid-ev.de) make this topic a priority.
With new auto-antibody specificities relevant to clinicians at stake the laboratory is faced with GLP validation procedures of these new tests, and the generation of normal values by establishing reference intervals and day-to-day stability [30]; ISO 15189 standards need to be applied to autoantibody analysis wherever possible. To cite an example of a time consuming introduction into the routine laboratory, epitope recognition of domain I-specific anti-β2-glycoprotein-I antibodies allows estimating a pathognomonic index for antiphospholipd syndrome whereas the immunological profile for SS includes only anti-Ro autoantibodies → ANA and RhF anti-La being excluded in the diagnostic procedure due to lack of specificity [31, 32].
New 2016 ACR/EULAR ranking of primary SS includes simplified criteria. Firstly, either salivary gland biopsy or anti-Ro must be reactive in order to support the inflammatory and autoimmune nature of the disease. In addition, the diagnosis must confirm the systemic nature of SS: patients without salivary or ocular glandular symptoms, but with extraglandular manifestations and B cell activation markers also need to be treated.
It turns out, that ACPA-reactive, compared to non-reactive, represent distinct disease, grades the former with a higher rate of severe joint destruction, requiring more agressive treatment so clearly as to suggest a different genetic and environmental risk factor pattern [33].
Half of the patients with polymyositis or dermatomyositis express myositis-specific antibodies (MSA), so when the clinical exam suggests the possibility of these diseases, their presence becomes strong supporting evidence for the diagnosis. There are many mysositis-associated specific antibodies, the best-known are anti-aminoacyl-tRNA synthetases, anti-signal recognition particle (SRP), and anti-Mi-2: chromodomain helicase DNA binding protein 4; those associated with sclerodermia (anti-RNP, Ku and PmScl) are also found in myositis and present as an overlap. Several studies now purport that different serological types can be associated to differences in presentation and prognosis.
The immune-mediated necrotizing myopathy induced by statin medication over years [34] calls for such detection assays in specialized labs to identify anti-HMGCR (3-hydroxy-3-methylglutaryl coenzyme A reductase) antibodies [35].
For certain groups of autoimmune diseases multiplex line/dot blots test sytems might have advantages, e.g. automation, numerical reading and reproducibility. The applicability is currently under scrutiny and multiplex autoantibody patterns to certain diseases, e.g. of the gastrointestinal tract, with autoimmune hepatitis, primary biliary cirrhosis or autoimmune gastritis are being tested in multicenter studies.
Multiplex immunoassays [36], introduced at the outset of the current century support the immediate identification of multiple autoantibodies from a single determination but to the best of the authors knowledge have not made their way to being much used.
When inefficiently designed medical software relates to non-evidence based medical practice, the result can create unnecessary costs and hurts the patient. We have ourselves scrutinized multiplex systems but with only two analyses at stake, both making sense for similar sexually transmitted infectious diseases [37]. We cannot set apart multiplexing with its large spectrum of autoantibodies currently offered by providers as a useful approach. However, we still lack clinical experience with the lab results stemming from such type of screening and clinical presentation of autoimmune disease would narrow the number of specificities involved in a given patient. The American College of Rheumatology and its ANA Task Force insists on the indirect immuno-fluorescence to remain the gold standard in antinuclear antibody (ANA) testing on human epithelial cells (Hep-2) now completed using computer-aided diagnosis [38].
Autoimmune disease diagnostics is incomplete using autoantibody measurements alone for two reasons:
Immunopathogenic mechanisms incompletely explored:
The pathogenetic roles of autoantibodies largely remain elusive without a priori knowledge of disease-specific autoantigens. Reasonable endeavors to identify auto-epitopes come from quantitative proteogenomics: identification of full-length antibody variable heavy chain sequences are now possible. Recently, immune complexes from clinical specimens obtained from a patient with hepatitis C virus-induced cryoglobulinemia (HCV-CG) were taken as a source of antibodies. Reconstructed single-domain antibodies were reactive to both HCV antigens and potentially liver-derived human proteins [39]. Approaches like these may provide a proof-of-concept usage of autoantibodies to pinpoint the corresponding autoantigen which in turn is a candidate of a disease-specific expression. Highly epitope-restricted autoantibodies may then serve as novel biomarkers and for the development of antigen-specific immunotherapy against various autoantibody-related disorders. It is well known, that RhF non-reactive patients may nevertheless suffer from classical rheumatoir factor (RA) which underlines the sensitivity and specificity shortfalls of most autoantibodies described to date. Regarding the prognosis of a given autoimmune disorder, the presence of some autoantibodies may provide important information. Thus, antibodies against cyclic citrullinated peptides derived from filagrin (anti-CCP1), when considered in conjunction with RhF, are a criterion to evaluate the prognosis and development of erosions.
Non-autoantibody based lab analyses in autoimmune diseases:
These abound and include complement system exploration, inflammatory parameters and hematologic manifestations due and/or secondary to autoimmune disorders. The addition of such types of analyses to the lab profile of a patient have been outlined recently [28]. The presence of circulating immune complexes (www.immune-complex.ch), cryoglobulins and reactive direct or indirect antiglobulin (DAT, IAT) assays on red blood cells and platelets are relatively rarely ordered lab assays but indispensable in some rare diseases [40]. Similarly, CD markers on lymphocytes, natural killer cells or monocytes macrophages are second-line assays.
Monoclonal antibodies (mAbs) in the therapy and diagnosis of autoimmune diseases and other disorders
Systematic reviews on efficacy of second-line biologic choice in RA, PsA, and ankylosing spondylitis (AS) are now available [41]. They extract data from randomized, controlled trials, national healthcare databases, post-marketing surveys and open-label observational studies. Much attention is directed to mAbs (Table 3). Infliximab (trade names Remicade among others) is a chimeric mAb biologic drug that works against tumor necrosis factor alpha (TNF-α) or its bio-similar (bs-IFX) allow choosing among different routes and frequency of administration used to treat autoimmune diseases. Approved for the treatment of Crohn’s disease, ulcerative colitis, psoriasis, psoriatic arthritis, ankylosing spondylitis, and RA, infliximab is now widely used. It is used off-label outside its Federal Drug Administration (FDA) approval for Behçet’s disease and other conditions. Infliximab is administered by intravenous infusion, typically at 6–8-week intervals. It cannot be given by mouth because the digestive system would destroy the drug. Some variables in use during the last decade such as patients’ preference, treatments with anti-TNF monotherapy solely in potential childbearing women, and the intravenous route with dose titration in obese subjects were valid for all of the three rheumatic conditions. In RA, golimumab as a second-line biologic is now considered a good option in the case of anti-TNF failure with lab assays pointing the way. The switching strategy is preferable for responder patients who experience an adverse event, whereas more severe or class-specific side effects should be managed by the choice of a differently targeted drug. After two or more anti-TNF failures, swapping to a different mode of action may be necessary, especially in cases where RhF titers and excessive complement activation do not improve.
Some therapeutic mAbs requiring laboratory assays.
| Generic name | Trade name | Target | Clinical context |
|---|---|---|---|
| Bevacizumab | Avastin | Anti-VEGF | Retinal edema in leukoencephalopathy |
| Idarucizumab | Praxbind | Reverses dabigatran | Reverses dabigatran Hemorrhagic side effects |
| Cetuximab | Campto | Colon cancer | |
| Rituximab | Mabthera | Anti-CD20 | Immune mediated renal disease |
| Risanckizumab | IL23-Inhibitor | Crohn’s disease | |
| Adalimumab, infliximab | Humira Inflectra | Anti-TNFα | Psoriasis, juvenile uveitis |
| Golimumab | Simponi | Anti-TNFα | Rheumatoid arthritis |
| Epratuzumab | SLE | ||
| Blisimod | Inhibits BAFF | Nephropathy | |
| Barictinib | Olumiant | Inhibits Janus-kinase | Rhematoid arthritis |
| Lanadelumab | Inhibits kallikrein | Hereditory angionevrotic edema | |
| Secukinumab | Cosentyx | Inhibits IL-17 | |
| Imfinzi | Durvalumab | Blocks PD-L1 | Carcinoma |
| Checkpoint inhibitor | |||
| Natalizumab | Tysabri | Cell adhesion | Multiple sclerosis |
| Eculizumab | Soliris | CD55, CD59 | PNH, HUS |
| Mepolizumab | Nucala | Multiple sclerosis | |
| Tocilizumab | Actemra | Systemic sclerosis | |
| Imatinib | Tryptase | KIT inhibitor | Asthma |
| Ustekinumab | Subunit of IL 12 and IL23 | Psoriasis | |
| Ipilimumab | Yervoy | Checkpoint inhibitor enhance T cell responses (immune-related adverse events) irAE | Malignancies, prostate cancer |
The monoclonal antibody market makes it the dominant product class within the biopharmaceutical sector. The selection listed here makes clear that administration of mAbs requires diagnostic laboratory assays to ascertain their indication, often as a second-line option. Therapeutic drug monitoring and side effects with mAbs are dependent of reproducible lab assays [42].
Along these lines, the therapeutic mAb market received a boost when chimeric, humanized or fully humanized antibodies became approved by health authorities around the world. The second wave of approval and ongoing phase III clinical studies is due, in addition to better tolerance/less unexpected side effects, to the behavior of the target antigens/epitopes which are sensitive to pharmaceutical modulation of their functional expression, in this case upon binding to mAbs. Close to 100 mAbs can now be listed – Table 3 selects a few, prescription of which substantially depends on the results of laboratory assays. Lack of response to mAbs has been associated with inadequate mAb serum concentrations. TDM of mAbs has the potential to guide a more effective dosing in individual patients. The interpatient variability of mAb pharmacokinetics can be monitored by interval-timed lab assays. With mAbs being macromolecules, ligand-binding assays are at the forefront of reproducible test procedures still constrained by a substantial variation in detection and quantification. The optimal avoidance strategy towards formation of anti-drug, i.e. anti-mAb antibodies by patients adaptive immune response is not yet fully mapped out [43, 44].
The use of immune checkpoint inhibitors to treat cancer is expanding. Case-per-case success using anti-CTLA-4 (cytotoxic T lymphocyte antigen) monoclonal Abs (ipilimumab) used to treat advanced melanoma has fostered usage of this mAb, especially mAbs to target programmed death 1 protein (PD-1) and its ligand PD-L1, to also treat other cancer types: non-small cell-type of the lung, bladder, Hodgkin lymphoma, renal and squamous cell carcinoma of the head and neck. With somtimes spectacular remissions, approval by authorities was faster than usual and side effects now become known using postmerketing surveillance registers.
In fact, immune checkpoint inhibitors may elicit the irAE (immune related adverse events): rash, colitis, hepatitis, endocrinopathies. Nivolumab and iplimumab can cause deadly myocarditis or induce neurologic syndromes [45, 46].
To trace the drug upon administration, assessment of serum or tissue appearance of the administered mAb or its outcome on various lab assays likely reflect their administration. For example, mAbs specific for B-lymphocytic antigen CD20 (rituximab) can be followed beyond clinical efficacy by lab assays on CD19+ cell counts or by IgG serum concentrations [47]. Indeed, elimination half-lives of anti-CD20 varied as widely as between 1 and 17 days when measured by a two-compartment model assessing first-order distribution.
In a cohort of 64 patients suffering from RA the rituximab-elimination rate increased in speed with the number of CD19+ cell counts and increasing IgG serum concentrations which might be explained, at least in part, by different target-antigen loads in the patients. T cell activation can be identified using a calcium flux assay by flow cytometry – an original and progress promising approach [48]. Adverse events with adjuvant therapeutic measures, e.g. with glucocorticoids, in this context, would follow their own evaluations [49]. The same is true to estimate the therapeutic efficacy of IVIG – next to subtle exploration of the action mechanisms [50] inflammatory mediators such as TFN-α can be assessed in the diagnostic laboratory to estimate potency of IVIG treatment protocols [51].
Post-marketing surveillance programs with mAb therapeutic attempts are mandatory the more so as clinical studies designed for authority-approval may have included insufficient numbers of patients for a wise judgment of the unexpected side-effect ratio [52]. TDM of mAbs is used for personalized medical protocols and dosage schedules in individual patients. Interpatient variability of therapeutic mAb have been observed mainly in the treatment of autoimmune, inflammatory and malignant disease [42]. Therefore, individual adjustment of dosage with biopharmaceuticals is a must – whilst the problem is well recognized, only few randomized controlled TDM studies are available.
In a nutshell, the ongoing progress in diagnosis involving fast and scalable AI protocols and treatment of autoimmune disease is closely followed and depends on reliable lab assays.
Beyond diagnosis, the analyses help to direct treatment protocols and to explain action mechanisms as well as to adjust therapeutic protocols in personalized medicine.
Acknowledgments
To the family of Dr. Gert Risch for continuous support. English linguistic revision by Dr. Benjamin Sakem. Secretarial help by Simone Inderbitzin.
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
Research funding: In-house grant from labormedizinisches zentrum Dr. Risch.
Employment or leadership: None declared.
Honorarium: None declared.
Competing interests: The funding organization(s) played no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the report for publication.
References
1. Eyer K, Doineau R, Castrillon C, Jensen A, Griffiths AD, Bibette J, et al., editors. High-throughput single-cell deep phenotyping of immunoglobulin G secreting cells for high-resolution immune monitoring. Systems Biology of Adaptive Immunology, vol 1, abstract nr. 36. Zurich, Switzerland: ETH Zurich, 2017.Search in Google Scholar
2. Nydegger UE. Nature bundles immunoglobulin Iso-, all- and idiotype to target adaptive immune response. Transfus Med Hemother 2004;31:143–50.10.1159/000079073Search in Google Scholar
3. Turchaninova MA. High-quality full-length immunoglobulin profiling with unique molecular barcoding. Nat Protoc 2016;11:1599–616.10.1038/nprot.2016.093Search in Google Scholar PubMed
4. Reynolds JM, Martinez GJ, Chung Y, Dong C. Toll-like receptor 4 signaling in T cells promotes autoimmune inflammation. Proc Natl Acad Sci USA 2012;109:13064–9.10.1073/pnas.1120585109Search in Google Scholar PubMed PubMed Central
5. Piccoli L, Campo I, Fregni CS, Rodriguez BM, Minola A, Sallusto F, et al. Neutralization and clearance of GM-CSF by autoantibodies in pulmonary alveolar proteinosis. Nat Commun 2015;6:7375.10.1038/ncomms8375Search in Google Scholar PubMed PubMed Central
6. Friedensohn S, Khan TA, Reddy ST. Advanced methodologies in high-throughput sequencing of immune repertoires. Trends Biotechnol 2017;35:203–14.10.1016/j.tibtech.2016.09.010Search in Google Scholar PubMed
7. Steri M, Orru V, Idda ML, Pitzalis M, Pala M, Zara I, et al. Overexpression of the cytokine BAFF and autoimmunity risk. N Engl J Med 2017;376:1615–26.10.1056/NEJMoa1610528Search in Google Scholar PubMed PubMed Central
8. Joachim ML, coauthors. Single cell analysis of glandular T cell receptors in Sjögren’s syndrome. JCI Insight 2016;1. pli: 85609.10.1172/jci.insight.85609Search in Google Scholar PubMed PubMed Central
9. Segal Y, Calabro M, Kanduc D, Shoenfeld Y. Human papilloma virus and lupus: the virus, the vaccine and the disease. Curr Opin Rheumatol 2017;29:331–42.10.1097/BOR.0000000000000398Search in Google Scholar PubMed
10. Nyati KK, Nyati R. Role of Campylobacter jejuni infection in the pathogenesis of Guillain-Barre syndrome: an update. Biomed Res Int 2013;2013:852195.10.1155/2013/852195Search in Google Scholar
11. Di Zenzo G, Zambruno G, Borradori L. Endemic pemphigus foliaceus: towards understanding autoimmune mechanisms of disease development. J Invest Dermatol 2012;132:2499–502.10.1038/jid.2012.369Search in Google Scholar PubMed
12. Lossius A, Johansen JN, Torkildsen O, Vartdal F, Holmoy T. Epstein-Barr virus in systemic lupus erythematosus, rheumatoid arthritis and multiple sclerosis-association and causation. Viruses 2012;4:3701–30.10.3390/v4123701Search in Google Scholar PubMed PubMed Central
13. Soldevilla HF, Briones SF, Navarra SV. Systemic lupus erythematosus following HPV immunization or infection? Lupus 2012;21:158–61.10.1177/0961203311429556Search in Google Scholar PubMed
14. Watad A, Quaresma M, Brown S, Cohen Tervaert JW, Rodriguez-Pint I, Cervera R, et al. Autoimmune/inflammatory syndrome induced by adjuvants (Shoenfeld’s syndrome) – an update. Lupus 2017;26:675–81.10.1177/0961203316686406Search in Google Scholar PubMed
15. Bogdanos DP, Sakkas LI. From microbiome to infectome in autoimmunity. Curr Opin Rheumatol 2017;29:369–73.10.1097/BOR.0000000000000394Search in Google Scholar PubMed
16. Colpitts SL, Kasper LH. Influence of the gut microbiome on autoimmunity in the central nervous system. J Immunol 2017;198:596–604.10.4049/jimmunol.1601438Search in Google Scholar PubMed
17. Lardone RD, Yuki N, Irazoqui FJ, Nores GA. Individual restriction of fine specificity variability in anti-GM1 IgG antibodies associated with guillain-barre syndrome. Sci Rep 2016;6:19901.10.1038/srep19901Search in Google Scholar PubMed PubMed Central
18. Mentis AA, Dardiotis E, Grigoriadis N, Petinaki E, Hadjigeorgiou GM. Viruses and endogenous retroviruses in multiple sclerosis: from correlation to causation. Acta Neurologica Scandinavica 2017. doi: 10.1111/ane .12775.10.1111/ane .12775Search in Google Scholar
19. McSorley HJ, Maizels RM. Helminth infections and host immune regulation. Clin Microbiol Rev 2012;25:585–608.10.1128/CMR.05040-11Search in Google Scholar PubMed PubMed Central
20. Grando SA. Pemphigus autoimmunity: hypotheses and realities. Autoimmunity 2012;45:7–35.10.3109/08916934.2011.606444Search in Google Scholar PubMed PubMed Central
21. Pirker R, Dummer R. Editorial introductions. Curr Opin Oncol 2017;28:1–2.10.1097/ICU.0000000000000348Search in Google Scholar
22. Rolland DCR. Functional proteogenomics reveals biomarkers and therapeutic targets in lymphomas. Proc Natl Acad Sci USA 2017;114:6581–6.10.1073/pnas.1701263114Search in Google Scholar PubMed PubMed Central
23. Ansari MJ, Salama AD, Chitnis T. The programmed death-1 (PD-1) pathway regulates autoimmune diabetes in nonobese diabetic (NOD) mice. J Exp Med 2003;198:63–9.10.1084/jem.20022125Search in Google Scholar PubMed PubMed Central
24. Mozaffarian N, Wiedeman AE, Stevens AM. Active systemic lupus erythematosus is associated with failure of antigen-presenting cells to express programmed death ligand-1. Rheumatology (Oxford) 2008;47:1335–41.10.1093/rheumatology/ken256Search in Google Scholar PubMed PubMed Central
25. Scanzi F, Andreoli L, Martinelli M, Taraborelli M, Cavazzana I, Carabellese N, et al. Are the autoimmune/inflammatory syndrome induced by adjuvants (ASIA) and the undifferentiated connective tissue disease (UCTD) related to each other? A case-control study of environmental exposures. Immunol Res 2017;65:150–6.10.1007/s12026-017-8912-4Search in Google Scholar PubMed
26. Gaston JS. Recent advances in understanding spondyloarthritis. F1000Res 2017;6:304.10.12688/f1000research.10739.1Search in Google Scholar PubMed PubMed Central
27. Tsou PS, Sawalha AH. Unfolding the pathogenesis of scleroderma through genomics and epigenomics. J Autoimmun 2017. doi: 10.1016/j.jaut.2017.05.004.10.1016/j.jaut.2017.05.004Search in Google Scholar PubMed PubMed Central
28. Nydegger U, Lung T, Risch L, Risch M, Medina Escobar P, Bodmer T. Inflammation thread runs across medical laboratory specialities. Mediators Inflamm 2016;2016:4121837.10.1155/2016/4121837Search in Google Scholar PubMed PubMed Central
29. Trouw LA, Rispens T, Toes RE. Beyond citrullination: other post-translational protein modifications in rheumatoid arthritis. Nat Rev Rheumatol 2017;113:331–9.10.1038/nrrheum.2017.15Search in Google Scholar PubMed
30. Bizzaro N, Bossuyt X, Haapala AM, Shoenfeld Y, Sack U. Accreditation in autoimmune diagnostic laboratories. A position paper of the European Autoimmunity Standardisation Initiative (EASI). Autoimmun Rev 2017;16:81–6.10.1016/j.autrev.2016.09.021Search in Google Scholar PubMed
31. Franceschini F, Cavazzana I, Andreoli L, Tincani A. The 2016 classification criteria for primary Sjogren’s syndrome: what’s new? BMC Med 2017;15:69.10.1186/s12916-017-0837-1Search in Google Scholar PubMed PubMed Central
32. Fried R. CRP-Bestimmung in den Schweizer Praxislaboratorien. Pipette 2017:2.Search in Google Scholar
33. Huizinga TW. Personalized medicine in rheumatoid arthritis: is the glass half full or half empty? J Intern Med 2015;277:178–87.10.1111/joim.12319Search in Google Scholar PubMed
34. Christopher-Stine L, Basharat P. Statin-associated immune-mediated myopathy: biology and clinical implications. Curr Opin Lipidol 2017;28:186–92.10.1097/MOL.0000000000000399Search in Google Scholar PubMed
35. Ghirardello A, Bettio S, Bassi N, Gatto M, Beggio M, Lundberg I, et al. Autoantibody testing in patients with myositis: clinical accuracy of a multiparametric line immunoassay. Clin Exp Rheumatol 2017;35:176–7.10.26226/morressier.56e174d5d462b8028d88aa63Search in Google Scholar
36. Binder SR. Autoantibody detection using multiplex technologies. Lupus 2006;15:412–21.10.1191/0961203306lu2326oaSearch in Google Scholar PubMed
37. Sakem B, Michel R, Nydegger UE, Radjenovic D, Wydler M, Risch M, et al. Diagnostic relevance of simultaneous testing for Chlamydia trachomatis and Neisseria gonorrhoeae. Infection 2011;39:231–7.10.1007/s15010-011-0110-zSearch in Google Scholar PubMed
38. Infantino M, Meacci F, Grossi V, Manfredi M, Benucci M, Mercone M, et al. The burden of the variability introduced by the HEp-2 assay kit and the CAD system in ANA indirect immunofluorescence test. Immunol Res 2017;65:345–54.10.1007/s12026-016-8845-3Search in Google Scholar PubMed
39. Ogishi M, Yotsuyanagi H, Moriya K, Koike K. Delineation of autoantibody repertoire through differential proteogenomics in hepatitis C virus-induced cryoglobulinemia. Sci Rep 2016;6:29532.10.1038/srep29532Search in Google Scholar PubMed PubMed Central
40. Lambert JF, Nydegger UE. Geoepidemiology of autoimmune hemolytic anemia. Autoimmun Rev 2010;9:A350–4.10.1016/j.autrev.2009.11.005Search in Google Scholar PubMed
41. Cantini F, Niccoli L, Nannini C, Cassara E, Kaloudi O, Giulio Favalli E, et al. Second-line biologic therapy optimization in rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis. Semin Arthritis Rheum 2017. doi: 10.1016/j.semarthrit.2017.03.008.10.1016/j.semarthrit.2017.03.008Search in Google Scholar PubMed
42. Oude Munnink TH, Henstra MJ, Segerink LI, Movig KL, Brummelhuis-Visser P. Therapeutic drug monitoring of monoclonal antibodies in inflammatory and malignant disease: translating TNF-alpha experience to oncology. Clin Pharmacol Ther 2016;99:419–31.10.1002/cpt.211Search in Google Scholar PubMed
43. Darrouzain F, Bian S, Desvignes C, Bris C, Watier H, Paintaud G, et al. Immunoassays for measuring serum concentrations of monoclonal antibodies and anti-biopharmaceutical antibodies in patients. Ther Drug Monit 2017;39:316–21.10.1097/FTD.0000000000000419Search in Google Scholar PubMed
44. Paintaud G, Passot C, Ternant D, Antonio B, Bejan-Angoulvant T, Pascual-Salcedo D, et al. Rationale for therapeutic drug monitoring of biopharmaceuticals in inflammatory diseases. Ther Drug Monit 2017;39:339–43.10.1097/FTD.0000000000000410Search in Google Scholar PubMed
45. Friedman CF, Clark V, Raikhel AV, Barz T, Shoushtari AN, Momtaz P, et al. Thinking Critically About Classifying Adverse Events: Incidence of Pancreatitis in Patients Treated With Nivolumab + Ipilimumab. J Natl Cancer Inst 2017;109. doi: 10.1093/jnci/djw260.10.1093/jnci/djw260Search in Google Scholar PubMed PubMed Central
46. Friedman CF, Proverbs-Singh TA, Postow MA. Treatment of the immune-related adverse effects of immune checkpoint inhibitors: a review. JAMA Oncol 2016;2:1346–53.10.1001/jamaoncol.2016.1051Search in Google Scholar PubMed
47. Lioger B, Edupuganti SR, Mulleman D, Passot C, Desvignes C, Bejan-Angoulvant T, et al. Antigenic burden and serum IgG concentrations influence rituximab pharmacokinetics in rheumatoid arthritis patients. Br J Clin Pharmacol 2017;83:1773–81.10.1111/bcp.13270Search in Google Scholar PubMed PubMed Central
48. Kibbi N, Hong E, Ezaldein H, Hanlon D, Fahmy T, Edelson R. Quantifying in vivo murine antigen-specific T cell responses without requirement for prior knowledge of antigen identity. Transfus Apher Sci 2017;56:179–89.10.1016/j.transci.2016.11.004Search in Google Scholar PubMed
49. Miloslavsky EM, Naden RP, Bijlsma JW, Brogan PA, Brown ES, Brunetta P, et al. Development of a Glucocorticoid Toxicity Index (GTI) using multicriteria decision analysis. Ann Rheum Dis 2017;76:543–6.10.1136/annrheumdis-2016-210002Search in Google Scholar PubMed
50. Winter M, Baksmeier C, Steckel J, Barman S, Malviya M, Harrer-Kuster M, et al. Dose-dependent inhibition of demyelination and microglia activation by IVIG. Ann Clin Transl Neurol 2016;3: 828–43.10.1002/acn3.326Search in Google Scholar PubMed PubMed Central
51. Hu P, Jiang GM, Wu Y, Huang BY, Liu SY, Zhang DD, et al. TNF-alpha is superior to conventional inflammatory mediators in forecasting IVIG nonresponse and coronary arteritis in Chinese children with Kawasaki disease. Clin Chim Acta 2017;471:76–80.10.1016/j.cca.2017.05.019Search in Google Scholar PubMed
52. Ninomiya H, Obara N, Chiba S, Usuki K, Nishiwaki K, Matsumura I, et al. Interim analysis of post-marketing surveillance of eculizumab for paroxysmal nocturnal hemoglobinuria in Japan. Int J Hematol 2016;104:548–58.10.1007/s12185-016-2065-4Search in Google Scholar PubMed
Article Note:
Text written as a consequence of the 4th International Congress on Controversies in Rheumatology and Autoimmunity, Bologna, Italy, 9–11 March 2017.
©2017 Walter de Gruyter GmbH, Berlin/Boston
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Articles in the same Issue
- Frontmatter
- Allergie und Autoimmunität/Allergy and Autoimmunity
- Molekulare und Zelluläre Möglichkeiten in der Allergiediagnostik
- ICAP – ein Versuch zur einheitlichen Beschreibung der Fluoreszenzmuster von antizellulären Antikörpern auf HEp-2-Zellen
- Medical laboratory in autoimmunity 2017
- Erhebliche Diskrepanzen zwischen qualitativen Troponin-Schnelltesten („Karten-Testen“) und klassischer Laboranalytik: ist der Einsatz solcher Schnellteste vertretbar?
- Analyses of exhaled breath condensate cytokines for identification of lung cancer
- Molekulargenetische und zytogenetische Diagnostik/Molecular-Genetic and Cytogenetic Diagnostics
- Optimized short digestion protocol for free fetal DNA detection using methylation-dependent markers
Articles in the same Issue
- Frontmatter
- Allergie und Autoimmunität/Allergy and Autoimmunity
- Molekulare und Zelluläre Möglichkeiten in der Allergiediagnostik
- ICAP – ein Versuch zur einheitlichen Beschreibung der Fluoreszenzmuster von antizellulären Antikörpern auf HEp-2-Zellen
- Medical laboratory in autoimmunity 2017
- Erhebliche Diskrepanzen zwischen qualitativen Troponin-Schnelltesten („Karten-Testen“) und klassischer Laboranalytik: ist der Einsatz solcher Schnellteste vertretbar?
- Analyses of exhaled breath condensate cytokines for identification of lung cancer
- Molekulargenetische und zytogenetische Diagnostik/Molecular-Genetic and Cytogenetic Diagnostics
- Optimized short digestion protocol for free fetal DNA detection using methylation-dependent markers

