Abstract
Objectives
This study aims to reveal the distinctions between uterine rupture (UR) that occurs before onset of labor and during labor.
Methods
This multicenter study was conducted across three tertiary hospitals in South China involving obstetrical UR from January 2009 to April 2022. Cases were categorized into two groups based on the timing of UR: prelabor and labor.
Results
Among the cases of UR, 42 were classified in the prelabor group, and 27 in the labor group. The prelabor group had a higher prevalence of histories of myomectomies, ectopic pregnancy surgeries, congenital uterine anomalies, and prior URs. Among these ruptures, 45.2 % were located at cesarean section (CS) scars, compared to 81.5 % of cases during labor. Ruptures before onset of labor occurred across all gestational intervals, whereas those during labor, except one, occurred after 32 weeks. The median gestational age was 33 and 39 weeks in each group. Notably, prelabor cases demonstrated longer symptom-to-delivery intervals. Placenta percreta was more common in the prelabor group (16.7 vs. 3.7 % in the labor group). Among labor-related cases, 16 of the 27 occurred during trial of labor after cesarean (TOLAC), and four were identified following vaginal birth after cesarean (VBAC).
Conclusions
URs differ by timing relative to labor. Ruptures before onset of labor more often resulted from scars other than CS and placenta percreta, occurring at earlier gestational weeks. This finding highlighted challenges in diagnosis and management. In contrast, ruptures during labor mostly occurred after 32 weeks and were strongly associated with TOLAC/VBAC.
Introduction
Rupture of the gravid uterus, involving all layers of the uterine wall, poses a significant threat to both mother and neonate. Over recent years, the prevalence of uterine rupture (UR) has increased, driven by changes in maternal demographics and rising cesarean section (CS) rates [1]. In 2020, the overall CS rate among Chinese primiparous women exceeded 40.0 % [2]. The incidence of UR varies significantly between scarred and unscarred uteri, with rates of 22 per 10,000 and 0.2 per 10,000 deliveries, respectively [3], 4]. However, UR remains elusive to diagnose, often being misinterpreted as other obstetric problems, such as placental abruption, threatened labor, or even as gastrointestinal disorders [5]. Misdiagnosis and delayed diagnosis are critical contributors to poor pregnancy outcomes [6]. While cases of UR during labor typically benefit from intensive monitoring that allows for timely identification, those occurring before onset of labor are more likely to be misdiagnosed or neglected due to less frequent surveillance [7]. Thus, this study aims to address the distinctions between UR before onset of labor and during labor, with the goal of improving early recognition and facilitating prompt intervention.
Materials and methods
The data was retrieved from three tertiary medical centers in Guangdong Province, South China, covering a total of 261,435 deliveries from January 2009 to April 2022. The involved centers were The Third Affiliated Hospital of Guangzhou Medical University, Shenzhen Guangming District People’s Hospital, and Dongguan Maternal and Child Health Care Hospital. This study adhered to the Declaration of Helsinki and was approved by the Institutional Review Board [IRB (2024)-126]. We identified obstetrical UR cases by the first author reviewing maternal medical records. The cases were stratified into two groups based on the timing of uterine rupture: prelabor and labor. The labor group was defined as UR after the onset of regular uterine contractions with cervical dilation ≥1 cm, whereas cases before this stage were categorized as prelabor. Baseline characteristics, predisposing factors, perioperative conditions, and maternal-fetal outcomes were systematically documented.
Variables and definition
Scarred uteri can be categorized into CS-related and other types, depending on the origin of surgical intervention. For this study, either clinical or pathological diagnoses of placenta increta and percreta are considered under the umbrella term of PAS disorder. Static UR means a fully symptomless rupture without any prior noticeable signs, often get diagnosed in elective CS [8]. Grand multiparity refers to a woman who has had ≥3 births at ≥20 weeks. Pre-hospital UR refers to cases occurring before hospital admission. In such situations, limited resources, compared to those available after admission, restrict opportunities for timely surgical intervention. Preoperative duration was measured in hours from the onset of symptoms (e.g., abdominal pain) to the decision to operate. Uterine repair involved restoring structural integrity by repairing the rupture site rather than excising it. Partial excision was performed to remove the gravid uterus in uterine didelphys or a uterus with severe invasive placenta. Hysterectomy referred to the surgical removal of the gravid uterine corpus, with or without the cervix, during the peripartum period. According to the Chinese Expert Consensus, severe neonatal asphyxia was: 1-min Apgar score 0–3 or 5-min Apgar score 0–5, with umbilical cord artery pH<7.0. Perinatal death includes stillbirth and neonatal death.
Statistical analysis
The statistical analysis was performed using IBM SPSS 22.0 software. Categorical variables were analyzed with the chi-square test, while continuous variables were assessed using either the t-test for normally distributed data or the Kruskal–Wallis test for non-normally distributed data. Numerical data were presented as mean±standard deviation for normally distributed variables, and as median (P25, P75) for non-normally distributed variables. Categorical data were expressed as numbers (percentages). A p-value <0.05 was considered statistically significant.
Results
The estimated prevalence of UR was 2.6 per 10,000 deliveries (69/261,435) over a span of 14 years. Among the cases of UR, 23 (33.3 %) resulted in perinatal death, 21 (30.4 %) involved preterm neonates, 20 (29.0 %) led to maternal hemorrhagic shock, and 11 (15.9 %) required hysterectomies. No maternal death was attributed to UR. Out of the 69 cases, 42 (60.9 %) occurred before onset of labor and 27 (39.1 %) occurred during labor. Maternal characteristics and pregnancy outcomes between the two groups were presented in Table 1. Compared to the labor group, the prelabor group had a higher proportion of nulliparous women and a relatively lower rate of scarred uteri. As shown in Figure 1, we calculated the percentages based on 32 cases with scarred uteri in the prelabor group and 22 cases in the labor group. CS was the predominant source of uterine scars in both groups; however, the prelabor group exhibited more diverse scar origins, with significant contributions from previous myomectomy, ectopic pregnancy surgery, and ruptured uterine repair. Three recurrent URs were noted: The first occurred after a rupture at term and resulted in intrauterine fetal death at 34+4 weeks. The second case had a history of uterine rupture at 29 weeks due to congenital didelphys uterus, this time terminated a pregnancy at 35+4 weeks with a live birth. The third case involved a cornual rupture at 33 weeks caused by placenta percreta, this time got diagnosed at 26 weeks for left upper quadrant abdominalgia. Fortunately, expectant management prolonged this pregnancy by 20 days, resulting in a delivery at 28+5 weeks of a 1,350 g neonate, with Apgar score of 3-7-8. Six women had placenta previa, with two complicated by placenta increta and three by placenta percreta. In terms of neonatal outcomes, the median gestational age (GA) at delivery was 33 weeks in the prelabor group and 39 weeks in the labor group. Figure 2 demonstrates the number of URs and rate of live birth at each gestational interval. UR before onset of labor could be observed across all intervals, while all labor-related URs, except one, occurred after 32 weeks of gestation. The live birth rate increased progressively with GA, rising from 50.0 % at 24–27 weeks to 90.9 % at 37–41 weeks. Among infants delivered at ≥32 weeks, 90.0 % (45/50) were live births. CS was the predominant mode of delivery in both groups, while the four vaginal deliveries were associated with vaginal birth after cesarean (VBAC). The prelabor group had a significantly higher rate of preterm birth compared to the labor group (60.7 vs. 17.4 %, p=0.002). The incidence of hemorrhagic shock and perinatal death were also higher in the prelabor group, though with no significant difference.
Baseline characteristics and pregnancy outcomes in cases of UR occurred before onset of labor and during labor.
| Prelabor (n=42) | Labor (n=27) | p-Value | |
|---|---|---|---|
| Maternal age, y | 33 (26, 37) | 31 (29, 35) | 0.61 |
| Pre-pregnancy BMI, kg/m2 | 21.8 ± 3.6 | 21.9 ± 3.0 | 0.95 |
| In vitro fertilization | 4 (9.5) | 2 (7.4) | 0.76 |
| Regular prenatal visit | 26 (61.9) | 21 (77.8) | 0.17 |
| Parity status | 0.04 | ||
| Nulliparous | 9 (21.4) | 1 (3.7) | |
| Multiparous | 33 (78.6) | 26 (96.3) | |
| Scarred uteri | 32 (76.2) | 22 (81.5) | 0.60 |
| Previous CS | 27 (64.3) | 21 (77.8) | 0.24 |
| Previous ectopic pregnancy surgery | 3 (7.1) | 1 (3.7) | 0.55 |
| Previous myomectomy | 5 (11.9) | 0 (0.0) | 0.06 |
| Previous UR | 3 (7.1) | 0 (0.0) | 0.16 |
| PAS history | 2 (4.8) | 0 (0.0) | 0.25 |
| Uterine malformations | 5 (11.9) | 1 (3.7) | 0.24 |
| Placenta previa | 5 (11.9) | 1 (3.7) | 0.24 |
| Baseline Hb, g/dL | 11.4 ± 0.9 | 11.8 ± 0.9 | 0.18 |
| GA at delivery, weeks | 33.0 (27.0, 37.5) | 39.0 (37.5, 40.1) | <0.001 |
| Mode of delivery | 0.01 | ||
| Vaginal | 0 (0.0) | 4 (14.8) | |
| Cesarean | 31 (100.0) | 23 (85.2) | |
| Postpartum hemorrhage | 18 (42.9) | 12 (44.4) | 0.90 |
| Hemorrhagic shock | 14 (33.3) | 6 (22.2) | 0.32 |
| Inpatient days, d | 9.4 ± 6.2 | 8.4 ± 4.9 | 0.49 |
| Preterm birth | 17 (60.7) | 4 (17.4) | 0.00 |
| Perinatal death | 17 (40.5) | 6 (22.2) | 0.12 |
| Severe neonatal asphyxia | 3 (10.7) | 3 (13.0) | 0.57 |
| NICU admission | 14 (50.0) | 7 (30.4) | 0.16 |
-
UR, uterine rupture; BMI, body mass index; CS, cesarean section; UR, uterine rupture; PAS, placenta accreta spectrum; Hb, haemoglobin; GA, gestational age; NICU, neonatal intensive care unit. Items with significant differences are shown in bold.
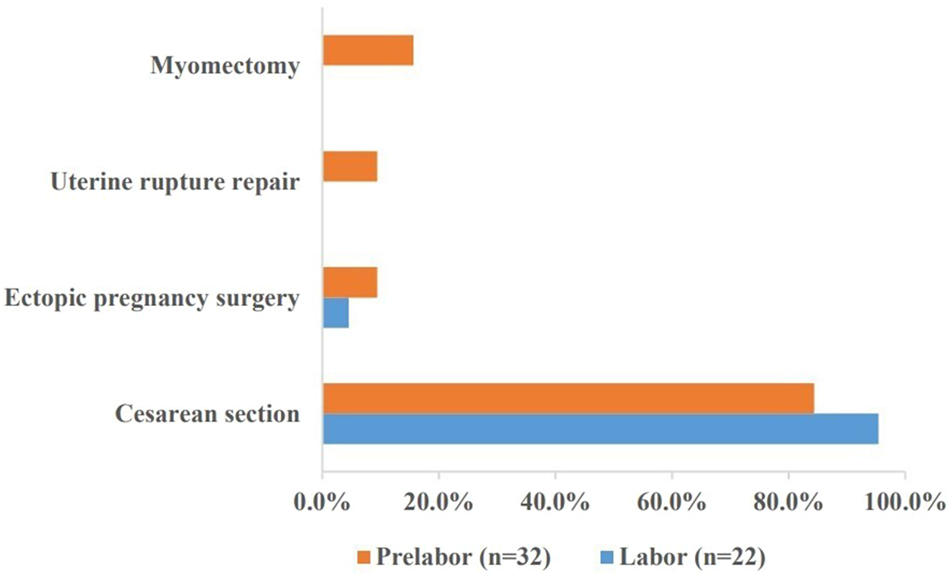
Origin of scars in ruptured scarred uterus in the prelabor and labor groups.

The number of URs and rate of live birth among women delivered at each gestational interval.
As rupture onset, acute abdomen and cardiotocographic abnormalities (primarily bradycardia) were observed more often in the labor group, which also exhibited a higher rate of vaginal bleeding (70.4 vs. 26.2 %, p<0.001). Notably, URs before onset of labor were more likely to be diagnosed before hospital admission (59.5 vs. 7.4 %, p=0.001) and had a longer symptom-to-delivery interval compared to those during labor (6 vs. 4 h). The GA and hemoglobin (Hb) at admission was both significantly lower in the prelabor group (Table 2). In the labor group, 16 ruptures were detected during trial of labor after cesarean (TOLAC), and four happened right after VBAC, resulting in two hysterectomies and two stillbirths. Eight cases were associated with labor induction and two with oxytocin augmentation during TOLAC. A total of 41 ruptures were located at the CS scar of the anterior lower uterine segment, which were significantly more common in the labor group than in the prelabor group (81.5 vs. 45.2 %, p=0.026). Table 3 showed that ruptures occurring at the CS car were associated with better outcomes compared to those at other sites, with a later delivery week and less estimated blood loss. In Table 2, one-third of URs before onset of labor correlated with abnormally invasive placenta, with 11 of the 14 cases occurring before 32 weeks of gestation. All five partial excisions were performed on women with placenta increta, two of whom had concomitant uterine didelphys. Estimated blood loss and the need for RBC transfusion were both higher in the prelabor group.
Clinical presentation in cases of UR occurred before onset of labor and during labor.
| Prelabor (n=42) | Labor (n=27) | p-Value | |
|---|---|---|---|
| Rupture symptoms | |||
| Abdominalgia | 34 (81.0) | 26 (96.3) | 0.07 |
| Vaginal bleeding | 11 (26.2) | 19 (70.4) | <0.001 |
| Abnormal FHR | 16 (38.1) | 13 (48.1) | 0.41 |
| Unstable vital signs | 8 (19.0) | 4 (14.8) | 0.65 |
| Pre-hospital diagnosis | 25 (59.5) | 2 (7.4) | 0.00 |
| Preoperative duration, h | 6.0 (3.8, 14.3) | 4.0 (2.0, 6.0) | 0.05 |
| Admission GA, weeks | 33.1 (26.8, 37.3) | 39.0 (37.5, 39.6) | 0.00 |
| Admission Hb, g/dL | 10.5 (8.4, 11.9) | 11.9 (10.6, 12.3) | 0.02 |
| Underwent TOLAC | 0 (0.0) | 20 (74.1) | <0.001 |
| Induction or augmentation of labor | 7 (16.7) | 3 (11.1) | 0.52 |
| Rupture sites | 0.03 | ||
| Uterine horn | 7 (16.7) | 1 (3.7) | |
| Uterine fundus | 6 (14.3) | 1 (3.7) | |
| Uterine body | 10 (23.8) | 3 (11.1) | |
| Cesarean scar | 19 (45.2) | 22 (81.5) | |
| PAS disorder | 14 (33.3) | 3 (11.1) | 0.04 |
| Placenta percreta | 7 (16.7) | 1 (3.7) | 0.10 |
| Surgical procedure | 0.40 | ||
| Uterine repair | 30 (71.4) | 23 (85.2) | |
| Partial uterine resection | 4 (9.5) | 1 (3.7) | |
| Hysterectomy | 8 (19.0) | 3 (11.1) | |
| Estimated blood loss, mL | 850 (475, 2,350) | 400 (300, 1,100) | 0.03 |
| RBC transfusion, U | 4.9 ± 7.8 | 2.8 ± 3.6 | 0.13 |
| RBC transfusion ≥4U | 18 (42.9) | 9 (33.3) | 0.43 |
| Postoperative Hb, g/dL | 8.9 ± 1.6 | 8.5 ± 1.6 | 0.30 |
-
FHR, fetal heart rate; GA, gestational age; Hb, haemoglobin; TOLAC, trial of labor after cesarean; RBC, red blood cell. Items with significant differences are shown in bold.
Maternal and perinatal outcomes following uterine rupture at four anatomical sites.
| Cornua (n=8) | Fundus (n=7) | Body (n=13) | CS scar (n=41) | |
|---|---|---|---|---|
| GA at delivery, weeks | 27.2 ± 5.8a | 31.8 ± 6.1b | 31.4 ± 5.0c | 36.6 ± 5.2a,b,c |
| Estimated blood loss, mL | 1,800.0 ± 1,019.8 | 2,485.7 ± 1,532.4 | 2,367.3 ± 2,372.8 | 1,112.2 ± 1,862.7 |
| Hemorrhagic shock | 4 (50.0) | 4 (57.1) | 4 (30.8) | 8 (19.5) |
| Hysterectomy | 1 (12.5) | 2 (28.6) | 3 (23.1) | 5 (12.2) |
| Perinatal death | 4 (50.0) | 3 (42.9) | 5 (38.5) | 11 (26.8) |
-
GA, gestational age; CS, cesarean section. aRepresents p<0.001, brepresents p= 0.03, crepresents p=0.00.
Discussion
Obstetrical UR is a rare and severe complication of pregnancy, encompassing a heterogeneous group of conditions. Few studies have differentiated UR between prelabor and labor for the lack of a consistent definition. A study [4] defined ≥4 cm of cervical dilatation as UR during labor, whereas we adopted a more conservative criterion of ≥1 cm, as it marked the initiation of labor monitoring. As reported in previous studies, UR after the start of labor was generally more common than that before onset of labor (1.1 vs. 0.1 per 10,000 deliveries) [7], 9]. However, the proportion of URs before onset of labor in our dataset reached 60.9 %. This reverse pattern can be attributed to two major factors. First, referral bias: half of the cases were collected from an Obstetrics Critical Care Center in South China, where complex and severe cases (mostly prelabor) were referred for advanced treatment. Second, the small sample size may not adequately represent real-world prevalence.
It’s well known that scarred uterus was the most common risk factor for UR [10]. This study included 48 with CS scars and 11 with other types of scars (five myomectomies, four ectopic pregnancy surgeries, three rupture histories, and two PAS histories). Among the 69 ruptures, 59.4 % were located at the cesarean scar of the anterior lower uterine segment, consistent with a recent report [11]. URs before onset of labor more frequently occurred at unusual sites, such as the cornual region, fundus, and uterine body, which were associated with significantly worse outcomes [4]. This study found three cases of cornual rupture in women who had previously undergone laparoscopic surgery for ectopic pregnancy. Minimally invasive surgery and the use of energy devices increase the potential damage to the uterine myometrium. Cornual rupture occurred at the earliest GA compared to other sites and was associated with a higher estimated blood loss (Table 3). Women with a history myomectomy or UR usually experienced ruptures before onset of labor, consistent with previous literature [4]. Compared with abdominal myomectomy, the laparoscopic approach may increase the risk of UR in a subsequent pregnancy. Gil et al. reported a 0.43 % incidence of UR among pregnant women with previous myomectomy, with ruptures after laparoscopic myomectomy being 2.5 times more common than those after laparotomy [12]. To date, longitudinal studies remain insufficient to assess the impact of different surgical techniques and interventions on UR prevention.
PAS disorder was associated with an increased risk of UR before onset of labor and was often observed in pregnancies remote from term. We identified nine cases of placenta increta and eight of placenta percreta. The number of PAS-related ruptures in our data was significantly higher than in other reports, as one institution served as a PAS referral center. Over the past two decades, the incidence of PAS disorders has increased tenfold [13]. Prenatal B-ultrasound remains the preferred diagnostic tool for detecting PAS disorders, with magnetic resonance imaging serving as an additional aid when the maternal condition is stable [14]. A primigravida at 23 weeks was brought to emergency with acute abdomen, and an ultrasound examination reported fluid accumulation in the pelvis. As hemoglobin declined from 8.5 to 5.7 g/dL, a prompt laparotomy was performed. Cornual rupture and placenta percreta were identified, but no image of invasive placenta was detected before the operation. It is noteworthy that the woman had undergone two surgeries in the right tubo-uterine angle for ectopic pregnancy. Another primigravida who conceived via embryo transfer technology, presented with right lower abdominal dull pain and oligohydramnios at 35+4 weeks. Magnetic resonance image revealed extensive placenta percreta in the left anterior uterine wall. Following transabdominal access, obstetricians noticed a 1.0 cm breach at the uterus’s left anterior wall [15]. A large population-based study in China revealed that placenta percreta was the strongest risk for UR at 28–33 weeks, especially in those without a CS scar [16]. In our study, 57.9 % of URs before 32 weeks were associated with PAS. All five partial uterine resections were performed on patients with PAS, and 7 out of 11 total hysterectomies were related to PAS. Further investigations are needed into prenatal screening to prevent PAS-related UR.
Besides ruptures associated with scarred uteri, 15 cases were identified in uteri that were intact and unscarred. Relevant backgrounds included PAS disorders, congenital uterine anomalies, grand multiparity, a history of conservatively managed PAS, pelvic inflammatory disease, previous hysteroscopic procedures or multiple abortions, and the inappropriate use of oxytocin or prostaglandin analogues. Six cases of congenital uterine anomalies were confirmed, including three didelphys uteri, two septate uteri, and one bicornuate uterus. In such cases, uncoordinated uterine contractions may result from structural defects and aberrant distribution of oxytocin receptors. Prolonged exposure to oxytocin or prostaglandin analogues may increase risk of UR, particularly in cases of obstructed labor or with excessive dosages [17]. Eight ruptures in the second and third trimesters were linked to labor induction, including four with rivanol, one with oxytocin following two cesarean sections at term, and three with unknown dosages. Improper medication or lax indications may be triggers of UR. None of them received prenatal examinations, and they presented with severe consequences from 19 to 34 weeks. Ruptures in unscarred uteri are extremely rare but prone to extensive uterine injury and catastrophic outcomes [18], 19]. According to a large-scale study by Al-Zirqi et al., unscarred URs showed a 2.6-fold higher risk of peripartum hysterectomy than scarred uteri [9]. Our study noted that women with unscarred uteri had 26.7 % of hysterectomies and 46.7 % of perinatal mortalities, both higher than the rates in women with scarred uteri (13.0 and 29.6 %, respectively).
According to a multi-country prospective study in Europe, URs before onset of labor accounted for 57.7 % of atypical cases (unscarred, preterm or prelabor) [4]. Abdominal pain is a common clinical sign of UR [20]. Atypical abdominalgia may exist under some rare circumstance [21]. High frequency of vaginal bleeding in the labor group can be explained by labor progress. No correlation was found between the amount of blood loss and the severity of rupture. In some cases, severe intra-abdominal hemorrhage may occur without vaginal bleeding, except when cervical lacerations were present. Abnormal cardiotocographic findings were slightly less common in the prelabor group compared to the labor group. However, this comparison showed limited, as more women in the prelabor group had not yet reached the gestational age required for continuous electronic fetal heart rate monitoring. URs in the second trimester resulted in 50.0 % hysterectomies, whereas this rate decreased to 8.8 % in the third trimester. Hypovolemic shock remains the primary cause of maternal death secondary to UR [22]. Surgical removal of the uterus is the most effective approach to control persistent intra-peritoneal hemorrhage when other interventions fail. The likelihood of live birth was significantly higher in deliveries after 32 weeks. Fetal mortality can be minimized if an immediate CS is performed, with delivery completed within 20 minutes. In contrast, the risk of perinatal death increases 16-fold if delivery is delayed beyond 30 min (OR, 16.7; 95 % CI, 6.4–43.5) [23]. Placental separation and fetal extrusion were the main causes of perinatal death and severe asphyxia [24]. Currently, no reliable methods exist to predict UR. Some studies suggested that a lower uterine segment thickness of ≥2.3 mm near-term, assessed by ultrasound, may safely allow TOLAC [25]. However, this approach has shown poor specificity and remains unreliable for clinical application [26]. Further exploration of novel predictive markers for UR is warranted to improve early detection and intervention.
Conclusions
This study showed the distinct features and outcomes of UR before and during labor. UR before onset of labor was more likely to be associated with scarred uteri not originating from CS, and those with PAS disorder. They were characterized by early onset and relatively longer symptom-to-delivery intervals, resulting in higher maternal-fetal morbidity and fetal mortality. URs during labor mostly occurred in the CS scar after 32 weeks and were generally associated with favorable outcomes. These disparities emphasized the importance of heightened awareness and diagnostic vigilance in managing suspected UR before onset of labor.
Funding source: National Key Research and Development Program of China
Award Identifier / Grant number: 2021YFC2701500
Funding source: Guangzhou fundamental research project jointly funded by School (Institution) (high-level university)
Award Identifier / Grant number: 202102010131
-
Research ethics: The study was approved by the Institutional Review Board of the Third Affiliated Hospital of Guangzhou Medical University on June 23, 2024. Approval ID: IRB (2024)-126. It was conducted in accordance with the Declaration of Helsinki.
-
Informed consent: Not applicable.
-
Author contributions: F He, QW Nie: Project development. Qw Nie, Sj Luo, L Wang: Data collection or management. Qw Nie, Bj Chen: Data analysis. Qw Nie, Sj Luo and Bj Chen: Manuscript writing and revising. All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Use of Large Language Models, AI and Machine Learning Tools: We used ChatGPT for improving language quality in the later revision. The LLM was not involved in the research design, data collection, or the drafting of the original manuscript.
-
Conflict of interest: The authors state no conflict of interest.
-
Research funding: This work was supported by grants from the National Key Research and Development Program of China (2021YFC2701500) and Guangzhou fundamental research project jointly funded by School (Institution) (high-level university) No. 202102010131.
-
Data availability: The raw data can be obtained on request from the corresponding author.
References
1. Zhou, Y, Mu, Y, Chen, P, Xie, Y, Zhu, J, Liang, J. The incidence, risk factors and maternal and foetal outcomes of uterine rupture during different birth policy periods: an observational study in China. BMC Pregnancy Childbirth 2021;21:360. https://doi.org/10.1186/s12884-021-03811-8.Search in Google Scholar PubMed PubMed Central
2. Yin, S, Zhou, Y, Yuan, P, Wei, Y, Chen, L, Guo, X, et al.. Hospital variations in caesarean delivery rates: an analysis of national data in China, 2016–2020. J Glob Health 2023;13:4029. https://doi.org/10.7189/jogh.13.04029.Search in Google Scholar PubMed PubMed Central
3. Vandenberghe, G, Bloemenkamp, K, Berlage, S, Colmorn, L, Deneux-Tharaux, C, Gissler, M, et al.. The International Network of Obstetric Survey Systems study of uterine rupture: a descriptive multi-country population-based study. BJOG 2019;126:370–81. https://doi.org/10.1111/1471-0528.15271.Search in Google Scholar PubMed
4. Vandenberghe, G, Vierin, A, Bloemenkamp, K, Berlage, S, Colmorn, L, Deneux-Tharaux, C, et al.. Incidence and outcomes of uterine rupture in women with unscarred, preterm or prelabour uteri: data from the international network of obstetric survey systems. BJOG 2023;12:1493–501. https://doi.org/10.1111/1471-0528.17517.Search in Google Scholar PubMed
5. Shi, L, Lin, X, Sha, S, Yao, L, Zhu, X, Shao, Y. Delayed presentation of uterine rupture in a didelphys uterus misdiagnosed as appendicitis: a case report and review of the literature. Arch Gynecol Obstet 2017;296:1015–16. https://doi.org/10.1007/s00404-017-4522-6.Search in Google Scholar PubMed
6. Rottenstreich, M, Rotem, R, Hirsch, A, Farkash, R, Rottenstreich, A, Samueloff, A, et al.. Delayed diagnosis of intrapartum uterine rupture - maternal and neonatal consequences. J Matern Fetal Neonatal Med 2021;34:708–13. https://doi.org/10.1080/14767058.2019.1613366.Search in Google Scholar PubMed
7. Al-Zirqi, I, Vangen, S. Prelabour uterine rupture: characteristics and outcomes. BJOG 2020;127:1637–44. https://doi.org/10.1111/1471-0528.16363.Search in Google Scholar PubMed
8. Langhe, R, Shah, UF, Alfathil, A, Gannon, M. Silent uterine rupture in scarred uterus. BMJ Case Rep 2017;2017:bcr2016218189. https://doi.org/10.1136/bcr-2016-218189.Search in Google Scholar PubMed PubMed Central
9. Al-Zirqi, I, Daltveit, AK, Vangen, S. Maternal outcome after complete uterine rupture. Acta Obstet Gynecol Scand 2019;98:1024–31. https://doi.org/10.1111/aogs.13579.Search in Google Scholar PubMed
10. Savukyne, E, Bykovaite-Stankeviciene, R, Machtejeviene, E, Nadisauskiene, R, Maciuleviciene, R. Symptomatic uterine rupture: a fifteen year review. Medicina (Kaunas) 2020;56:574. https://doi.org/10.3390/medicina56110574.Search in Google Scholar PubMed PubMed Central
11. Wan, S, Yang, M, Pei, J, Zhao, X, Zhou, C, Wu, Y, et al.. Pregnancy outcomes and associated factors for uterine rupture: an 8 years population-based retrospective study. BMC Pregnancy Childbirth 2022;22:91. https://doi.org/10.1186/s12884-022-04415-6.Search in Google Scholar PubMed PubMed Central
12. Gil, Y, Badeghiesh, A, Suarthana, E, Mansour, F, Capmas, P, Volodarsky-Perel, A, et al.. Risk of uterine rupture after myomectomy by laparoscopy or laparotomy. J Gynecol Obstet Hum Reprod 2020;49:101843. https://doi.org/10.1016/j.jogoh.2020.101843.Search in Google Scholar PubMed
13. Jauniaux, E, Grønbeck, L, Bunce, C, Langhoff-Roos, J, Collins, SL. Epidemiology of placenta previa accreta: a systematic review and meta-analysis. BMJ Open 2019;9:e031193. https://doi.org/10.1136/bmjopen-2019-031193.Search in Google Scholar PubMed PubMed Central
14. Aboughalia, H, Basavalingu, D, Revzin, MV, Sienas, LE, Katz, DS, Moshiri, M. Imaging evaluation of uterine perforation and rupture. Abdom Radiol (NY) 2021;46:4946–66. https://doi.org/10.1007/s00261-021-03171-z.Search in Google Scholar PubMed
15. Yang, Y, He, F. Placenta percreta complicated with uterine rupture. Arch Gynecol Obstet 2022;305:291–2. https://doi.org/10.1007/s00404-021-06340-z.Search in Google Scholar PubMed
16. Tao, J, Mu, Y, Chen, P, Xie, Y, Liang, J, Zhu, J. Pregnancy complications and risk of uterine rupture among women with singleton pregnancies in China. BMC Pregnancy Childbirth 2022;22:131. https://doi.org/10.1186/s12884-022-04465-w.Search in Google Scholar PubMed PubMed Central
17. Al-Zirqi, I, Daltveit, AK, Forsén, L, Stray-Pedersen, B, Vangen, S. Risk factors for complete uterine rupture. Am J Obstet Gynecol 2017;216:161–5. https://doi.org/10.1016/j.ajog.2016.10.017.Search in Google Scholar PubMed
18. Gibbins, KJ, Weber, T, Holmgren, CM, Porter, TF, Varner, MW, Manuck, TA. Maternal and fetal morbidity associated with uterine rupture of the unscarred uterus. Am J Obstet Gynecol 2015;213:381–2. https://doi.org/10.1016/j.ajog.2015.05.048.Search in Google Scholar PubMed
19. McEvoy, A, Corbett, GA, Nolan, C, Daly, R, Murnane, M, Higgins, S, et al.. Outcomes of uterine rupture in the setting of the unscarred compared with the scarred uterus. Obstet Gynecol 2023;141:854–6. https://doi.org/10.1097/AOG.0000000000005108.Search in Google Scholar PubMed
20. Liao, YC, Tsang, LL, Yang, TH, Lin, YJ, Chang, YW, Hsu, TY, et al.. Unscarred uterine rupture with catastrophic hemorrhage immediately after vaginal delivery: diagnosis and management of six consecutive cases. J Matern Fetal Neonatal Med 2023;36:2243366. https://doi.org/10.1080/14767058.2023.2243366.Search in Google Scholar PubMed
21. Hruban, L, Jouzova, A, Janku, P, Weinberger, V, Seidlova, D, Juren, T, et al.. Conservative management of complete fetal expulsion into the abdominal cavity after silent uterine rupture - case report. BMC Pregnancy Childbirth 2023;23:500. https://doi.org/10.1186/s12884-023-05812-1.Search in Google Scholar PubMed PubMed Central
22. Astatikie, G, Limenih, MA, Kebede, M. Maternal and fetal outcomes of uterine rupture and factors associated with maternal death secondary to uterine rupture. BMC Pregnancy Childbirth 2017;17:117. https://doi.org/10.1186/s12884-017-1302-z.Search in Google Scholar PubMed PubMed Central
23. Al-Zirqi, I, Daltveit, AK, Vangen, S. Infant outcome after complete uterine rupture. Am J Obstet Gynecol 2018;219:101–9. https://doi.org/10.1016/j.ajog.2018.04.010.Search in Google Scholar PubMed
24. Bujold, E, Gauthier, RJ. Neonatal morbidity associated with uterine rupture: what are the risk factors? Am J Obstet Gynecol 2002;186:311–14. https://doi.org/10.1067/mob.2002.119923.Search in Google Scholar PubMed
25. Bujold, E, Jastrow, N, Simoneau, J, Brunet, S, Gauthier, RJ. Prediction of complete uterine rupture by sonographic evaluation of the lower uterine segment. Am J Obstet Gynecol 2009;201:320–1. https://doi.org/10.1016/j.ajog.2009.06.014.Search in Google Scholar PubMed
26. Rozenberg, P, Sénat, MV, Deruelle, P, Winer, N, Simon, E, Ville, Y, et al.. Evaluation of the usefulness of ultrasound measurement of the lower uterine segment before delivery of women with a prior cesarean delivery: a randomized trial. Am J Obstet Gynecol 2022;226:251–3. https://doi.org/10.1016/j.ajog.2021.08.005.Search in Google Scholar PubMed
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Reviews
- Vasa previa guidelines and their supporting evidence
- Fetal origins of adult disease: transforming prenatal care by integrating Barker’s Hypothesis with AI-driven 4D ultrasound
- Original Articles – Obstetrics
- Postpartum remote blood pressure monitoring and risk of hypertensive-related readmission: systematic review and meta-analysis of randomized controlled trials
- Proposal of a novel index in assessing perinatal mortality in prenatal diagnosis of Sacrococcygeal teratoma
- Maternity staff views on implementing a national perinatal mortality review tool: understanding barriers and facilitators
- Prenatal care for twin pregnancies: analysis of maternal and neonatal morbidity and mortality
- Hematological indicators and their impact on maternal and neonatal outcomes in pregnancies with thalassemia traits
- The reference ranges for fetal ductus venosus flow velocities and calculated waveform indices and their predictive values for right heart diseases
- Risk factors and outcomes of uterine rupture before onset of labor vs. during labor: a multicenter study
- Feasibility and reproducibility of speckle tracking echocardiography in routine assessment of the fetal heart in a low-risk population
- Enhancing external cephalic version success: insights from an Israeli tertiary center
- Original Articles – Fetus
- Comparative sonographic measurement of the fetal thymus size in singleton and twin pregnancies
- Transversal cardiac diameter is increased in fetuses with dextro-transposition of the great arteries older than 28th weeks of gestation
- Short Communications
- Severe maternal morbidity in twin pregnancies: the impact of body mass index and gestational weight gain
- Trends in gestational age and short-term neonatal outcomes in the United States
- Letter to the Editor
- Mechanisms of hypoxaemia in late pulmonary hypertension associated with bronchopulmonary dysplasia
Articles in the same Issue
- Frontmatter
- Reviews
- Vasa previa guidelines and their supporting evidence
- Fetal origins of adult disease: transforming prenatal care by integrating Barker’s Hypothesis with AI-driven 4D ultrasound
- Original Articles – Obstetrics
- Postpartum remote blood pressure monitoring and risk of hypertensive-related readmission: systematic review and meta-analysis of randomized controlled trials
- Proposal of a novel index in assessing perinatal mortality in prenatal diagnosis of Sacrococcygeal teratoma
- Maternity staff views on implementing a national perinatal mortality review tool: understanding barriers and facilitators
- Prenatal care for twin pregnancies: analysis of maternal and neonatal morbidity and mortality
- Hematological indicators and their impact on maternal and neonatal outcomes in pregnancies with thalassemia traits
- The reference ranges for fetal ductus venosus flow velocities and calculated waveform indices and their predictive values for right heart diseases
- Risk factors and outcomes of uterine rupture before onset of labor vs. during labor: a multicenter study
- Feasibility and reproducibility of speckle tracking echocardiography in routine assessment of the fetal heart in a low-risk population
- Enhancing external cephalic version success: insights from an Israeli tertiary center
- Original Articles – Fetus
- Comparative sonographic measurement of the fetal thymus size in singleton and twin pregnancies
- Transversal cardiac diameter is increased in fetuses with dextro-transposition of the great arteries older than 28th weeks of gestation
- Short Communications
- Severe maternal morbidity in twin pregnancies: the impact of body mass index and gestational weight gain
- Trends in gestational age and short-term neonatal outcomes in the United States
- Letter to the Editor
- Mechanisms of hypoxaemia in late pulmonary hypertension associated with bronchopulmonary dysplasia

