Transversal cardiac diameter is increased in fetuses with dextro-transposition of the great arteries older than 28th weeks of gestation
-
Oskar Sylwestrzak
, Aleksandra Piórecka
and Maria Respondek-Liberska
Abstract
Objectives
In majority of congenital heart defects the size of the fetal heart is normal (without cardiomegaly). Aim of this study was to establish normal ranges of fetal transversal cardiac diameter (4CV TW ED) and to compare fetal dextro-transposition of the great arteries (d-TGA) with normal ranges for 4CV TW ED.
Methods
Retrospectively of 3,553 records we analyzed and included 1,154 healthy singleton fetuses as a control group. Consecutive percentiles for 4CV TW ED diameter according to the gestational age (GA) were calculated. 74 fetuses with d-TGA were analyzed in 3rd trimester.
Results
The −2, −1, +1 and +2 Z-scores of 4CV TW ED between weeks 18 and 37 of gestation were calculated. In the group of fetuses with d-TGA 68 % of them had 4CV TW ED > +2 Z-score fitted for GA. Rashkind procedure during first 24 h after birth was performed in 63 % cases. Increased 4CV TW ED was more frequently seen in fetuses who needed Rashkind procedure after birth, but without statistical significance.
Conclusions
4CV TW ED measurement during the third trimester scans as a cardiac screening tool in obstetrical practice may potentially help to detect d-TGA and indicate further echocardiographic examination in case of d-TGA suspicion. 4CV TW ED was not helpful to predict the necessity for neonatal Rashkind procedure.
Introduction
Fetal heart size (FHS) measurement is a fundamental and essential part of fetal echocardiographic examination [1], 2]. The fetal heart should be assessed during every fetal anatomical ultrasound. Nevertheless, as highlighted by prof. DeVore it would be preferable to directly measure the end-diastolic size of the heart in the four-chamber view rather than to assume that its size is increased or decreased based on the cardiothoracic area ratio [3]. According to recommendations in many guidelines, abnormal heart size values should indicate the need for a detailed fetal echocardiography [1], 4]. With the continued advancement of prenatal diagnosis, an increasing number of pregnancies complicated by fetal congenital heart defects (CHD) are being detected [5]. Cardiomegaly might be present in certain conditions, such as Ebstein anomaly and ventricular diverticulum (Table 1) [6], [7], [8]. Polish Society of Gynecologists and Obstetricians recommends the last fetal ultrasound between 28th to 32nd week of gestation. However, pregnancies may continue until the 40th week of gestation, and the longer pregnancy duration lacks ultrasound evaluation. The so-called “third trimester ultrasound” could be the last opportunity to detect a fetal heart defect. In clinical practice, the quality of late ultrasound and echocardiography depends significantly on maternal and fetal conditions, especially maternal weight, amniotic fluid volume, and fetal position. It also typically requires experience and favourable clinical skills. As presented by DeVore G. et al. fetal end-diastolic transversal width of the heart in four chamber view (4CV TW ED) might be a simple screening tool to evaluate fetuses who may have undetected dextro-transposition of the great arteries (d-TGA) [9]. Therefore, the aim of this study was to reevaluate the potential for late detection of d-TGA based on simple fetal heart measurement.
| Congenital heart defect |
| Absent aortic valve syndrome |
| Aneurysm/diverticulum |
| Aortic stenosis |
| Ebstein’s anomaly |
| Mitral atresia |
| Myocardial dysplasia or noncompaction |
| Pulmonary arteriovenous malformation |
| Pulmonary atresia |
| Pulmonary stenosis |
| Tricuspid valve atresia |
| Tricuspid valve dysplasia |
Materials and methods
This was a single-centre, retrospective study, based on a database of ultrasound and echocardiographic examination records, performed at a tertiary fetal cardiology center. Fetal ultrasound scans and fetal echocardiographic examinations were conducted by fetal medicine specialists using the Samsung HERA w10, GE Voluson E8, GE Voluson 10, and Phillips iU22.
Estimation of normal values of four chamber view end-diastolic transverse width (4CV TW ED)
Gestational age (GA) at the time of examination (based on the last menstrual period if confirmed by CRL measurement from the first trimester ultrasound), 4CV TW ED measured at the level of the four-chamber view (in diastole, just before atrioventricular valves open), cardiac problems and extracardiac problems were all collected. All fetuses underwent at least one echocardiographic examination. Inclusion criteria included: normal fetal biometry, normal heart anatomy and function, no extracardiac malformations (defined as abnormalities requiring surgery after birth), and no extracardiac anomalies (defined as abnormalities not requiring surgery after birth, such as single umbilical artery). Fetuses with maternal diabetes, maternal Hashimoto, maternal pharmacotherapy, maternal heart problems, maternal hypertension, functional anomalies, oligo- or polyhydramnios, a two-vessel cord or any other fetal abnormality were excluded. These fetuses constituted the reference group for the normal 4CV TW ED diameter analysis. Data from all patients were de-identified. The data were collected from our unit’s database and reevaluated for this analysis.
Ethical approval is not applicable due to the retrospective nature of the study and the absence of any intervention. Additional approval from the Ethics Committee was not necessary, as the focus was on the interpretation of previously collected data.
4CV TW ED values in d-TGA fetuses
The study group consisted of fetuses with d-TGA, without extracardiac malformations, and older than 28th weeks of gestation (third trimester of pregnancy). The analyzed parameters were identical to those in the control group and included 4CV TW ED of the heart. Information about GA at delivery, type of delivery, birthweight, Apgar score, and whether a Rashkind procedure was performed on the 1st day of postnatal life were also analyzed.
Statistical analysis
For statistical analysis Statistica 13.1 and Excel 2007 programs were used. Continuous variables were expressed as means, and qualitative variables as numbers (%). Minimal and maximal values, as well as confidence intervals, were presented where applicable. The normality of the distribution of continuous variables was checked using Shapiro–Wilk test. For each consecutive gestational week, the mean 4CV TW ED diameter was estimated and presented with confidence interval CI 0.95 and 5th, 50th and 95th percentile. Pearson’s linear regression was used for the 4CV TW ED TCD analysis. Z-scores for 4CV TW ED were calculated [10].
Results
Control group
The age of the fetuses ranged from min. 18 to max. 37 weeks of gestation according to fetal biometry in singleton pregnancies. Of 3,553 records for estimation of 4CV TW ED nomograms 1,154 healthy fetuses met inclusion criteria and were included in the control group.
The regression equation for 4CV TW ED as a function of GA in weeks was:
Linear regression demonstrated statistically significant correlation between 4CV TW ED and GA.
Z-score values of 4CV TW ED were calculated according to DeVore [10] and presented in Figures 1 and 2 and Table 2.

Scatter graph of integrated normal 4CV TW ED of singleton healthy fetuses between 18th and 37th week of gestation.
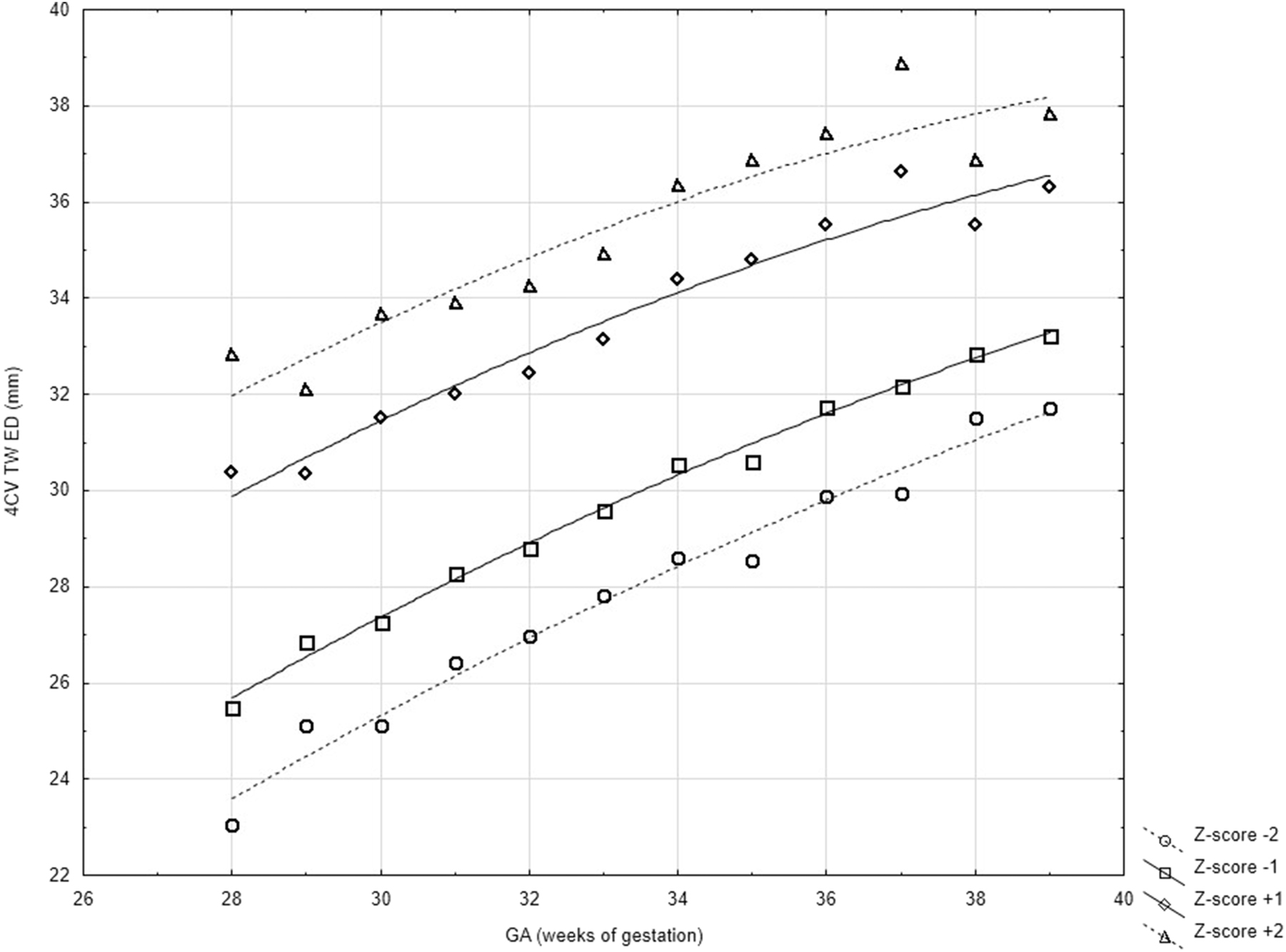
Normal values of 4CV TW ED of singleton healthy fetuses between 18th and 37th week of gestation using fractional polynomial regression analysis.
Normal 4CV TW ED during gestation presented as Z-scores.
| GA, week | No. of fetuses | Mean | −2 Z-score | −1 Z-score | +1 Z-score | +2 Z-score |
|---|---|---|---|---|---|---|
| 18 | 35 | 20 | 18 | 19 | 22 | 23 |
| 19 | 28 | 21 | 17 | 19 | 23 | 25 |
| 20 | 48 | 22 | 18 | 20 | 24 | 26 |
| 21 | 30 | 22 | 20 | 21 | 24 | 25 |
| 22 | 41 | 23 | 18 | 21 | 26 | 28 |
| 23 | 41 | 24 | 21 | 22 | 26 | 27 |
| 24 | 44 | 25 | 19 | 22 | 28 | 31 |
| 25 | 50 | 26 | 21 | 24 | 28 | 30 |
| 26 | 56 | 27 | 22 | 24 | 29 | 31 |
| 27 | 52 | 27 | 23 | 25 | 29 | 31 |
| 28 | 55 | 28 | 23 | 25 | 30 | 33 |
| 29 | 59 | 29 | 25 | 27 | 30 | 32 |
| 30 | 92 | 29 | 25 | 27 | 32 | 34 |
| 31 | 72 | 30 | 26 | 28 | 32 | 34 |
| 32 | 73 | 31 | 27 | 29 | 32 | 34 |
| 33 | 63 | 31 | 28 | 30 | 33 | 35 |
| 34 | 57 | 32 | 29 | 31 | 34 | 36 |
| 35 | 47 | 33 | 29 | 31 | 35 | 37 |
| 36 | 33 | 34 | 30 | 32 | 36 | 37 |
| 37 | 40 | 34 | 30 | 32 | 37 | 39 |
4CV TW ED in D-transposition of the great arteries fetuses-study group
There were 74 fetuses with d-TGA, without extracardiac malformations, between 18th and 40th weeks of gestation. These 74 fetuses had 152 fetal echocardiographic examinations (range 1–7). 63 cases had 128 echocardiographic examinations performed between 28th to 40th week of gestation. There were 18 cases of d-TGA had additionally small ventricular septal defect. There were 43 male and 20 female fetuses. In the study group 43 cases (68 %) presented 4CV TW ED measurement greater than +2 Z-score fitted to gestational age [Figure 3]. Mean gestational age at birth was 38 ± 1 week. There were 34 (54 %) cesarean sections, 28 (44 %) vaginal deliveries and 1 (1 %) forceps delivery. Mean birth weight was 3,257 ± 452 g. Median Apgar score for 1st and 5th minute was 9 and 9. Data for Rashkind procedure was available for 47 cases (75 %).

4CV TW ED values of d-TGA cases on 0.95 CI scatter graph. 30 cases (68 %) of d-TGA cases presented increased 4CV TW ED measurement during pregnancy according to normal values presented in Table 1.
Baloon atrial septostomy
Of these 47 cases Rashkind procedure during first 24 h after birth was performed in 30 (63 %) cases. Of these 30 cases who had Rashkind procedure, 23 cases (76 %) had 4CV TW ED value greater than Z-score +2 fitted for GA during pregnancy and 7 (23 %) cases had normal 4CV TW ED (Figure 4). Of 17 cases who did not have Rashkind procedure, 9 (53 %) cases had 4CV TW ED value greated than Z-score +2 fitted for GA and 8 (47 %) cases had normal 4CV TW ED. Increased 4CV TW ED was more frequently seen in fetuses who needed Rashkind procedure after birth, but without statistical significance (p=0.09).

4CV TW ED values of d-TGA cases with and without Rashkind on 0.95 CI scatter graph. 4CV TW ED is not predictive for Rashkind procedure.
Discussion
FHS assessment can be performed in several ways [11], [12], [13], [14]. The literature includes numerous articles on fetal heart measurements [Table 3] [11], 13], [15], [16], [17], [18]. Here, we present a continuation and development of previously published study [16]. Respondek et al. in 1992 described methods for assessing the FHS by calculating the ratio of the area of the four chambers to the chest area when viewed in the same plane. Additionally, She presented a method involving the measurement of the 4CV TW ED of the heart. In this second method, dimensions are measured in the longitudinal plane of the fetal trunk divided by the 4CV TW ED diameter of the chest just above the liver [16]. In her study, fetal echocardiograms were obtained from 99 women between the 22nd and 38th weeks of pregnancy. In our another study conducted in 2018, we analyzed fetal heart measurements including ratio of heart area to chest area (HA/CA) and 4CV TW ED, and their correlation with GA. Our study group consisted of 609 fetuses, and we concluded that the hearts’ transverse diameter correlates with GA, while HA/CA ratio remained relatively constant with slight increase as GA progressed [18]. 4CV TW ED was found to be a simple method for measuring FHS (Figure 4). As shown in our new nomograms, the 4CV TW ED correlates with GA (r=0.91). Our nomograms presented the Z-scores of 4CV TW ED between weeks 18 and 37 of gestation and can be used in clinical practice to confirm normal FHS. For further analysis, dimensions above Z-score +2 were considered increased. These nomograms may be useful in evaluating both normal and abnormal fetuses and in assessing the progression of heart defect.
Methods of FHS assessment in various studies.
| Studies about FHS measurements | Year of publication | Methods of FHS assessment | Numbers of fetuses |
|---|---|---|---|
| Sylwestrzak & Respondek-Liberska et al. | 2024 | 4CV TW ED | 1,154 |
| Sylwestrzak & Respondek-Liberska [18]. | 2018 | 4CV TW ED HA/CA | 609 |
| Lussier et al. [17] | 2015 | HC | 575 |
| Luewan et al. [13] | 2011 | Heart volume (cardio-STIC-M) | 657 |
| Gembruch et al. [11] | 2000 | 4CV TW ED ventricular dimensions, interventricular septal thickness, HA HC |
136 |
| Respondek et al. [16] | 1992 | 4CV TW ED | 99 |
| Jeanty et al. [15] | 1984 | 4CV TW ED AP L Heart volume |
695 |
-
4CV TW ED, transverse diameter of heart; AP L, longitudinal diameter of heart; HA, heart area; HA/CA, heart area to chest area; HD, heart diameter; HC, heart circumference; HL, heart length; LA/RA, width of left/right atrium; LV/RV, width of left/right ventricle.

Four-chamber view of fetal heart. The same time 4CV TW ED (measurement D1), longitudinal fetal heart diameter, heart area and chest area measurements may be obtained.
CHD are the most common congenital defects. Depending on type of defect, study population, and geographic variations, the prevalence of CHD ranges from 3 to 12 per 1,000 pregnancies [19], 20]. Since the 1980s, echocardiographic examination has become the most important technique for the prenatal detection and diagnosis of CHD. Every fetus with CHD, particularly those with abnormal fetal heart structure, has its own unique haemodynamic physiology. In these cases, cardiac remodeling may depend on the type of defect and haemodynamic stability of the fetus. Known patterns of remodeling include changes in shape (response to pressure overload), myocardial hypertrophy (response to pressure overload), cavity dilation (improving contractility as response to volume overload) or hypoplasia. Cardiac remodeling in structure and shape is usually correlated with changes in heart function. This process can lead to heart dysfunction, which may then affect systolic and/or diastolic function [21], [22], [23]. Mild cardiomegaly defined as 4CV TW ED greater than Z-score +2 for GA, may indicate fetal heart remodeling as a compensatory response. In cases of d-TGA abnormal ventriculo-arterial connections results in pathological conditions for both the left and right ventricles, which normally pump blood to the correct parts of the fetus. This type of response, including myocardial hypertrophy, may increase fetal inotropic capability, and in some cases, protect the fetal cardiovascular system from increased intracardiac pressure and circulatory failure caused by abnormal fetal heart anatomy. However, future, more profound studies are needed.
In 1984, Jeanty and colleagues created nomograms of transverse and longitudinal diameters of the fetal heart, which were useful in diagnosis various congenital anomalies [15]. They developed nomograms for 12–40 weeks of gestation the mean value of 4CV TW ED increases by approximately 1–2 mm each week, which was also confirmed by our study. The highest value recorded in the range of 18–37 weeks was 45 mm located at the 95 percentile. In our research, the highest value was also observed at 37 weeks, which was 39 mm, located in Z-score +2. The lowest value in Jeanty and other studies was 10 mm, located in 5 percentile, examined in 18 weeks. In contrast, we observed 12 mm in 18 weeks, located under Z-score −2. These dissimilarities likely have no significant clinical value.
According to ISUOG guidelines, FHS measurement is recommended between 18 and 22 weeks of pregnancy. However, as we have demonstrated, examining the fetal heart in the third trimester of pregnancy, particularly near term, is an important component of prenatal diagnosis and may be even considered mandatory [24]. A similar result was presented by DeVore et al., suggesting that the late third trimester could be the last chance not to miss d-TGA cases [9]. Some signs of circulatory failure in the fetus may appear at the end of pregnancy, often preceded by heart dysfunction and remodeling, which can be detected through echocardiographic examination [25]. Moreover, not all CHD are detected during second-trimester ultrasound. Abnormal fetal heart dimensions could potentially help to identify patients who will require further specialized evaluation and both prenatal and postnatal care [26]. Certain forms of heart defect, such as Ebstein’s anomaly, are particularly associated with cardiac enlargement. Nevertheless, diagnosis of Ebstein’s anomaly is relatively straightforward compared to the d-TGA. Diagnosing d-TGA requires obtaining images of the outflow tracts and visualizing the ventriculo-arterial connections. The prenatal detection rate of d-TGA in obstetrical ultrasound varies between 50 and 75 %, depending on the center and the examiner. Because many examiners are not comfortable with the outflow tract examination, as demonstrated in our study, 4CV TW ED may serve as a screening tool for further echocardiographic evaluation (Figure 5). This simple method identified increased 4CV TW ED values in 68 % of d-TGA. Mild cardiomegaly is often the only feature visible in the four-chamber view of fetal d-TGA. The prenatal detection of d-TGA is crucial for several reasons: medical (it may be beneficial for the newborn), legal (in case of late postnatal detection and newborn transportation to referral center, one may avoid problem of malpractice, as the newborn eventually would be appropriately treated) and family-related (in cases where the mother wishes to terminate the pregnancy based on prenatal diagnosis). For d-TGA, prenatal detection is particularly valuable due to the urgent need for balloon atrial septostomy after birth in about half of the cases. Missing the prenatal diagnosis may lead to postnatal hemodynamic deterioration, hypoxia and an unfavorable prognosis. The detection of CHD can also be used to assess the quality and experience of the primary care obstetrician performing routine ultrasound scans.
Advances in ultrasound technology have made it possible to detect congenital heart defects as early as the first trimester or between 18 and 20 weeks of gestation. Abnormal heart position in the chest (such as dextrocardia), abnormal heart axis (such as zero axis or 90° axis), or abnormal four-chamber view or three-vessels view may suggest the presence of CHD. The detection rate of CHD in routine obstetric ultrasound is around 40 % in low-risk populations [27]. Numerous factors can hinder or distort the examination, but there is a significant difference between basic obstetrical ultrasound and targeted fetal echocardiography. In specialized centers, the detection rate of CHD increases with fetal echocardiography. Image quality substantially decreases with increasing maternal BMI, and detection rate is unlikely to exceed 20 % in such cases [28]. Additionally, fetal position can affect the accuracy of CHD diagnosis [29]. Our simple 4CV TW ED measurement was incorrect in up to 68 % of d-TGA cases. This suggests that 4CV TW ED could be an effective method for indicating the need for further targeted echocardiographic examination in the third-trimester ultrasonography. Prenatal diagnosis improves the quality of perinatal care and allows for better preparation for a complicated postnatal period [30], 31]. The Polish Society of Gynecologists and Obstetricians recommends the last fetal anatomical ultrasound between 28th to 32nd week of gestation. They recommend assessing FHS, noting that the heart area should be about one-third of the fetal thorax area [32]. In case of d-TGA, the heart-to-chest area ratio may still be within normal values. The 4CV TW ED method seems much quicker and easier, making it especially useful for late scans after 28 weeks. In this context, our method could have a potential impact on d-TGA detection because even an inexperienced sonographer can easily classify a fetus to high-risk (the group that need further echocardiographic evaluation) using simple graph estimation. There are many causes of fetal heart mild cardiomegaly. One potential cause is d-TGA, but regardless of the underlying cause, all cases of cardiomegaly should be further evaluated with targeted fetal echocardiography. A similar finding was firstly presented by DeVore et al., although in this study, they focused on the Tetralogy of Fallot [33]. Our findings suggest that even simple ultrasound machines-without special fetal echocardiography software (such as 4D scans or artificial intelligence) [34], 35] – remain essential in daily practice for CHD screening. Given its potential clinical value, 4CV TW ED measurement could be incorporated into every fetal ultrasound examination, especially after the 28th week of gestation.
An interesting observation was that fetuses with d-TGA who showed cardiomegaly prenatally more often required an urgent Rashkind procedure after birth. The prenatal prediction of the need for an urgent Rashkind procedure in the context of fetal d-TGA has been studied before. However, 4CV TW ED was not as satisfactory a predictor as other parameters and did not present statistical significance [36], [37], [38], [39] (Supplementary 1).
Conclusions
4CV TW ED measurement is a simple parameter that directly shows fetal heart size. Using 4CV TW ED measurement during third-trimester scans as a cardiac screening tool in obstetric practice may help to detect d-TGA and indicate the need for further echocardiographic examination in case of d-TGA suspicion. However, 4CV TW ED was not useful in predicting the necessity for a neonatal Rashkind procedure.
-
Research ethics: The local Institutional Review Board deemed the study exempt from review.
-
Informed consent: Informed consent was obtained from all individuals included in this study.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Use of Large Language Models, AI and Machine Learning Tools: None declared.
-
Conflict of interest: The authors state no conflict of interest.
-
Research funding: None declared.
-
Data availability: Not applicable.
References
1. Respondek-Liberska, M, Sklansky, M, Wood, D, Słodki, M, Weiner, S, Cuneo, B, et al.. Recommendations for fetal echocardiography in singleton pregnancy in 2015. Prenatal Cardiol 2015;2:28–34.Search in Google Scholar
2. Moon-Grady, AJ, Donofrio, MT, Gelehrter, S, Hornberger, L, Kreeger, J, Lee, W, et al.. Guidelines and recommendations for performance of the fetal echocardiogram: an update from the American society of echocardiography. J Am Soc Echocardiogr 2023;36:679–723. https://doi.org/10.1016/j.echo.2023.04.014.Search in Google Scholar PubMed
3. DeVore, GR. Enhancement of evaluation of the fetal heart as proposed by ISUOG guidelines for third-trimester ultrasound examination. Ultrasound Obstet Gynecol 2024;64:696–9. https://doi.org/10.1002/uog.27660.Search in Google Scholar PubMed
4. American Institute of Ultrasound in Medicine. AIUM practice guideline for the performance of fetal echocardiography. J Ultrasound Med 2013;32:1067–82. https://doi.org/10.7863/ultra.32.6.1067.Search in Google Scholar PubMed
5. Hernandez-Andrade, E, Patwardhan, M, Cruz-Lemini, M, Luewan, S. Early evaluation of the fetal heart. Fetal Diagn Ther 2017;42:161–73. https://doi.org/10.1159/000477564.Search in Google Scholar PubMed
6. DeVore, GR, Tabsh, K, Polanco, B, Satou, G, Sklansky, M. Fetal heart size: a comparison between the point-to-point trace and automated ellipse methods between 20 and 40 Weeks’ gestation. J Ultrasound Med 2016;35:2543–62. https://doi.org/10.7863/ultra.16.02019.Search in Google Scholar PubMed
7. Płużańska, J, Respondek-Liberska, M. Fetal echocardiography in uncommon parental cardiac anomalies: right atrium diverticulum, interventricular septal aneurysm, left and right ventricle diverticulum – report from referral center for fetal cardiology in Poland. Prenatal Cardiol 2018;1:24–34.10.1515/pcard-2018-0004Search in Google Scholar
8. Rydzewska, K, Sylwestrzak, O, Krekora, M, Słodki, M, Respondek-Liberska, M. Ebstein’s anomaly: epidemiological analysis and presentation of different prenatal management. J Matern Fetal Neonatal Med 2022;35:3297–304. https://doi.org/10.1080/14767058.2020.1818207.Search in Google Scholar PubMed
9. DeVore, GR, Cuneo, B, Sklansky, M, Satou, G. Abnormalities of the width of the four-chamber view and the area, length, and width of the ventricles to identify fetuses at high-risk for D-transposition of the great arteries and Tetralogy of Fallot. J Ultrasound Med 2023;42:637–46. https://doi.org/10.1002/jum.16060.Search in Google Scholar PubMed
10. DeVore, GR. Computing the Z Score and Centiles for cross-sectional analysis: a practical Approach. J Ultrasound Med 2017;36:459–73. https://doi.org/10.7863/ultra.16.03025.Search in Google Scholar PubMed
11. Gembruch, U, Shi, C, Smrcek, JM. Biometry of the fetal heart between 10 and 17 weeks of gestation. Fetal Diagn Ther 2000;15:20–31. https://doi.org/10.1159/000020970.Search in Google Scholar PubMed
12. Rozmus-Warcholinska, W, Wloch, A, Acharya, G, Cnota, W, Czuba, B, Sodowski, K, et al.. Reference values for variables of fetal cardio circulatory dynamics at 11-14 weeks of gestation. Ultrasound Obstet Gynecol 2010;35:540–7. https://doi.org/10.1002/uog.7595.Search in Google Scholar PubMed
13. Luewan, S, Yanase, Y, Tongprasert, F, Srisupundit, K, Tongsong, T. Fetal cardiac dimensions at 14-40 weeks’ gestation obtained using cardio-STIC-M. Ultrasound Obstet Gynecol 2011;37:416–22. https://doi.org/10.1002/uog.8961.Search in Google Scholar PubMed
14. Li, X, Zhou, Q, Huang, H, Tian, X, Peng, Q. Z-score reference ranges for normal fetal heart sizes throughout pregnancy derived from fetal echocardiography. Prenat Diagn 2015;2:117–24. https://doi.org/10.1002/pd.4498.Search in Google Scholar PubMed
15. Jeanty, P, Romero, R, Cantraine, F, Cousaert, E, Hobbins, JC. Fetal cardiac dimensions: a potential tool for the diagnosis of congenital heart defects. J Ultrasound Med 1984;8:359–64. https://doi.org/10.7863/jum.1984.3.8.359.Search in Google Scholar PubMed
16. Respondek, M, Respondek, A, Huhta, JC, Wilczynski, J. 2D echocardiographic assessment of the fetal heart size in the 2nd and 3rd trimester of uncomplicated pregnancy. Eur J Obstet Gynecol Reprod Biol 1992;44:185–8. https://doi.org/10.1016/0028-2243(92)90096-h.Search in Google Scholar PubMed
17. Lussier, EC, Yeh, SJ, Chih, WL, Lin, SM, Chou, YC, Huang, SP, et al.. Reference ranges and Z-scores for fetal cardiac measurements from two-dimensional echocardiography in Asian population. PLoS One 2020;15:e0233179. https://doi.org/10.1371/journal.pone.0233179.Search in Google Scholar PubMed PubMed Central
18. Sylwestrzak, O, Respondek-Liberska, M. Echocardiographic methods of fetal heart size assessment- heart to chest area ratio and transversal heart diameter. Prenatal Cardiol 2018;8:20–3. https://doi.org/10.1515/pcard-2018-0003.Search in Google Scholar
19. Axt-Fliedner, R, Chiriac, A, Gembruch, U. First and early second trimester fetal heart scanning. Ultraschall Med 2009;30:364–75. https://doi.org/10.1055/s-0028-1109358.Search in Google Scholar PubMed
20. Ge, CJ, Mahle, AC, Burd, I, Jelin, EB, Sekar, P, Jelin, AC. Fetal CHD and perinatal outcomes. Cardiol Young 2020;30:686–91. https://doi.org/10.1017/s1047951120000785.Search in Google Scholar PubMed PubMed Central
21. Thakur, V, Fouron, JC, Mertens, L, Jaeggi, ET. Diagnosis and management of fetal heart failure. Can J Cardiol 2013;7:759–67. https://doi.org/10.1016/j.cjca.2013.02.001.Search in Google Scholar PubMed
22. Andrés-Delgado, L, Mercader, N. Interplay between cardiac function and heart development. Biochim Biophys Acta 2016:1707–16. https://doi.org/10.1016/j.bbamcr.2016.03.004.Search in Google Scholar PubMed PubMed Central
23. Crispi, F, Sepúlveda-Martínez, Á, Crovetto, F, Gómez, O, Bijnens, B, Gratacós, E. Main patterns of fetal cardiac remodeling. Fetal Diagn Ther 2020;47:337–44. https://doi.org/10.1159/000506047.Search in Google Scholar PubMed
24. Sokołowski, Ł, Słodki, M, Murlewska, J, Strzelecka, I, Kordjalik, I, Blitek, M, et al.. Fetal echocardiography in the 3rd trimester of pregnancy as an essential element of modern prenatal diagnostics and perinatal care – recommendations of Polish Society of Prenatal Cardiology 2020. Prenatal Cardiol 2020;1:5–12. https://doi.org/10.5114/pcard.2020.102272.Search in Google Scholar
25. Crispi, F, Gratacós, E. Fetal cardiac function: technical considerations and potential research and clinical applications. Fetal Diagn Ther 2012;32:47–64. https://doi.org/10.1159/000338003.Search in Google Scholar PubMed
26. Slodki, M, Janiak, K, Zarkowska, A, Forys, S, Respondek-Liberska, M. P04.06: cardiomegaly in fetus: a powerful indicator of fetal and neonatal demise. Ultrasound Obstet Gynecol 2009;34:192. https://doi.org/10.1002/uog.7060.Search in Google Scholar
27. Bravo-Valenzuela, NJ, Peixoto, AB, Araujo Júnior, E. Prenatal diagnosis of congenital heart disease: a review of current knowledge. Indian Heart J 2018;70:150–64. https://doi.org/10.1016/j.ihj.2017.12.005.Search in Google Scholar PubMed PubMed Central
28. Uhden, M, Knippel, AJ, Stressig, R, Hammer, R, Siegmann, H, Froehlich, S, et al.. Impact of maternal obesity and maternal overweight on the detection rate of fetal heart defects and the image quality of prenatal echocardiography. Ultraschall Med 2011;32:E108–14. https://doi.org/10.1055/s-0031-1281646.Search in Google Scholar PubMed
29. Karuga, FF, Szmyd, B, Respondek-Liberska, M. Fetal congenital heart disease and fetal position – are they related? Prenatal Cardiol 2019;1:33–6. https://doi.org/10.5114/pcard.2019.92544.Search in Google Scholar
30. Carvalho, JS, Mavrides, E, Shinebourne, EA, Campbell, S, Thilaganathan, B. Improving the effectiveness of routine prenatal screening for major congenital heart defects. Heart 2002;88:387–91. https://doi.org/10.1136/heart.88.4.387.Search in Google Scholar PubMed PubMed Central
31. Mai, CT, Isenburg, JL, Canfield, MA, Meyer, RE, Correa, A, Alverson, CJ, et al.. National Birth Defects Prevention Network. National population-based estimates for major birth defects, 2010-2014. Birth Defects Res 2019;111:1420–35. https://doi.org/10.1002/bdr2.1589.Search in Google Scholar PubMed PubMed Central
32. Borowski, D, Pietryga, M, Basta, P, Cnota, W, Czuba, B, Dubiel, M, et al.. Practice guidelines of the polish society of gynecologists and obstetricians – ultrasound section for ultrasound screening in uncomplicated pregnancy – 2020. Ginekol Pol 2020;91:490–501. https://doi.org/10.5603/gp.2020.0110.Search in Google Scholar
33. DeVore, GR, Satou, GM, Afshar, Y, Harake, D, Sklansky, M. Evaluation of fetal cardiac size and shape: a new screening tool to identify fetuses at risk for Tetralogy of Fallot. J Ultrasound Med 2021;40:2537–48. https://doi.org/10.1002/jum.15639.Search in Google Scholar PubMed
34. Yeo, L, Romero, R. How to acquire cardiac volumes for sonographic examination of the fetal heart: Part 2. J Ultrasound Med 2016;35:1043–66. https://doi.org/10.7863/ultra.16.01082.Search in Google Scholar PubMed PubMed Central
35. Yeo, L, Luewan, S, Markush, D, Gill, N, Romero, R. Prenatal diagnosis of dextrocardia with complex congenital heart disease using fetal intelligent navigation echocardiography (FINE) and a literature review. Fetal Diagn Ther 2018;43:304–16. https://doi.org/10.1159/000468929.Search in Google Scholar PubMed PubMed Central
36. Słodki, M, Axt-Fliedner, R, Zych-Krekora, K, Wolter, A, Kawecki, A, Enzensberger, C, et al.. New method to predict need for Rashkind procedure in fetuses with dextro-transposition of the great arteries. Ultrasound Obstet Gynecol 2018;51:531–6. https://doi.org/10.1002/uog.17469.Search in Google Scholar PubMed
37. Sylwestrzak, O, Respondek-Liberska, M. Functional assessment of atrial M-mode in 3rd trimester in the context of postnatal balloon atrial septostomy in fetuses with simple dextro-transposition of the great arteries. J Matern Fetal Neonatal Med 2022;35:9864–9. https://doi.org/10.1080/14767058.2022.2061346.Search in Google Scholar PubMed
38. DeVore, GR, Satou, G, Sklansky, M, Cuneo, B. Speckle tracking analysis to evaluate the size, shape, and function of the atrial chambers in fetuses with d-transposition of the great arteries to predict the need for neonatal urgent balloon atrial septostomy. Echocardiography 2023;40:204–16. https://doi.org/10.1111/echo.15533.Search in Google Scholar PubMed
39. DeVore, GR, Satou, G, Sklansky, M, Cuneo, B. Speckle tracking analysis in fetuses with D-transposition: predicting the need for urgent neonatal balloon atrial septostomy. Pediatr Cardiol 2023;44:1382–96. https://doi.org/10.1007/s00246-023-03131-y.Search in Google Scholar PubMed
Supplementary Material
This article contains supplementary material (https://doi.org/10.1515/jpm-2024-0621).
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Reviews
- Vasa previa guidelines and their supporting evidence
- Fetal origins of adult disease: transforming prenatal care by integrating Barker’s Hypothesis with AI-driven 4D ultrasound
- Original Articles – Obstetrics
- Postpartum remote blood pressure monitoring and risk of hypertensive-related readmission: systematic review and meta-analysis of randomized controlled trials
- Proposal of a novel index in assessing perinatal mortality in prenatal diagnosis of Sacrococcygeal teratoma
- Maternity staff views on implementing a national perinatal mortality review tool: understanding barriers and facilitators
- Prenatal care for twin pregnancies: analysis of maternal and neonatal morbidity and mortality
- Hematological indicators and their impact on maternal and neonatal outcomes in pregnancies with thalassemia traits
- The reference ranges for fetal ductus venosus flow velocities and calculated waveform indices and their predictive values for right heart diseases
- Risk factors and outcomes of uterine rupture before onset of labor vs. during labor: a multicenter study
- Feasibility and reproducibility of speckle tracking echocardiography in routine assessment of the fetal heart in a low-risk population
- Enhancing external cephalic version success: insights from an Israeli tertiary center
- Original Articles – Fetus
- Comparative sonographic measurement of the fetal thymus size in singleton and twin pregnancies
- Transversal cardiac diameter is increased in fetuses with dextro-transposition of the great arteries older than 28th weeks of gestation
- Short Communications
- Severe maternal morbidity in twin pregnancies: the impact of body mass index and gestational weight gain
- Trends in gestational age and short-term neonatal outcomes in the United States
- Letter to the Editor
- Mechanisms of hypoxaemia in late pulmonary hypertension associated with bronchopulmonary dysplasia
Articles in the same Issue
- Frontmatter
- Reviews
- Vasa previa guidelines and their supporting evidence
- Fetal origins of adult disease: transforming prenatal care by integrating Barker’s Hypothesis with AI-driven 4D ultrasound
- Original Articles – Obstetrics
- Postpartum remote blood pressure monitoring and risk of hypertensive-related readmission: systematic review and meta-analysis of randomized controlled trials
- Proposal of a novel index in assessing perinatal mortality in prenatal diagnosis of Sacrococcygeal teratoma
- Maternity staff views on implementing a national perinatal mortality review tool: understanding barriers and facilitators
- Prenatal care for twin pregnancies: analysis of maternal and neonatal morbidity and mortality
- Hematological indicators and their impact on maternal and neonatal outcomes in pregnancies with thalassemia traits
- The reference ranges for fetal ductus venosus flow velocities and calculated waveform indices and their predictive values for right heart diseases
- Risk factors and outcomes of uterine rupture before onset of labor vs. during labor: a multicenter study
- Feasibility and reproducibility of speckle tracking echocardiography in routine assessment of the fetal heart in a low-risk population
- Enhancing external cephalic version success: insights from an Israeli tertiary center
- Original Articles – Fetus
- Comparative sonographic measurement of the fetal thymus size in singleton and twin pregnancies
- Transversal cardiac diameter is increased in fetuses with dextro-transposition of the great arteries older than 28th weeks of gestation
- Short Communications
- Severe maternal morbidity in twin pregnancies: the impact of body mass index and gestational weight gain
- Trends in gestational age and short-term neonatal outcomes in the United States
- Letter to the Editor
- Mechanisms of hypoxaemia in late pulmonary hypertension associated with bronchopulmonary dysplasia

