Abstract
Objectives
The aim of this study is to compare the obstetric, neonatal, and hematological indicators of pregnant women with thalassemia traits with those of pregnant women without such traits.
Methods
This retrospective cohort study was conducted from January 2017 to October 2023 at the Department of Obstetrics and Gynecology, The First Affiliated Hospital of Dali University. The study included 185 cases of thalassemia traits and 185 control cases. Data were analysis using the SPSS program (Version 27.0).
Results
Significant differences were observed in gravidity and parity histories (p<0.05). Significant differences were also observed in the rates of gestational diabetes mellitus (GDM), hypertensive disorder of pregnancy (HDP), cesarean delivery, adherent placenta, and anemia in the second and third trimesters following the number of RR (95 % CI): 2.182 (1.101–4.324), 9.000 (1.152–70.325), 2.091 (1.555–2.811), 3.401 (1.280–9.009), 4.222 (2.102–8.481), and 2.053 (1.476–2.855), respectively (p<0.05). However, no significant differences were noted in the rates of preterm birth, low birth weight, macrosomia, intrauterine growth restriction, fetal distress, fetal malformation, and stillbirth (p>0.05). Furthermore, significant differences were noted in the levels of hemoglobin (Hb), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), and red cell distribution width (RDW) during the first, second, and third trimesters (p<0.05).
Conclusions
As pregnancy progresses, the levels of Hb tend to decrease, while the MCH and RDW levels increase. On the other hand, the level of MCV remain the same overtime. Thalassemia traits are significantly associated with anemia during pregnancy, particularly in the second and third trimesters. Furthermore, thalassemia traits are related to an increased incidence of GDM, HDP, and cesarean delivery.
Introduction
Thalassemia is characterized by the absence or point mutation of the globin gene in hemoglobin, leading to impaired synthesis of globin peptide chains. This results in insufficient hemoglobin production, ineffective erythropoiesis, hemolysis, and varying degrees of microcytic hypochromic hemolytic anemia, thereby decreasing the oxygen-carrying capacity of red blood cells. Chronic anemia in thalassemia can lead to excessive iron absorption. This can damage the liver, spleen, heart, and endocrine systems [1], [2], [3].
The World Health Organization (WHO) published the World Report on Hemoglobinopathies Epidemiology in 2008, revealing that among 229 countries, approximately 71 % face issues with hemoglobin diseases, affecting 330,000 newborns annually. Of these hemoglobin diseases, sickle cell anemia accounts for 83 %, while thalassemia constitutes 17 %. Globally, around 7 % of pregnant women are carriers of hemoglobin diseases (including α-thalassemia, β-thalassemia, Hb C, Hb D-Punjab, and Hb E), and over 1 % of couples are at risk [4].
In 1925, thalassemia was first described and identified in the Mediterranean population by an Italian physician, who reported five cases exhibiting unique symptoms of microcytic hypochromic hemolytic anemia. This specific form of anemia was subsequently named thalassemia. Thalassemia represents the most prevalent autosomal recessive monogenic genetic disease globally, affecting the highest cumulative number of individuals. It is estimated that approximately 350 million people worldwide are carriers of the thalassemia gene [5]. Of these carriers, around 260 million carry the α-thalassemia gene, constituting approximately 5 % of the global population, while approximately 80–90 million carry the β-thalassemia gene, accounting for about 1.5 % of the global population [6], 7].
Thalassemia is widely distributed, prevalent not only in Mediterranean coastal regions but also across Northern Africa, the Middle East, Southeast Asia, and southern China. It is the most common and highest incidence single-gene genetic disease worldwide [8]. The Southeast Asian population, in particular, exhibits a high incidence of α-thalassemia, β-thalassemia, Hb E, and Hb CS [9], [10], [11], [12], [13].
Based on the Zeng and Huang study conducted in 1987, the national prevalence of α-thalassemia and β-thalassemia was determined to be 0.66 and 2.64 %, respectively, across several laboratories [14]. Recent large-scale surveys on thalassemia conducted in various regions of China have indicated continued high incidence rates [15], 16]. Lai et al. reported an overall prevalence of 7.88 % for β-thalassemia and 2.21 % for α-thalassemia [17]. In Southern China, including Guangxi, Guangdong, Hainan, Yunnan, Sichuan, Chongqing, Fujian, and other areas, carrier rates vary significantly, ranging from 3.3 to 24.07 %. Among these regions, Guangxi and Guangdong exhibit the highest incidence of thalassemia [17], 18].
Thalassemia is the most common and prevalent single-gene genetic disorder globally. Despite its widespread effect, effective curative treatments applicable in clinical practice remain elusive. The high cost of treatment and challenges in finding suitable donors further complicate management efforts. This disease imposes a growing burden on healthcare systems and economies, contributing to significant psychological stress and substantial financial strain on the families of patients and society at large. Thalassemia presents serious public health challenges and can impede the development of a country. In China, treating severe thalassemia costs approximately RMB 1 million per case on average, often pushing affected families into poverty or back into poverty [19], [20], [21], [22].
Thalassemia encompasses four primary types: α, β, δ, and δβ, with α and β-thalassemia being the most prevalent [23]. α-thalassemia is further categorized into four degrees: silent carrier, mild, intermediate, and severe. Silent carriers are asymptomatic and lead normal lives. Mild cases are healthy and asymptomatic, although they may experience mild anemia or microcytosis. Intermediate cases present with symptoms including anemia, fatigue, hepatosplenomegaly, and mild jaundice, typically developing these symptoms after infancy. Severe cases in fetuses often lead to fetal death due to severe edema during development or shortly after birth. β-thalassemia includes mild, intermediate, and severe forms. Mild cases may be asymptomatic or present with mild anemia. Intermediate cases indicate symptoms in early childhood like mild to moderate splenomegaly and mild bone changes. Severe cases experience progressive anemia starting 3–6 months after birth, necessitating lifelong transfusions and iron chelation therapy, leading to severe hepatosplenomegaly. Without proper treatment, these patients typically do not survive beyond the age of 5 [24], [25], [26].
As the global population expands and migrates, the number of thalassemia gene carriers rises, spreading the disease to diverse regions and making it a significant global public health concern affecting nearly every country worldwide. Effective strategies for prevention and control of adverse pregnancy outcomes, antenatal care enhancement for women of childbearing age, and enhancement of birth population quality need to be considered [25].
Most thalassemia-related studies in China have predominantly focused on Guangxi and Guangdong provinces. However, Yunnan stands out as the province with the largest number of minority nationalities in China, each with distinct customs and lifestyles. Geographically adjacent to Southeast Asian countries, which have high incidences of thalassemia, Yunnan presents a unique setting where various types of thalassemia with complex genetic underpinnings may affect individuals, particularly pregnant women [17], [18], [19], [20]. Previous studies have often examined only specific subsets of hematological indicators or pregnancy outcomes, potentially leading to misdiagnoses among patients with mild thalassemia, who may be asymptomatic or experience mild anemia. Pregnancy can exacerbate symptoms, heightening adverse effects for both the mother and child. Consequently, research on pregnancy with mild thalassemia remains limited and controversial [21], [22], [23], [24], [25], [26]. Therefore, the objective of this research was to assess the association between thalassemia traits and pregnancy outcomes among women without thalassemia, focusing on various adverse pregnancy outcomes and complications including gestational diabetes mellitus (GDM), hypertensive disorders of pregnancy (HDP), preeclampsia, eclampsia, intrahepatic cholestasis of pregnancy (ICP), placental abruption, hydramnios, oligohydramnios, premature rupture of membranes (PROM), chorioamnionitis, postpartum hemorrhage, cesarean delivery, meconium-stained amniotic fluid, adherent placenta, and anemia during pregnancy. Additionally, the objective of this study was to assess perinatal outcomes like fetal weight, preterm birth, low birth weight, macrosomia, intrauterine growth restriction (IUGR), neonatal asphyxia, fetal distress, fetal malformation, and stillbirth. Furthermore, the goal of the study was to indicate the combined effect of maternal blood routine indicators including hemoglobin (Hb), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), and red cell distribution width (RDW).
Materials and methods
The retrospective group study was approved by the Dali Institutional Review Board. From January 2017 to October 2023, data were collected from the In-patient Ward database at the Department of Obstetrics and Gynecology, The First Affiliated Hospital of Dali University. A total of 292 cases of pregnant women with thalassemia were diagnosed, of which 185 cases met the specified criteria, comprising of 112 cases of α-thalassemia trait and 73 cases of β-thalassemia trait. The process for screening cases among pregnant women is depicted in Figure 1. Additionally, 185 pregnant women without thalassemia were selected as the study participants of the control group. Data were retrieved from the electronic medical record system of the hospital.
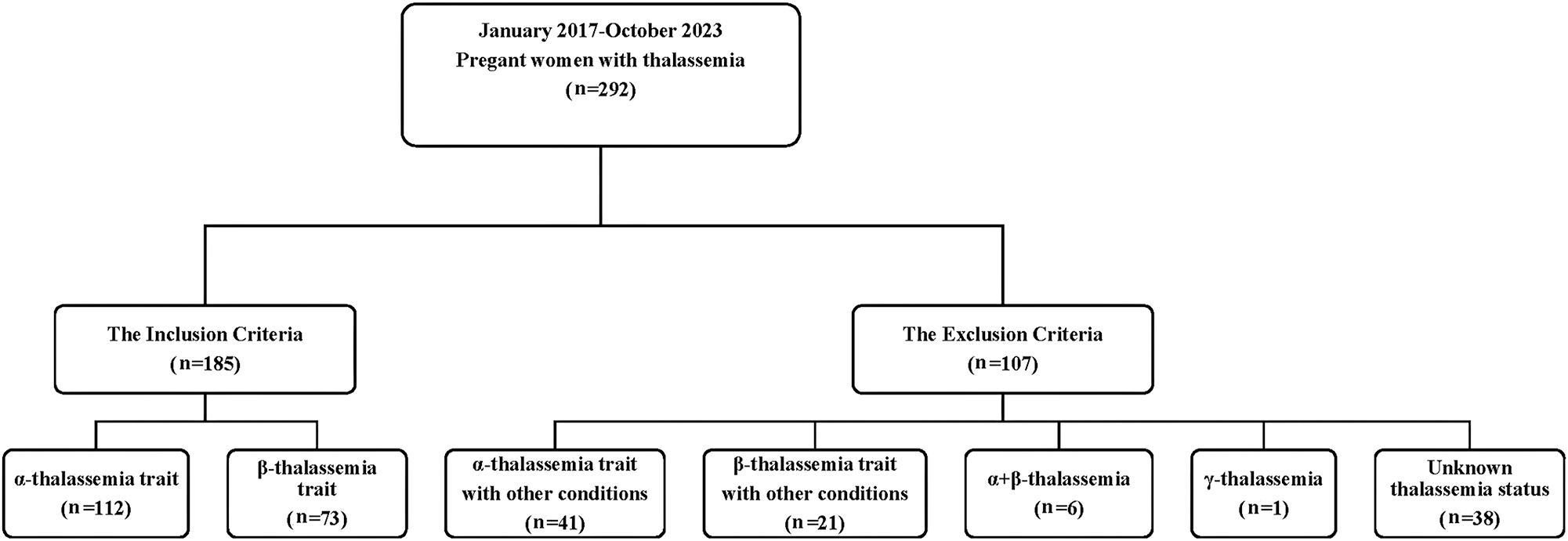
The screening process of pregnant women with thalassemia.
Inclusion and exclusion criteria
The study group included patients diagnosed with α-thalassemia and β-thalassemia minor or those having the traits through genetic testing either before or during pregnancy. Conversely, the control group was randomly selected during the same period with a 1:1 ratio, based on similar baseline characteristics.
The inclusion criteria were as follows: (1) singleton pregnancies; (2) attendance for prenatal care and delivery at the F*** H; (3) no other medical or surgical conditions such as pre-gestational diabetes, chronic hypertension, or anemia from any causes; (4) availability of data on pregnancy outcomes; (5) no history of blood transfusion; and (6) pregnant women with good mental health, normal cognitive function, and high compliance.
The exclusion criteria were as follows: (1) pregnant women with other medical or surgical complications during pregnancy, like overt diabetes mellitus (DM), chronic hypertension, and renal disease; (2) fetuses affected by thalassemia major; (3) fetuses with significant chromosomal abnormalities or structural defects; (4) inadequate clinical data and pregnancy outcomes, including expectant mothers with unclear thalassemia status; (5) multiple pregnancies; (6) incomplete data.
The controls were randomly selected based on similar baseline characteristics and study duration as the study group. The inclusion and exclusion criteria for pregnant women without thalassemia were the same as for the study group.
Research methodology
This study uses a 1:1 sampling ratio, dividing the selected cases into two groups: the study group, comprising of pregnant women with thalassemia trait (α-thalassemia and β-thalassemia), and the control group, comprising of pregnant women without thalassemia. The following data are compared:
Comparison of general data of pregnant women in both groups.
Comparison of obstetric outcomes in both groups and subgroups.
Comparison of neonatal outcomes in both groups and subgroups.
Comparison of hematological indicators tests during different stages of pregnancy like Hb, MCV, MCH, and RDW in both groups and subgroups.
Analysis of factors potentially influencing anemia in pregnant women with thalassemia.
Statistical methods
Statistical analyses were conducted using SPSS software, version 27.0 (SPSS Statistics for Windows, SPSS Inc., Chicago, IL, USA). In cases where quantitative data exhibit a normal distribution like Hb, MCV, MCH, and RDW, the results are presented as mean±standard deviation (x̄ ± SD). Alternatively, if the data do not conform to a normal distribution, they are expressed using the median and interquartile range (M±IQR). When comparing two groups, a t-test for independent sample is used for analysis if the data are normally distributed and exhibit homogeneity of variance. Conversely, if the data do not follow a normal distribution and display heterogeneity of variance, a non-parametric test (Mann-Whitney U Test) for two independent samples is used. For comparisons among three groups, a one-way ANOVA is applied under conditions of normal distribution and homogeneity of variance. Alternatively, if the data do not adhere to a normal distribution and exhibit heterogeneity of variance, either the Kruskal-Wallis H Test or Friedman test is used. For categorical data like obstetric history, pregnancy complications in pregnant women, and adverse neonatal events, frequencies or percentages are used for presentation. Statistical analyses use the Chi-square test or Fisher’s exact test, along with Pearson’s Chi-square, as appropriate. Relative risk (RR) and its 95 % confidence interval (CI) are calculated to quantify the risk ratio of adverse pregnancy outcomes associated with thalassemia traits. The significance level is set to 0.05 for all analyses.
Results
Comparison of general information between thalassemia trait and control group of pregnant women
Table 1 displays the baseline characteristics of the study and control groups. There were no statistically significant differences observed in maternal age, maternal BMI, gestational age, history of abortions, history of fetal arrest, or history of stillbirth between the two groups. However, significant differences were noted in gravidity (p=0.021) and parity history (p=0.001) between the groups.
Baseline characteristics of pregnant women with thalassemia trait and control group.
| Characteristics | Study group (n=185) | Control group (n=185) | p-Value |
|---|---|---|---|
| Maternal age x̄±SD | 29.08 ± 4.91 | 28.66 ± 4.19 | 0.381 |
| Maternal BMI (kg/m2) x̄±SD | 26.97 ± 4.04 | 26.34 ± 3.37 | 0.103 |
| Gestational age, n (%) | 0.134 | ||
| <36 weeks | 14 (66.7 %) | 7 (33.3 %) | |
| 36 w–39 weeks | 127 (51.2 %) | 121 (48.8 %) | |
| ≥40 weeks | 44 (43.6 %) | 57 (56.4 %) | |
| Gravidity, n (%) | 0.021 | ||
| 1 time | 68 (36.8 %) | 91 (49.2 %) | |
| ≥2 times | 117 (63.2 %) | 94 (50.8 %) | |
| Parity, n (%) | 0.001 | ||
| Nulliparity | 108 (58.4 %) | 143 (77.3 %) | |
| Primiparity | 70 (37.8 %) | 41 (22.2 %) | |
| Multiparity | 7 (3.8 %) | 1 (0.5 %) | |
| History of abortions, n (%) | 0.085 | ||
| Yes | 77 (41.6 %) | 60 (32.4 %) | |
| No | 108 (58.4 %) | 125 (67.6 %) | |
| History of fetal arrest, n (%) | 0.156 | ||
| Yes | 13 (7 %) | 6 (3.2 %) | |
| No | 172 (93 %) | 179 (96.8 %) | |
| History of stillbirth, n (%) | 0.123 | ||
| Yes | 4 (2.2 %) | 0 (0 %) | |
| No | 181 (97.8 %) | 185 (100 %) |
Comparison of obstetric outcomes between thalassemia traits and control group of pregnant women
Table 2 illustrates significant differences between the two groups in the prevalence rates of GDM, HDP, cesarean delivery, adherent placenta, anemia in the 2nd trimester, and anemia in the 3rd trimester, with corresponding RR and 95 % CI of 2.182 (1.101–4.324), 9.000 (1.152–70.325), 2.091 (1.555–2.811), 3.401 (1.280–9.009), 4.222 (2.102–8.481), and 2.053 (1.476–2.855), respectively. However, there were no statistically significant differences observed in the rates of preeclampsia, eclampsia, intrahepatic cholestasis of pregnancy (ICP), placental abruption, hydramnios, oligohydramnios, PROM, chorioamnionitis, postpartum hemorrhage, meconium-stained amniotic fluid, or anemia in the 1st trimester.
Obstetric outcomes between thalassemia trait and control group.
| Outcome | Study group (n=185) | Control group (n=185) | p-Value | Relative risk (95 % CI) |
|---|---|---|---|---|
| GDM, n (%) | 24 (13 %) | 11 (5.9 %) | 0.032 | 2.182 (1.101–4.324) |
| HDP, n (%) | 9 (4.9 %) | 1 (0.5 %) | 0.020 | 9.000 (1.152–70.325) |
| Preeclampsia, n (%) | 4 (2.2 %) | 1 (0.5 %) | 0.372 | 4.000 (0.451–35.449) |
| Eclampsia, n (%) | 2 (1.1 %) | 0 (0 %) | 0.499 | – |
| ICP, n (%) | 4 (2.2 %) | 3 (1.6 %) | 1.000 | 1.333 (0.303–5.875) |
| Placental abruption, n (%) | 6 (3.2 %) | 4 (2.2 %) | 0.751 | 1.500 (0.430–5.228) |
| Hydramnios, n (%) | 1 (0.5 %) | 2 (1.5 %) | 1.000 | 0.500 (0.046–5.467) |
| Oligohydramnios, n (%) | 13 (7 %) | 10 (5.4 %) | 0.668 | 1.300 (0.585–2.890) |
| PROM, n (%) | 35 (18.9 %) | 50 (27 %) | 0.083 | 0.700 (0.478–1.024) |
| Chorioamnionitis, n (%) | 30 (16.2 %) | 31 (16.8 %) | 1.000 | 0.968 (0.612–1.531) |
| Postpartum hemorrhage, n (%) | 16 (8.6 %) | 19 (17.5 %) | 0.723 | 0.842 (0.447–1.586) |
| Cesarean delivery, n (%) | 92 (49.7 %) | 44 (23.8 %) | <0.001 | 2.091 (1.555–2.811) |
| Meconium-stained amniotic fluid, n (%) | 21 (11.4 %) | 28 (24.5 %) | 0.358 | 0.750 (0.442–1.271) |
| Adherent placenta, n (%) | 5 (2.7 %) | 17 (9.2 %) | 0.014 | 3.401 (1.280–9.009) |
| Anemia at the 1st trimester, n (%) | 12 (6.5 %) | 4 (2.2 %) | 0.071 | 3.000 (0.986–9.131) |
| Anemia at the 2nd trimester, n (%) | 38 (20.5 %) | 9 (4.9 %) | <0.001 | 4.222 (2.102–8.481) |
| Anemia at the 3rd trimester, n (%) | 78 (42.2 %) | 38 (20.5 %) | <0.001 | 2.053 (1.476–2.855) |
Comparisons of the obstetric outcomes between the sub groups α-thalassemia trait, β-thalassemia trait and control group
Table 3 presents the results of subgroup analysis concerning significant obstetric outcomes across various types of thalassemia characteristics. Significant differences were observed in the incidence rates of HDP, cesarean delivery, adherent placenta, anemia in the 2nd trimester, and anemia in the 3rd trimester when comparing the three groups. Conversely, no statistically significant differences were found among the three groups regarding the incidence rates of GDM, preeclampsia, eclampsia, ICP, placental abruption, hydramnios, oligohydramnios, PROM, chorioamnionitis, postpartum hemorrhage, meconium-stained amniotic fluid, or anemia in the 1st trimester.
Comparisons of the obstetric outcomes between the sub groups.
| Outcomes | α-thalassemia (n=112) | β-thalassemia (n=73) | Control group (n=185) | p-Value |
|---|---|---|---|---|
| GDM, n (%) | 15 (13.4 %)a | 9 (12.3 %) | 11 (5.9 %) | 0.056 |
| HDP, n (%) | 6 (5.4 %)a | 3 (4.1 %) | 1 (0.5 %) | 0.018 |
| Preeclampsia, n (%) | 4 (3.6 %) | 0 (0 %) | 1 (0.5 %) | 0.073 |
| Eclampsia, n (%) | 1 (0.9 %) | 1.1.4 (%) | 0 (0 %) | 0.249 |
| ICP, n (%) | 2 (1.8 %) | 2 (2.7 %) | 3 (1.6 %) | 0.782 |
| Placental abruption, n (%) | 6 (5.4 %) | 0 (0 %) | 4 (2.2 %) | 0.091 |
| Hydramnios, n (%) | 0 (0 %) | 1 (1.4 %) | 2 (1.1 %) | 0.592 |
| Oligohydramnios, n (%) | 8 (7.1 %) | 5 (6.8 %) | 10 (5.4 %) | 0.811 |
| PROM, n (%) | 24 (21.4 %) | 11 (15.1 %) | 50 (27.0 %) | 0.116 |
| Chorioamnionitis, n (%) | 15 (15.2 %) | 13 (17.8 %) | 31 (16.8 %) | 0.867 |
| Postpartum hemorrhage, n (%) | 7 (6.3 %) | 9 (12.3 %) | 19 (10.3 %) | 0.315 |
| Cesarean delivery, n (%) | 58 (51.8 %)a | 34 (46.6 %)b | 44 (23.8 %) | <0.0001 |
| Meconium-stained amniotic fluid, n (%) | 11 (9.8 %) | 10 (13.7 %) | 28 (15.1 %) | 0.433 |
| Adherent placenta, n (%) | 1 (0.9 %)a | 4 (5.5 %) | 17 (9.2 %) | 0.006 |
| Anemia at the 1st trimester, n (%) | 8 (7.1 %) | 4 (5.5 %) | 4 (2.2 %) | 0.103 |
| Anemia at the 2nd trimester, n (%) | 17 (15.2 %)a | 21 (28.8 %)b | 9 (4.9 %) | <0.0001 |
| Anemia at the 3rd trimester, n (%) | 40 (35.7 %)a | 38 (52.1 %)b | 38 (20.5 %) | <0.0001 |
-
aIndicates comparison between the α-thalassemia trait pregnant women group and the control group pregnant women group, p<0.05; bindicates comparison between the β-thalassemia trait pregnant women group and the control group pregnant women group, p<0.05.
Comparisons of the obstetric outcomes between the sub groups α-thalassemia and β-thalassemia with control group
Table 4 presents the subgroup analysis among different types of thalassemia traits for significant obstetric outcomes. Significant differences were observed between the α-thalassemia trait group and the control group in the rates of GDM, HDP, cesarean delivery, anemia in the 2nd trimester, and anemia in the 3rd trimester, with RR and 95 % CI of 2.252 (1.073–4.729), 9.911 (1.209–81.254), 2.177 (1.591–2.980), 0.097 (0.013–0.720), 3.120 (1.440–6.760), and 1.739 (1.193–2.535), respectively. In contrast, comparing the β-thalassemia group with the control group revealed significant differences in the rates of cesarean delivery, anemia in the 2nd trimester, and anemia in the 3rd trimester, with RR and 95 % CI of 1.958 (1.371–2.796), 5.913 (2.843–12.300), and 2.534 (1.770–3.628), respectively.
Comparisons of the obstetric outcomes between the sub groups α-thalassemia and β-thalassemia with control group.
| Outcomes | α-thalassemia (n=112) RR (95 % CI) | β-thalassemia (n=73) RR (95 % CI) |
|---|---|---|
| GDM, n (%) | 2.252 (1.073–4.729)a | 2.073 (0.897–4.795) |
| HDP, n (%) | 9.911 (1.209–81.254)a | 7.603 (0.804–71.909) |
| Preeclampsia, n (%) | 6.607 (0.748–58.373) | – |
| Eclampsia, n, % | – | – |
| ICP, n (%) | 1.101 (0.187–6.489) | 1.689 (0.288–9.905) |
| Placental abruption, n (%) | 2.478 (0.715–8.589) | – |
| Hydramnios, n (%) | – | 1.267 (0.117–13.761) |
| Oligohydramnios, n (%) | 1.321 (0.537–3.249) | 1.267 (0.448–3.581) |
| PROM, n (%) | 0.793 (0.518–1.214) | 0.558 (0.308–1.010) |
| Chorioamnionitis, n (%) | 0.906 (0.526–1.559) | 1.063 (0.590–1.914) |
| Postpartum hemorrhage, n (%) | 0.609 (0.264–1.401) | 1.2 (0.570–2.530) |
| Cesarean delivery, n (%) | 2.177 (1.591–2.980)a | 1.958 (1.371–2.796)b |
| Meconium-stained amniotic fluid, n (%) | 0.649 (0.336–1.251) | 0.905 (0.463–1.768) |
| Adherent placenta, n (%) | 0.097 (0.013–0.720)a | 0.596 (0.208–1.712) |
| Anemia at the 1st trimester, n (%) | 3.304 (1.018–10.720) | 2.534 (0.651–9.866) |
| Anemia at the 2nd trimester, n (%) | 3.120 (1.440–6.760)a | 5.913 (2.843–12.300)b |
| Anemia at the 3rd trimester, n (%) | 1.739 (1.193–2.535)a | 2.534 (1.770–3.628)b |
-
aIndicates comparison between the α-thalassemia trait pregnant women group and the control group, p<0.05; bindicates comparison between the β-thalassemia trait pregnant women group and the control group, p<0.05.
Comparisons of obstetric outcomes between the α-thalassemia trait and β-thalassemia trait
Table 5 indicates significant differences between α-thalassemia trait and β-thalassemia groups in the anemia rates of the 2nd trimester and 3rd trimester, with RR and 95 % CI of 1.894 (1.074–3.344) and 1.458 (1.046–2.033), respectively. However, there were no statistically significant differences observed between the two groups in the rates of GDM, HDP, cesarean delivery, and adherent placenta, with RR and 95 % CI of 1.086 (0.502–2.351), 1.304 (0.337–5.050), 1.112 (0.821–1.507), and 0.163 (0.019–1.429), respectively.
Comparisons of obstetric outcomes between the α-thalassemia and β-thalassemia.
| Outcomes | α-thalassemia (n=112) | β-thalassemia (n=73) | p-Value | Relative risk (95 % CI) |
|---|---|---|---|---|
| GDM, n (%) | 15 (13.4 %) | 9 (12.3 %) | 1.000 | 1.086 (0.502–2.351) |
| HDP, n (%) | 6 (5.4 %) | 3 (4.1 %) | 1.000 | 1.304 (0.337–5.050) |
| Cesarean delivery, n (%) | 58 (51.8 %) | 34 (46.6 %) | 0.548 | 1.112 (0.821–1.507) |
| Adherent placenta, n (%) | 1 (0.9 %) | 4 (5.5 %) | 0.080 | 0.163 (0.019–1.429) |
| Anemia at the 2nd trimester, n (%) | 17 (15.2 %) | 21 (28.5 %) | 0.040 | 1.894 (1.074–3.344) |
| Anemia at the 3rd trimester, n (%) | 40 (35.7 %) | 38 (52.1 %) | 0.033 | 1.458 (1.046–2.033) |
Comparison of neonatal outcomes between thalassemia traits and control group of pregnant women
Following the exclusion of five cases of stillbirth and four cases of fetal malformation in both the thalassemia trait group and control group, neonatal outcomes were analyzed and are presented in Table 6. No statistically significant differences were found between the study and control groups in the rates of preterm birth, low birth weight, macrosomia, IUGR, neonatal asphyxia, fetal distress, fetal malformation, or stillbirth.
Comparisons of neonatal outcomes between the two groups.
| Outcomes | Study group (n=185) | Control group (n=185) | p-Value | Relative risk (95 % CI) |
|---|---|---|---|---|
| Preterm birth, n (%) | 16 (8.6 %) | 10 (5.4 %) | 0.309 | 1.600 (0.746–3.433) |
| Low birth weight, n (%) | 14 (7.6 %) | 7 (3.8 %) | 0.176 | 2.000 (0.826–4.842) |
| Macrosomia, n (%) | 3 (1.6 %) | 4 (2.2 %) | 1.000 | 0.750 (0.170–3.305) |
| IUGR, n (%) | 4 (2.2 %) | 2 (1.1 %) | 0.685 | 2.000 (0.371–10.786) |
| Fetal distress, n (%) | 18 (9.7 %) | 16 (8.6 %) | 0.857 | 1.125 (0.592–2.137) |
| Neonatal asphyxia, n (%) | 5 (2.7 %) | 6 (3.2 %) | 1.000 | 0.833 (0.259–2.683) |
| Fetal malformation, n (%) | 4 (2.2 %) | 0 (0 %) | 0.123 | – |
| Stillbirth, n (%) | 4 (2.2 %) | 1 (0.5 %) | 0.372 | 4.000 (0.451–35.449) |
Comparisons of the neonatal outcomes between the sub groups α-thalassemia trait, β-thalassemia trait and control group
Table 7 presents the subgroup analysis among different types of thalassemia traits for neonatal outcomes. Statistically significant differences were observed in the incidence rates of low birth weight and fetal malformation among the three groups. However, no statistically significant differences were found in the incidence rates of preterm birth, macrosomia, IUGR, neonatal asphyxia, fetal distress, and stillbirth.
Comparisons of neonatal outcomes between the α-thalassemia, β-thalassemia and control group.
| Outcomes | α-thalassemia (n=112) | β-thalassemia (n=73) | Control group (n=185) | p-Value |
|---|---|---|---|---|
| Preterm birth, n (%) | 11 (9.8 %) | 5 (6.8 %) | 10 (5.4 %) | 0.361 |
| Low birth weight, n (%) | 12 (10.7 %)a | 2 (2.7 %) | 7 (3.8 %) | 0.034 |
| Macrosomia, n (%) | 2 (1.8 %) | 1 (1.4 %) | 4 (2.2 %) | 1.000 |
| IUGR, n (%) | 3 (2.7 %) | 1 (1.4 %) | 2 (1.1 %) | 0.574 |
| Fetal distress, n (%) | 10 (8.9 %) | 8 (11.0 %) | 16 (8.6 %) | 0.839 |
| Neonatal asphyxia, n (%) | 3 (2.7 %) | 2 (2.7 %) | 6 (3.2 %) | 1.000 |
| Fetal malformation, n (%) | 4 (3.6 %)a | 0 (0 %) | 0 (0 %) | 0.010 |
| Stillbirth, n (%) | 1 (0.9 %) | 3 (4.1 %) | 1.05 (%) | 0.096 |
-
aIndicates comparison between the α-thalassemia trait pregnant women group and the control group, p<0.05.
Comparisons of neonatal outcomes between α-thalassemia and β-thalassemia with control group
Table 8 presents the subgroup analysis among different types of thalassemia traits for neonatal outcomes. Significant differences were observed between the α-thalassemia group and the control group in the rate of low birth weight, with an RR and 95 % CI of 2.832 (1.149–6.980).
Comparisons of neonatal outcomes between α-thalassemia and β-thalassemia with control group.
| Outcomes | α-thalassemia (n=112) vs. control group (n=185) | β-thalassemia (n=73) vs. control group (n=185) | α-thalassemia (n=112) vs. β-thalassemia (n=73) |
|---|---|---|---|
| Preterm birth, n (%) | 1.817 (0.797–4.140) | 1.267 (0.448–3.581) | – |
| Low birth weight, n (%) | 2.832 (1.149–6.980)a | 0.724 (0.154–3.404) | 3.911 (0.901–16.968) |
| Macrosomia, n (%) | 0.826 (0.154–4.436) | 0.634 (0.073–5.574) | 1.304 (0.120–14.117) |
| IUGR, n (%) | 2.478 (0.420–14.601) | 1.267 (0.117–13.761) | 1.955 (0.207–18.439) |
| Fetal distress, n (%) | 1.032 (0.485–2.195) | 1.267 (0.567–2.832) | 0.815 (0.337–1.968) |
| Neonatal asphyxia, n (%) | 0.826 (0.211–3.237) | 0.845 (0.174–4.090) | 0.978 (0.167–8.710) |
| Fetal malformation, n (%) | –a | – | – |
| Stillbirth, n (%) | 1.652 (0.104–26.146) | 7.603 (0.804–71.909) | 0.217 (0.023–2.049) |
-
aIndicates comparison between the α-thalassemia trait pregnant women group and the control group, p<0.05.
Comparisons of hematological indicators between thalassemia traits and control group
Table 9 presents comparisons of hematological indicators between the thalassemia trait and control groups. Significant differences were found in Hb, MCV, MCH, and RDW levels between the two groups during the 1st, 2nd, and 3rd trimesters. On the other hand Table 10 presents significant differences of Hb, MCH, and RDW levels and no statistically significant differences of MCV levels during the 1st, 2nd, and 3rd trimesters for the thalassemia trait.
Comparisons of hematological indicators between the two groups during the 1st trimester, 2nd trimester and 3rd trimester.
| Variables | Trimester | Study group (n=185) | Control group (n=185) | p-Value |
|---|---|---|---|---|
| Hb, g/L, x̄ ±SD | 1st | 126.74 ± 11.61 | 134.51 ± 11.59 | <0.001 |
| 2nd | 114.87 ± 13.77 | 123.45 ± 10.11 | <0.001 | |
| 3rd | 111.89 ± 14.31 | 127.09 ± 10.72 | <0.001 | |
| MCV, fL, x̄ ±SD | 1st | 81.58 ± 7.05 | 89.59 ± 4.99 | <0.001 |
| 2nd | 81.18 ± 7.70 | 92.42 ± 4.90 | <0.001 | |
| 3rd | 81.04 ± 7.58 | 92.42 ± 5.13 | <0.001 | |
| MCH, pg, x̄ ±SD | 1st | 26.97 ± 2.80 | 30.42 ± 1.99 | <0.001 |
| 2nd | 26.32 ± 2.99 | 30.91 ± 1.97 | <0.001 | |
| 3rd | 26.16 ± 2.98 | 32.47 ± 1.65 | <0.001 | |
| RDW, %, x̄ ±SD | 1st | 14.30 ± 1.78 | 13.38 ± 1.91 | <0.001 |
| 2nd | 14.97 ± 1.85 | 13.69 ± 1.11 | <0.001 | |
| 3rd | 15.22 ± 1.85 | 13.99 ± 1.51 | <0.001 |
Comparisons of hematological indicators of study group (n=185) during the 1st trimester, 2nd trimester and 3rd trimester.
| Variables | Trimester 1st | Trimester 2nd | Trimester 3rd | p-Value |
|---|---|---|---|---|
| Hb, g/L, x̄ ±SD | 126.74 ± 11.61 | 114.87 ± 13.77 | 111.89 ± 14.31 | <0.001 |
| MCV, fL, x̄ ±SD | 81.58 ± 7.05 | 81.18 ± 7.70 | 81.04 ± 7.58 | 0.769 |
| MCH, pg, x̄ ±SD | 26.97 ± 2.80 | 26.32 ± 2.99 | 26.16 ± 2.98 | 0.020 |
| RDW, %, x̄ ±SD | 14.30 ± 1.78 | 14.97 ± 1.85 | 15.22 ± 1.85 | <0.001 |
Discussion
The effect of thalassemia traits on hematological indices during pregnancy
Patients with thalassemia traits typically do not exhibit clinical symptoms; however, they are susceptible to develop more severe anemia during pregnancy, posing risks to maternal and fetal health [27]. Pregnant women with thalassemia constitute a distinct group requiring ongoing monitoring of routine blood tests from early pregnancy stages to assess maternal and fetal risks. Monitoring parameters like Hb, MCV, MCH, and RDW is crucial for indicating anemia [28]. Thalassemia-associated anemia is characterized by microcytic hypochromic morphology, typically presenting with lower levels of Hb, MCH, and MCV, and elevated or increasing RDW [29], 30]. This study revealed that pregnant women with thalassemia traits experience significant declining levels of Hb, MCV, and MCH, alongside increasing RDW as pregnancy progresses, underscoring their association with pregnancy outcomes and emphasizing the importance of early discovery in this population. Liang et al. similarly reported lower Hb, MCV, and MCH, and higher RDW in pregnant women carrying the thalassemia gene, which is consistent with our findings [31]. Numerous domestic and international studies have highlighted the adverse effect of thalassemia on pregnancy [28], [32], [33], [34], [35]. Genetic mutations associated with thalassemia disrupt globin synthesis, commonly leading to microcytic hypochromic anemia. Therefore, assessing Hb, MCV, MCH, and RDW levels in pregnant women has become pivotal for screening thalassemia, providing valuable diagnostic insights and guiding subsequent management and delivery decisions [36]. Furthermore, this study advocates for the routine measurement of Hb, MCV, MCH, and RDW levels as a straightforward method to screen pregnant women for thalassemia trait.
The effect of thalassemia trait on neonatal outcomes
The effect of thalassemia trait on the incidence of neonatal asphyxia
Neonatal asphyxia refers to the inability of the newborn to initiate breathing at birth, often caused by complications during delivery [37]. However, the study found no statistically significant difference in the incidence of neonatal asphyxia between the thalassemia trait group and the control group. Sheiner et al. reported a significant increase in the rate of neonatal asphyxia with increasing severity of maternal anemia [38]. Considering that pregnant women with thalassemia traits rarely experience moderate to severe anemia, it may be the reason why there is minimal adverse effect on fetal outcomes.
The effect of thalassemia trait on low birth weight in newborns
A newborn’s birth weight serves as a key indicator for assessing neonatal health and assessing the effectiveness of prenatal care. It not only reflects intrauterine nutrition and fetal growth during pregnancy but also plays a key role in determining future physical and cognitive development [39], 40]. Throughout pregnancy, numerous factors influence fetal weight, including maternal nutritional intake, placental function in nutrient transport, maternal oxygen-carrying capacity, and the genetic potential of the fetus. In cases of mild anemia during pregnancy, the mother and the fetus’ bone marrow both compete for serum iron (Fe). Since Fe is transported from the mother to the fetus through the placenta in a unidirectional manner, fetal tissues have a preferential uptake of Fe, thereby mitigating severe fetal Fe deficiency. However, moderate to severe maternal anemia compromises the placental delivery of oxygen and nutrients to the fetus, potentially leading to fetal growth restriction, fetal distress, preterm birth, or stillbirth [41].
Studies have identified gestational age as the foremost determinant of newborn birth weight, followed by factors like fetal number, gender, maternal education level, maternal age, maternal nutritional status, residential location, parity, and obstetric history [42], 43]. Some authors argue that thalassemia affects newborn birth weight and poses a risk factor for low birth weight [44], 45]. This is attributed to reduced hemoglobin levels in pregnant women with thalassemia, which diminishes nutrient transfer to the fetus through the placenta, thereby affecting newborn birth weight. The results of this study found a lower incidence of low birth weight in the thalassemia trait group compared to the control group, although this difference was not statistically significant. However, subgroup analysis indicated a statistically significant increase in low birth weight incidence in α-thalassemia trait, while no significant difference was found in β-thalassemia trait compared to the control group, consistent with the findings of Pang et al. [46]. Conversely, a systematic review in China proposed that α-thalassemia is not associated with increased risk of low birth weight in newborns, whereas β-thalassemia is a significant risk factor, conflicting with the conclusions of our study [47]. This inconsistency may be influenced by regional dietary habits; variations in dietary practices can effect maternal nutrition and subsequently affect fetal intrauterine development, contributing to regional disparities in research outcomes.
The effect of thalassemia trait on maternal outcomes
The effect of thalassemia trait on anemia in pregnant women
In pregnant women with thalassemia, genetic mutations impair globin chain synthesis due to α or β gene deficiencies, resulting in ineffective red blood cell production. Additionally, the influence of human chorionic gonadotropin (HCG) exacerbates hemolytic anemia, intensifying the severity of thalassemia during pregnancy when compared to normal pregnancies. Patients with thalassemia trait often exhibit no obvious symptoms due to compensatory effects from unaffected genes, and their hemoglobin levels may appear normal. However, during illness or pregnancy, these compensatory mechanisms are limited and anemia symptoms and its related complications can manifest [48]. Research indicates that physiological changes leading to anemia during pregnancy may exacerbate anemia in pregnant women with thalassemia trait, adversely affecting maternal and fetal health and potentially leading to long-term effects on newborns [49]. Our study found an RR and 95 % CI of 3.000 (0.986–9.131) in the incidence of anemia between the thalassemia trait and control groups in early pregnancy, which slightly failed to reach statistical significance. However, significant differences were observed in the incidence of anemia between the two groups during mid and late pregnancy. Subgroup analysis further indicated significantly higher incidence of anemia in the β-thalassemia trait group compared to the α-thalassemia trait group during mid and late pregnancy. Li et al. similarly reported significantly lower Hb levels in late pregnancy among women with thalassemia trait compared to those without, which is consistent with our findings [50]. Without appropriate interventions, the prevalence of anemia among pregnant women with thalassemia trait is likely to escalate as pregnancy progresses.
The effect of thalassemia trait on gestational diabetes mellitus
The occurrence of GDM is associated with changes in the endocrine environment during pregnancy. As blood volume increases, pregnant women often experience relatively insufficient insulin secretion. In early pregnancy, maternal blood glucose levels tend to decrease with advancing gestational age. However, in mid and late pregnancy, the levels of anti-insulin substances like progesterone and estrogen, increase, reducing insulin sensitivity. This reduction in insulin sensitivity leads to insufficient insulin secretion to meet the physiological demands of pregnancy, resulting in abnormal glucose metabolism [49], 50].
There are both domestic and international controversies regarding thalassemia and whether it adversely affects pregnancy outcomes. The results of this study indicate that pregnant women with thalassemia trait are more likely to develop GDM compared to normal pregnant women, similar to the findings of Gérardin et al. [51]. Furthermore, the subgroup analysis in this study revealed a statistically significant difference between pregnant women with α-thalassemia and normal pregnant women, which is consistent with the study by Lao and Ho [52]. However, no statistically significant difference was found between pregnant women with β-thalassemia and normal pregnant women, aligning with the results reported by Tsatalas et al. [53]. Some literature reports indicate that the occurrence of GDM in pregnant women with thalassemia trait is not statistically significant compared to normal pregnant women [54], 55]. This discrepancy may result because these studies do not differentiate between types of thalassemia gene mutations, indicating that the differences in results might be related to the diversity of thalassemia types. Additionally, variations in lifestyle and dietary habits among pregnant women could also contribute to these differing outcomes.
The effect of thalassemia traits on hypertensive disorders of pregnancy, preeclampsia, and eclampsia
HDP are a group of complications commonly seen during pregnancy and are a major cause of morbidity and mortality in pregnant women and newborns, accounting for 25 % of maternal deaths [56], [57], [58]. The global incidence of HDP is between 8 and 10 % [59]. HDP can be classified based on severity into mild, moderate, and severe forms. Severe HDP, also known as pre-eclampsia and eclampsia, is characterized by convulsions on the basis of hypertension, with severe pre-eclampsia accounting for about 39.96 % of cases [60]. Pregnancy complicated by thalassemia can lead to an increased risk of adverse pregnancy outcomes like fetal growth restriction, preterm birth, and even fetal death in utero, and may cause serious complications such as thromboembolism, cardiac abnormalities, and endocrine dysfunction [61]. Therefore, pregnancies complicated by thalassemia require proactive and effective prenatal screening and diagnosis along with routine prenatal examinations [62]. The results of this study, similar to those published by Luo et al., demonstrate that the incidence of HDP in pregnant women with thalassemia trait is significantly higher than in the normal pregnant group [63]. However, this study also reveals that the incidence of preeclampsia and eclampsia in pregnant women with thalassemia trait is not statistically different from that in normal pregnant women, which is consistent with the findings of Zhang et al. [64]. Some international studies have revealed that the incidence of preeclampsia in pregnant women with thalassemia trait is higher than in normal pregnant women. This difference may be related to the proportion of primiparas in the thalassemia group and may also be influenced by dietary, environmental, social, psychological, genetic, and economic factors [65]. Clinical calcium deficiency has been commonly considered a major inducer of HDP [66].
The effect of thalassemia traits on the mode of delivery
The decision for a cesarean section involves many confounding factors like advanced maternal age, multiple pregnancies, primiparity, abnormal fetal position, and fetal birth weight [67], 68]. Consequently, whether thalassemia increases the risk of cesarean section remains controversial. A systematic review by Lai et al. on the relationship between thalassemia and pregnancy outcomes indicated that β-thalassemia is a risk factor for cesarean section [17]. This may be due to anemia during pregnancy increasing the incidence of fetal distress in utero, thereby raising the cesarean section rate. However, α-thalassemia is not a risk factor for cesarean section in pregnant women. The review proposes a weak correlation between thalassemia and cesarean section, potentially due to an increased incidence of fetal distress in utero caused by anemia during pregnancy, which leads to a higher rate of cesarean sections. Moreover, this meta-analysis indicated that the cesarean section rate in the thalassemia group was higher than in the normal group [31]. According to Zhu et al., the cesarean section rate in women with thalassemia was significantly higher than that in non-thalassemic women, attributing this to the increased incidence of fetal distress in utero due to anemia during pregnancy [69]. The results of this study revealed that the cesarean section rate in the thalassemia trait group was significantly higher than in the normal pregnant women group, aligning with previous studies [47], 63], [69], [70], [71], [72], [73]. Furthermore, this study revealed no statistically significant difference in the incidence of fetal distress in utero between the thalassemia trait and normal pregnant women groups. Previous studies have indicated that patients with thalassemia trait during pregnancy have reduced body defense capabilities, decreased tolerance to vaginal delivery and cesarean section, and are prone to hemorrhagic shock, neonatal asphyxia, and restricted fetal growth, which differs from the findings of this study [74], 75]. This study indicates that pregnant women with thalassemia, when combined with other diseases during pregnancy, experience an increased incidence of fetal distress in utero and adverse fetal events, thereby raising the cesarean section rate.
The other effect of thalassemia traits on adverse pregnancy outcomes
Thalassemia is a chronic hereditary hemolytic disease resulting from the deletion or mutation of globin genes, leading to a globin synthesis disorder. It is also known as globin regeneration disorder anemia. Anemia, a high-risk factor during pregnancy, can increase pregnancy risks even in its mild form. In severe cases, it may lead to a reduction in effective circulating blood volume in the uterus, causing miscarriage, preterm birth, or fetal death due to ischemia and hypoxia. Research by Sheiner et al. demonstrated that pregnant women with mild β-thalassemia have a higher incidence of IUGR and oligohydramnios compared to non-thalassemic pregnant women [38]. However, there is no significant difference in the incidence of congenital malformations or perinatal death. Studies conducted by Wang et al. and Pang et al. indicated that thalassemia trait significantly increases the incidence of preterm birth and adverse postpartum pregnancy outcomes [47], 76]. Yang et al. revealed that chronic anemia combined with pregnancy can affect pregnancy outcomes, increasing the likelihood of preterm birth and postpartum hemorrhage [77]. Conversely, the study conducted by Zhang et al. found no statistically significant differences between thalassemia pregnant women and normal pregnant women in terms of macrosomia, polyhydramnios, oligohydramnios, or postpartum hemorrhage. Research by Chen et al. and Guo et al. demonstrated that patients with thalassemia trait exhibit weakened bodily defense capabilities, decreased tolerance to both vaginal delivery and cesarean section, and an increased susceptibility to hemorrhagic shock, neonatal asphyxia, and restricted fetal growth [74], 75]. Farmaki et al. further revealed that thalassemia gene carriers are at risk for late-term fetal death or neonatal death due to genetic deletions, underscoring the importance of SEA gene screening for pregnant women [78]. This study did not find statistically significant differences in maternal and perinatal outcomes, including ICP, placental abruption, hydramnios, oligohydramnios, premature rupture of membranes (PROM), chorioamnionitis, postpartum hemorrhage, meconium-stained amniotic fluid, adherent placenta, preterm birth, macrosomia, IUGR, fetal malformation, and stillbirth between the thalassemia trait and control groups. However, subgroup analysis revealed that the incidence of fetal malformation in pregnant women with α-thalassemia trait was significantly higher than in normal pregnant women. In contrast, the incidence in pregnant women with β-thalassemia trait was not statistically significant compared to normal pregnant women.
Limitations
This study has certain limitations. Firstly, as a retrospective study utilizing data from hospital electronic medical records, it lacks information on the dietary habits of the pregnant women involved. Variations in diet, cultural customs, and iron intake can influence pregnancy outcomes and may introduce regional differences in findings. Furthermore, the limited sample size may introduce bias, impacting the generalizability and accuracy of the results. Future studies with larger sample sizes and prospective data collection are recommended to validate these findings across diverse populations.
Conclusions
This study presents novel findings on the hematological changes during pregnancy, demonstrating that as pregnancy progresses, levels of Hb, MCV, and MCH decline, while RDW increases. Thalassemia traits significantly impact of on the development of anemia, particularly in the second and third trimesters, with β-thalassemia traits exhibiting a more pronounced effect compared to α-thalassemia traits. Furthermore, this study identifies thalassemia traits, especially α-thalassemia, as critical contributors to pregnancy complications such as GDM, HDP, and increased rates of cesarean deliveries. Notably, α-thalassemia trait emerges as a potential risk factor for low birth weight and fetal malformations, underscoring the need for careful monitoring and management of pregnant individuals with these traits. These findings contribute to a better understanding of how thalassemia traits influence maternal and fetal health outcomes and provide valuable insights for clinical practice and prenatal care.
Acknowledgments
We would like to acknowledge the hard and dedicated work of all the staff who implemented the intervention and evaluation components of the study.
-
Research ethics: This study was conducted with approval from the Ethics Committee of the First Affiliated Hospital of Dali University (No. DFY20230922001). This study was conducted in accordance with the declaration of Helsinki. Written informed consent was obtained from all participants.
-
Informed consent: Not applicable.
-
Author contributions: Conception and design of the research: Ratana Meng, Jifang Shi. Acquisition of data: Ratana Meng, Jifang Shi, Analysis and interpretation of the data: Ratana Meng, Chanrith Mork, Haining Bi. Statistical analysis: Ratana Meng,Jifang Shi, Haining Bi. Obtaining financing: None. Writing of the manuscript: Ratana Meng, Jifang Shi. Critical revision of the manuscript for intellectual content: Ratana Meng, Jifang Shi. All authors read and approved the final draft.
-
Use of Large Language Models, AI and Machine Learning Tools: None declared.
-
Conflict of interest: The authors declare that they have no competing interests.
-
Research funding: None declared.
-
Data availability: The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
1. Muncie, HL, Campbell, J. Alpha and beta thalassemia. Am Fam Physician 2009;80:339–44.Search in Google Scholar
2. Zervas, A, Katopodi, A, Protonotariou, A, Livadas, S, Karagiorga, M, Politis, C, et al.. Assessment of thyroid function in two hundred patients with beta-thalassemia major. Thyroid 2002;12:151–4. https://doi.org/10.1089/105072502753522383.Search in Google Scholar PubMed
3. Borgna-Pignatti, C, Gamberini, MR. Complications of thalassemia major and their treatment. Expert Rev Hematol 2011;4:353–66. https://doi.org/10.1586/ehm.11.29.Search in Google Scholar PubMed
4. Modell, B, Darlison, M. Global epidemiology of haemoglobin disorders and derived service indicators. Bull World Health Organ 2008;86:480–7. https://doi.org/10.2471/blt.06.036673.Search in Google Scholar PubMed PubMed Central
5. Weatherall, DJ. The evolving spectrum of the epidemiology of thalassemia. Hematol Oncol Clin N Am 2018;32:165–75. https://doi.org/10.1016/j.hoc.2017.11.008.Search in Google Scholar PubMed
6. Piel, FB, Weatherall, DJ. The α-thalassemias. N Engl J Med 2014;371:1908–16. https://doi.org/10.1056/nejmra1404415.Search in Google Scholar PubMed
7. Colah, R, Gorakshakar, A, Nadkarni, A. Global burden, distribution and prevention of β-thalassemias and hemoglobin E disorders. Expert Rev Hematol 2010;3:103–17. https://doi.org/10.1586/ehm.09.74.Search in Google Scholar PubMed
8. McLean, E, Cogswell, M, Egli, I, Wojdyla, D, de Benoist, B. Worldwide prevalence of anaemia, WHO vitamin and mineral nutrition information system, 1993–2005. Public Health Nutr 2008;12:444–54. https://doi.org/10.1017/s1368980008002401.Search in Google Scholar PubMed
9. Fucharoen, S, Winichagoon, P. Haemoglobinopathies in Southeast Asia. Indian J Med Res 2011;134:498–506.Search in Google Scholar
10. Filon, D, Oppenheim, A, Rachmilewitz, EA, Kot, R, Truc, DB. Molecular analysis of beta-thalassemia in Vietnam. Hemoglobin 2000;24:99–104. https://doi.org/10.3109/03630260009003428.Search in Google Scholar PubMed
11. Fucharoen, S, Fucharoen, G, Sriroongrueng, W, Laosombat, V, Jetsrisuparb, A, Prasatkaew, S, et al.. Molecular basis of beta-thalassemia in Thailand: analysis of beta-thalassemia mutations using the polymerase chain reaction. Hum Genet 1989;84:41–6. https://doi.org/10.1007/bf00210668.Search in Google Scholar PubMed
12. Verma, IC, Saxena, R, Kohli, S. Past, present & future scenario of thalassaemic cote & control in India. Indian J Med Res 2011;134:507–21.Search in Google Scholar
13. Premawardhena, A, De Silva, S, Arambepola, M, Olivieri, N, Merson, L, Muraco, J, et al.. Thalassemia in Sri Lanka: a progress report. Hum Mol Genet 2004;13:R203–6. https://doi.org/10.1093/hmg/ddh250.Search in Google Scholar PubMed
14. Zeng, YT, Huang, SZ. Disorders of haemoglobin in China. J Med Genet 1987;24:578–83. https://doi.org/10.1136/jmg.24.10.578.Search in Google Scholar PubMed PubMed Central
15. Tang, W, Zhang, C, Lu, F, Tang, J, Lu, Y, Cui, X, et al.. Spectrum of α-thalassemia and β-thalassemia mutations in the Guilin region of southern China. Clin Biochem 2015;48:1068–72. https://doi.org/10.1016/j.clinbiochem.2015.06.008.Search in Google Scholar PubMed
16. Lin, M, Zhong, TY, Chen, YG, Wang, JZ, Wu, JR, Lin, F, et al.. Molecular epidemiological characterization and health burden of thalassemia in Jiangxi Province, P. R. China. PLoS One 2014;9:e101505. https://doi.org/10.1371/journal.pone.0101505.Search in Google Scholar PubMed PubMed Central
17. Lai, K, Huang, G, Su, L, He, Y. The prevalence of thalassemia in mainland China: evidence from epidemiological surveys. Sci Rep 2017;7:920. https://doi.org/10.1038/s41598-017-00967-2.Search in Google Scholar PubMed PubMed Central
18. Yang, Y, Zhang, J. Research progress on thalassemia in Southern China - review. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2017;25:276–80. https://doi.org/10.7534/j.issn.1009-2137.2017.01.050.Search in Google Scholar PubMed
19. Xu, XM. Operational guidelines for prevention and control of Thalassemia. Beijing, China: People’s Military Medical Press; 2011:115–8 pp.Search in Google Scholar
20. Zhou, XZ, Zhang, MF, Lin, Y, Liu, K. A survey of treament compliance of children with thalassemia major. Chin Nurs Res 2008;22:3.Search in Google Scholar
21. Cai, Y, Yao, L, Zhang, XL, Li, JH, Xue, B, Li, XM. Nursing care of sequential psychological adaptation in children with umbilical cord blood stem cell transplantation. Chang Jiang Da Xue Xue Bao 2005;2:303.Search in Google Scholar
22. Yamashita, R, Sobota, A, Trachtenberg, F, Xu, Y, Pakbaz, Z, Odame, I, et al.. The impact of the child with thalassemia on the family: parental assessment by child health questionnaire. Blood 2009;114:1371–. https://doi.org/10.1182/blood.v114.22.1371.1371.Search in Google Scholar
23. Farashi, S, Harteveld, CL. Molecular basis of α-thalassemia. Blood Cells Mol Dis 2017;70:43–53. https://doi.org/10.1016/j.bcmd.2017.09.004.Search in Google Scholar PubMed
24. Casale, M, Meloni, A, Filosa, A, Cuccia, L, Caruso, V, Palazzi, G, et al.. Multiparametric cardiac magnetic resonance survey in children with thalassemia major: a multicenter study. Circ Cardiovasc Imaging 2015;8:e003230. https://doi.org/10.1161/circimaging.115.003230.Search in Google Scholar
25. Kurtoglu, AU, Kurtoglu, E, Temizkan, AK. Effect of iron overload on endocrinopathies in patients with beta-thalassaemia major and intermedia. Endokrynol Pol 2012;63:260–3.Search in Google Scholar
26. Pan, HF, Long, GF, Li, Q, Feng, YN, Lei, ZY, Wei, HW, et al.. Current status of thalassemia in minority populations in Guangxi, China. Clin Genet 2007;71:419–26. https://doi.org/10.1111/j.1399-0004.2007.00791.x.Search in Google Scholar PubMed
27. Mei, Z, Flores-Ayala, RC, Grummer-Strawn, LM, Brittenham, GM. Is erythrocyte protoporphyrin a better single screening test for iron deficiency compared to hemoglobin or mean cell volume in children and women. Nutrients 2017;9:557. https://doi.org/10.3390/nu9060557.Search in Google Scholar PubMed PubMed Central
28. Wan, LK, Chen, YH, Tian, M, He, B, Wang, L, Xu, CM. The value of ultrasonic detection of fetal umbilical movement and venous diameter in prenatal screening of thalassemia at 12–28 weeks of gestation. Zhong Hua Chao Sheng Ying Xiang Xue Za Zhi 2011;20:542–5.Search in Google Scholar
29. Tang, RQ, Li, B, Li, H. The significance of mean erythrocyte content, mean erythrocyte volume and erythrocyte volume distribution width in the differential diagnosis of iron deficiency anemia and thalassemia. Zhong Guo Lin Chuang Yi Sheng Za Zhi 2016;44:60–2.Search in Google Scholar
30. Tan, S, Li, QH, Chen, X, Li, CL. Clinical application value of screening for thalassemia. Jian Yan Yi Xue 2018;33:730–3.Search in Google Scholar
31. Liang, SL. Screening effect of hemoglobin electrophoresis on pregnant women with thalassemia. Zhong Guo Yi Yao Ke Xue 2018;8:92–4.Search in Google Scholar
32. Situ, WC, Que, GZ, Hu, YH, Yu, F. Value of MCV,RDW, and RBC fragility in prenatal screening for thalassemia. Shi Yong Yi Xue Za Zhi 201l;27:2976–7.Search in Google Scholar
33. He, Y, Zhang, YH, Wu, RX, Huang, YF. The value of mean volume, brittleness and capillary hemoglobin electrophoresis in the diagnosis of prenatal thalassemia. Guo Ji Jian Yan Yi Xue Za Zhi 2013;34:2521–3.Search in Google Scholar
34. Hanprasertpong, T, Kor-anantakul, O, Leetanaporn, R, Suntharasaj, T, Suwanrath, C, Pruksanusak, N, et al.. Pregnancy outcomes amongst thalassemia traits. Archiv Gynecol Obestet 2013;288:1051–4. https://doi.org/10.1007/s00404-013-2886-9.Search in Google Scholar PubMed PubMed Central
35. Tsianakas, V, Atkin, K, Calnan, MW, Dormandy, E, Marteau, TM. Offering antenatal sickle cell and thalassaemia screening to pregnant women in primary care: a qualitative study of women’s experiences and expectations of participation. Health Expect 2011;15:115–25. https://doi.org/10.1111/j.1369-7625.2011.00669.x.Search in Google Scholar PubMed PubMed Central
36. Chen, YB, Jiang, YC, Chen, ZX, Yang, W, Zhang, ZS. Establishment of diagnostic threshold of HbA2 screening for thalassemia and value of combined screening with MCV and MCH. Jian Yan Yi Xue 2019;34:318–21.Search in Google Scholar
37. Neonatal resuscitation Group, Perinatal Medicine Branch, Chinese Medical Association. Expert consensus on the diagnosis of neonatal asphyxia. Zhong Hua Wei Chan Yi Xue Za Zhi 2016;19:3–6.Search in Google Scholar
38. Sheiner, E, Levy, A, Yerushalmi, R, Katz, M. Beta-thalassemia minor during pregnancy. Obstet Gynecol 2004;103:1273–7. https://doi.org/10.1097/01.aog.0000126575.34482.fb.Search in Google Scholar PubMed
39. Dobbins, TA, Sullivan, EA, Roberts, CL, Simpson, JM. Australian national birthweight percentiles by sex and gestational age, 1998–2007. Med J Aust 2012;197:291–4. https://doi.org/10.5694/mja11.11331.Search in Google Scholar PubMed
40. DeBoer, MD, Lima, AA, Oría, RB, Scharf, RJ, Moore, SR, Luna, MA, et al.. Early childhood growth failure and the developmental origins of adult disease: do enteric infections and malnutrition increase risk for the metabolic syndrome. Nutr Rev 2012;70:642–53. https://doi.org/10.1111/j.1753-4887.2012.00543.x.Search in Google Scholar PubMed PubMed Central
41. Xie, X, Gou, WL. [Obstetrics and gynecology] [M], 8th ed. Beijing: People’s Medical Publishing House; 2013:92–3 pp.Search in Google Scholar
42. Hanke, K, Hartz, A, Manz, M, Bendiks, M, Heitmann, F, Orlikowsky, T, et al.. Preterm prelabor rupture of membranes and outcome of very-low-birth-weight infants in the German Neonatal Network. PLoS One 2015;10:e0122564. https://doi.org/10.1371/journal.pone.0122564.Search in Google Scholar PubMed PubMed Central
43. Nkwabong, E, Kamgnia Nounemi, N, Sando, Z, Mbu, RE, Mbede, J. Risk factors and placental histopathological findings of term born low birth weight neonates. Placenta 2014;36:138–41. https://doi.org/10.1016/j.placenta.2014.12.005.Search in Google Scholar PubMed
44. Charoenboon, C, Jatavan, P, Traisrisilp, K, Tongsong, T. Pregnancy outcomes among women with beta-thalassemia trait. Arch Gynecol Obstet 2015;293:771–4. https://doi.org/10.1007/s00404-015-3908-6.Search in Google Scholar PubMed
45. Mettananda, S, Suranjan, M, Fernando, R, Dias, T, Mettananda, C, Rodrigo, R, et al.. Anaemia among females in child-bearing age: relative contributions, effects and interactions of α- and β-thalassaemia. PLoS One 2018;13:e0206928. https://doi.org/10.1371/journal.pone.0206928.Search in Google Scholar PubMed PubMed Central
46. Pang, T, Guo, XF, Zhou, YH, Qiu, XQ, Li, M, Liang, ZR, et al.. Retrospective analysis of pregnancy outcomes of pregnant women with mild β-thalassemia in Pingguo, Guangxi Zhuang Autonomous Region. Zhonghua Liuxingbingxue Zazhi 2017;38:1620–3. https://doi.org/10.3760/cma.j.issn.0254-6450.2017.12.007.Search in Google Scholar PubMed
47. Wang, YT, Xu, YC, Liu, Y, Wang, RH, Zhang, LW. A systematic review of the effects of thalassemia on pregnancy outcomes in pregnant women. Chong Qing Yi Ke Da Xue Xue Bao 2015;40:569–75.Search in Google Scholar
48. Zhang, L, Wang, LJ. Changes of serum iron, zinc and ferritin in pregnant women with gestational diabetes during the second trimester and their correlation with glucose metabolism indexes. Zhong Guo Xian Dai Yi Xue Za Zhi 2017;27:71–5.Search in Google Scholar
49. Dewey, KG, Oaks, BM. U-shaped curve for risk associated with maternal hemoglobin, iron status, or iron supplementation. Am J Clin Nutr 2017;106:1694S–1702S. https://doi.org/10.3945/ajcn.117.156075.Search in Google Scholar PubMed PubMed Central
50. Li, MY, Wei, JX, Wu, HZ, He, QL. Changes of hemoglobin and ferritin levels and pregnancy outcomes in patients with mild thalassemia during pregnancy. Shan Dong Yi Yao 2014;54:17–19.Search in Google Scholar
51. Gérardin, P, Boumahni, B, Choker, G, Carbonnier, M, Gabrièle, M, Heisert, M, et al.. Twin pregnancies in southern Reunion Island: a three-year cross-sectional study of risk factors and complications. J Gynecol Obstet Biol Reprod (Paris) 2006;35:804–12. https://doi.org/10.1016/s0368-2315(06)76483-3.Search in Google Scholar PubMed
52. Lao, TT, Ho, LF. alpha-Thalassaemia trait and gestational diabetes mellitus in Hong Kong. Diabetologia 2001;44:966–71. https://doi.org/10.1007/s001250100594.Search in Google Scholar PubMed
53. Tsatalas, C, Chalkia, P, Pantelidou, D, Margaritis, D, Bourikas, G, Spanoudakis, E. Pregnancy in beta-thalassemia trait carriers: an uneventful journey. Hematology 2009;14:301–3. https://doi.org/10.1179/102453309x439791.Search in Google Scholar
54. Ma, Q, Chen, YB, Zhao, ZH, Kong, XD. Prenatal diagnosis and clinical analysis of thalassemia in Central China. Zhonghua Fu Chan Ke Za Zhi 2017;52:339–41.Search in Google Scholar
55. Hanprasertpong, T, Kor-anantakul, O, Leetanaporn, R, Suntharasaj, T, Suwanrath, C, Pruksanusak, N, et al.. Pregnancy outcomes amongst thalassemia traits. Arch Gynecol Obstet 2013;288:1051–4. https://doi.org/10.1007/s00404-013-2886-9.Search in Google Scholar PubMed PubMed Central
56. Duley, L. The global impact of pre-eclampsia and eclampsia. Semin Perinatol 2009;33:130–7. https://doi.org/10.1053/j.semperi.2009.02.010.Search in Google Scholar PubMed
57. Khan, KS, Wojdyla, D, Say, L, Gülmezoglu, AM, Van Look, PF. WHO analysis of causes of maternal death: a systematic review. Lancet 2006;367:1066–74. https://doi.org/10.1016/s0140-6736(06)68397-9.Search in Google Scholar
58. Tebeu, PM, Ngassa, P, Kouam, L, Major, AL, Fomulu, JN. Maternal mortality in Maroua Provincial Hospital, Cameroon (2003–2005). West Indian Med J 2007;56:502–7.Search in Google Scholar
59. Ghulmiyyah, L, Sibai, B. Maternal mortality from preeclampsia/eclampsia. Semin Perinatol 2012;36:56–9. https://doi.org/10.1053/j.semperi.2011.09.011.Search in Google Scholar PubMed
60. Ye, C, Ruan, Y, Zou, L, Li, G, Li, C, Chen, Y, et al.. The 2011 survey on hypertensive disorders of pregnancy (HDP) in China: prevalence, risk factors, complications, pregnancy and perinatal outcomes. PLoS One 2014;9:e100180. https://doi.org/10.1371/journal.pone.0100180.Search in Google Scholar PubMed PubMed Central
61. He, W, Wang, XD, Yu, HY. Research status of pregnancy complicated with thalassemia [EB/OL]. Zhong Hua Fu You Lin Chuang Yi Xue Za Zhi (Electronic edition) 2017;13:14–19.Search in Google Scholar
62. Luo, LL, Wang, Y, Xiao, L, Gan, YJ, Lv, F, Sun, JX, et al.. Research progress of pregnancy complicated with thalassemia. Zhong Guo You Sheng Yu Yi Chuan Za Zhi 2017;25:4–6.Search in Google Scholar
63. Luo, LL, Liang, XX, Ma, YH, Huang, Y. Serum ferritin level changes and pregnancy outcome in pregnant women with α-thalassemia of South-east Asian type deletion. Hainan Yi Xue 2021;32:4.Search in Google Scholar
64. Zhang, JY, Zhang, YY, Jia, J, Dai, L, Zhou, R. Analysis of pregnancy outcomes in women with thalassemia. Xian Dai Yu Fang Yi Xue 2013;40:29–31.Search in Google Scholar
65. Hanprasertpong, T, Kor-anantakul, O, Leetanaporn, R, Suntharasaj, T, Suwanrath, C, Pruksanusak, N, et al.. Pregnancy outcomes amongst thalassemia traits. Arch Gynecol Obstet 2013;288:1051–4. https://doi.org/10.1007/s00404-013-2886-9.Search in Google Scholar
66. Wei, JW, Wu, P. Analysis of related influencing factors of hypertensive diseases in pregnancy. Chong Qing Yi Xue 2016;45:3078–80.Search in Google Scholar
67. Tebeu, PM, Mboudou, E, Halle, G, Kongnyuy, E, Nkwabong, E, Fomulu, JN. Risk factors of delivery by caesarean section in Cameroon (2003–2004): a regional hospital report. ISRN Obstet Gynecol 2011;2011:791319. https://doi.org/10.5402/2011/791319.Search in Google Scholar PubMed PubMed Central
68. Mhaske, N, Agarwal, R, Wadhwa, RD, Basannar, DR. Study of the risk factors for cesarean delivery in induced labors at term. J Obstet Gynecol India 2014;65:236–40. https://doi.org/10.1007/s13224-014-0596-2.Search in Google Scholar PubMed PubMed Central
69. Zhu, Q, Cao, YL, Li, J. Analysis of pregnant women with thalassemia and its influence on fetus. Zhong Guo Fu You Wei Sheng Za Zhi 2018;9:59–61.Search in Google Scholar
70. Wang, ZX, Luo, X, Li, WJ. Screening of pregnancy complicated with mild thalassemia and retrospective analysis of pregnancy outcomes. Shi Yong Fu Chan Ke Za Zhi 2005;21:245–7.Search in Google Scholar
71. Ansari, S, Azarkeivan, A, Tabaroki, A. Pregnancy in patients treated for beta thalassemia major in two centers (Ali Asghar Children’s Hospital and Thalassemia Clinic): outcome for mothers and newborn infants. Pediatr Hematol Oncol 2006;23:33–7. https://doi.org/10.1080/08880010500313306.Search in Google Scholar PubMed
72. Origa, R, Piga, A, Quarta, G, Forni, GL, Longo, F, Melpignano, A, et al.. Pregnancy and beta-thalassemia: an Italian multicenter experience. Haematologica 2009;95:376–81. https://doi.org/10.3324/haematol.2009.012393.Search in Google Scholar PubMed PubMed Central
73. Nassar, AH, Naja, M, Cesaretti, C, Eprassi, B, Cappellini, MD, Taher, A. Pregnancy outcome in patients with beta-thalassemia intermedia at two tertiary care centers, in Beirut and Milan. Haematologica 2008;93:1586–7. https://doi.org/10.3324/haematol.13152.Search in Google Scholar PubMed
74. Chen, XM, Guo, Y, Liang, H, Zhu, PY, Luo, HQ, Jiang, FF. Application of hemoglobin electrophoresis combined with mean red cell volume and serum iron detection in screening pregnant women for thalassemia. Cheng Du Yi Xue Yuan Xue Bao 2020;15:76–9.Search in Google Scholar
75. Guo, LL, Li, WJ, Pan, XD, Yuan, L. Genotype analysis of thalassemia in pregnant women in Guangming New District, Shenzhen from 2016 to 2017. Zhong Guo Fu You Bao Jian 2019;34:1604–6.Search in Google Scholar
76. Pan, Y, Ge, HS, Chen, H. A clinical study of iron supplementation in pregnant women with thalassemia trait. Chinese J Family Plan Gynecol 2019;11:4.Search in Google Scholar
77. Yang, CR, Zhu, FH. Changes of hemoglobin and serum protein in pregnant women with mild thalassemia and their clinical significance. Zhong Guo Di Fang Bing Fang Zhi Za Zhi 2016;31:1165–6.Search in Google Scholar
78. Farmaki, K, Gotsis, E, Tzoumari, I, Berdoukas, V. Rapid iron loading in a pregnant woman with transfusion-dependent thalassemia after brief cessation of iron chelation therapy. Eur J Haematol 2008;81:157–9. https://doi.org/10.1111/j.1600-0609.2008.01092.x.Search in Google Scholar PubMed PubMed Central
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Reviews
- Vasa previa guidelines and their supporting evidence
- Fetal origins of adult disease: transforming prenatal care by integrating Barker’s Hypothesis with AI-driven 4D ultrasound
- Original Articles – Obstetrics
- Postpartum remote blood pressure monitoring and risk of hypertensive-related readmission: systematic review and meta-analysis of randomized controlled trials
- Proposal of a novel index in assessing perinatal mortality in prenatal diagnosis of Sacrococcygeal teratoma
- Maternity staff views on implementing a national perinatal mortality review tool: understanding barriers and facilitators
- Prenatal care for twin pregnancies: analysis of maternal and neonatal morbidity and mortality
- Hematological indicators and their impact on maternal and neonatal outcomes in pregnancies with thalassemia traits
- The reference ranges for fetal ductus venosus flow velocities and calculated waveform indices and their predictive values for right heart diseases
- Risk factors and outcomes of uterine rupture before onset of labor vs. during labor: a multicenter study
- Feasibility and reproducibility of speckle tracking echocardiography in routine assessment of the fetal heart in a low-risk population
- Enhancing external cephalic version success: insights from an Israeli tertiary center
- Original Articles – Fetus
- Comparative sonographic measurement of the fetal thymus size in singleton and twin pregnancies
- Transversal cardiac diameter is increased in fetuses with dextro-transposition of the great arteries older than 28th weeks of gestation
- Short Communications
- Severe maternal morbidity in twin pregnancies: the impact of body mass index and gestational weight gain
- Trends in gestational age and short-term neonatal outcomes in the United States
- Letter to the Editor
- Mechanisms of hypoxaemia in late pulmonary hypertension associated with bronchopulmonary dysplasia
Articles in the same Issue
- Frontmatter
- Reviews
- Vasa previa guidelines and their supporting evidence
- Fetal origins of adult disease: transforming prenatal care by integrating Barker’s Hypothesis with AI-driven 4D ultrasound
- Original Articles – Obstetrics
- Postpartum remote blood pressure monitoring and risk of hypertensive-related readmission: systematic review and meta-analysis of randomized controlled trials
- Proposal of a novel index in assessing perinatal mortality in prenatal diagnosis of Sacrococcygeal teratoma
- Maternity staff views on implementing a national perinatal mortality review tool: understanding barriers and facilitators
- Prenatal care for twin pregnancies: analysis of maternal and neonatal morbidity and mortality
- Hematological indicators and their impact on maternal and neonatal outcomes in pregnancies with thalassemia traits
- The reference ranges for fetal ductus venosus flow velocities and calculated waveform indices and their predictive values for right heart diseases
- Risk factors and outcomes of uterine rupture before onset of labor vs. during labor: a multicenter study
- Feasibility and reproducibility of speckle tracking echocardiography in routine assessment of the fetal heart in a low-risk population
- Enhancing external cephalic version success: insights from an Israeli tertiary center
- Original Articles – Fetus
- Comparative sonographic measurement of the fetal thymus size in singleton and twin pregnancies
- Transversal cardiac diameter is increased in fetuses with dextro-transposition of the great arteries older than 28th weeks of gestation
- Short Communications
- Severe maternal morbidity in twin pregnancies: the impact of body mass index and gestational weight gain
- Trends in gestational age and short-term neonatal outcomes in the United States
- Letter to the Editor
- Mechanisms of hypoxaemia in late pulmonary hypertension associated with bronchopulmonary dysplasia

