Daily monitoring of vaginal interleukin 6 as a predictor of intraamniotic inflammation after preterm premature rupture of membranes – a new method of sampling studied in a prospective multicenter trial
-
Gregor Seliger
, Michael Bergner
Abstract
Objectives
(A) To introduce a new technique for vaginal fluid sampling (biocompatible synthetic fiber sponge) and (B) evaluate the collected vaginal fluid interleukine-6 (IL-6vag)-concentration as a new diagnostic tool for daily monitoring of intrauterine inflammation after preterm premature rupture of membranes (PPROM). Secondary objectives were to compare the potential to predict an intrauterine inflammation with established inflammation parameters (e.g., maternal white blood cell count).
Methods
This prospective clinical case-control diagnostic accuracy multicenter study was performed with women after PPROM (gestational age 24.0/7 – 34.0/7 weeks). Sampling of vaginal fluid was performed once daily. IL-6vag was determined by electrochemiluminescence-immunoassay-kit. Neonatal outcome and placental histology results were used to retrospectively allocate the cohort into two subgroups: 1) inflammation and 2) no inflammation (controls).
Results
A total of 37 cases were included in the final analysis. (A): Measurement of IL-6 was successful in 86% of 172 vaginal fluid samples. (B): Median concentration of IL-6vag in the last vaginal fluid sample before delivery was significantly higher within the inflammation group (17,085 pg/mL) compared to the controls (1,888 pg/mL; p=0.01). By Youden’s index an optimal cut-off for prediction an intrauterine inflammation was: 6,417 pg/mL. Two days before delivery, in contrast to all other parameters IL-6vag remained the only parameter with a sufficient AUC of 0.877, p<0.001, 95%CI [0.670–1.000].
Conclusions
This study established a new technique for vaginal fluid sampling, which permits assessment of IL-6vag concentration noninvasively in clinical daily routine monitoring.
Introduction
Preterm premature rupture of membranes (PPROM), is still one of the great challenges in modern obstetrics. It occurs in approximately 3–5% of all pregnancies [1], [2], [3], [4] and 25–30% of all preterm deliveries are associated with this complication [2], [5], [6]. Depending on the definition, 5–24% of the neonates delivered after PPROM will suffer from neonatal sepsis [7], [8], [9]. Prematurity, inflammation and infectious morbidities contribute with increasing proportion to the world-wide neonatal mortality rate [10], [11].
While the prevention of PPROM is a desirable goal [12], [13], [14], for now, the major subject of controversy is the timing of delivery. This has been addressed by both national and international guidelines of obstetrical societies worldwide [15], [16]. The key issue is balancing the risks and benefits of prolonging intrauterine stay to reduce the risk of prematurity and at the same time taking into account the increased risk of intraamniotic infection. Most managing approaches consider gestational age and the risk of maternal and fetal infection or inflammation [15], [16], [17]. Gestational age is relatively easy to assess; however, the diagnosis of intraamniotic inflammation is far more difficult because clinical signs are of limited utility [18].
Whereas maternal clinical or blood parameters are easy to determine, they are limited in their possibility to detect an ongoing fetal inflammation [19], [20], [21]. Markers obtained directly from the fetus and amniotic cavity reported improved predictive value [22], [23], [24], but requires an invasive sampling procedure and is therefore unsuitable for daily routine. To identity and evaluate diagnostic tools usable for daily monitoring with a reliable diagnostic and prognostic validity for inflammatory complications of the amniotic cavity and/or the fetus after PPROM remains therefore the major challenge.
Many studies investigated the possibilities of vaginal fluid sampling [25], [26], [27], [28], [29], [30]. This approach offers two main advantages: vaginal fluid is at least partly of intraamniotic origin and can be sampled noninvasively. Nearly all studies conducted a single-point measurement and correlated the resulting concentration with amniotic fluid parameters obtained by amniocentesis [27], [29], [30], [31], [32]. The potential for daily monitoring in this study design is limited. To our knowledge, only one study used a setting for repeated vaginal fluid sampling [33].
We devised a new technique for vaginal fluid sampling through a so-called biocompatible synthetic fiber sponge. This method permits assessment of vaginal Interleukin-6 (IL-6vag) concentrations noninvasively in daily monitoring. The first purpose of this prospective case-control diagnostic accuracy study was to introduce, establish and assess the new technique for vaginal fluid sampling and to evaluate the IL-6vag concentrations obtained with it as a new potential diagnostic tool for detection and daily monitoring of intrauterine inflammation in pregnant women after PPROM. The primary outcome was the presence or absence of histological chorioamnionitis (HCA) and/or early onset neonatal sepsis (EONS), diagnosed after delivery and the IL-6vag concentration measured in the last vaginal fluid sample before delivery. The second aim was to compare the diagnostic and predictive potential with the established monitoring parameters c-reactive protein- (CRPblood), white blood cell count (WBC) and IL-6 concentration in maternal blood (IL-6blood).
Materials and methods
Study design
The MuMfI-PPROM-Trial (Multimodal Monitoring of Fetal Risk of Inflammation in Preterm Premature Rupture of Membranes, ClinicalTrials.gov: NCT02702297), a prospective multicenter case-control diagnostic accuracy study was performed between February 2016 and January 2018 in four German level III perinatal centers [34] following the Standards for Reporting of Diagnostic Accuracy checklist [35].
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study design was approved by the Institutional Ethics Review Board Committee of the University of Halle (Saale) (2015-121), the University of Leipzig (339/16-lk) and the University of Jena (5203-06/17) and the medical association of Saxony-Anhalt (51/16). Informed consent from all participants (pregnant women and parents/legally authorized representative of the neonates) was obtained from all individual participants included in the study.
Inclusion criteria were PPROM (diagnosed by one clinical sign (sterile speculum examination demonstrating liquor) and/or commercial amniotic fluid test (AmniSure ROM TestTM, QIAGEN GmbH, Hilden, Germany)), gestational age between 24.0/7 and 34.0/7 weeks (according to ultrasound in early pregnancy or last menstruation period), maternal age over 18 years and written informed consent in English or German. Pregnancies with lethal fetal malformations, fetal demise or indication for urgent delivery (for example non-reassuring fetal status, unstoppable labor or any other contraindication for prolongation of the pregnancy according to the applicable guidelines [36] were excluded from this study. Diagnostic and monitoring as well as general treatments were performed based on the current guidelines and the decisions of the attending physician. Treatment generally used the following substances a) antenatal steroids – Betamethasone (2 × 12 mg) administered intramuscularly at intervals of 24 h b) antibiotic prophylaxis – Ampicillin for two days followed by Amoxicillin for five days plus a single dose of Azithromycin at day one c) if tocolysis is necessary – Atosiban. During the prolongation period, a daily sampling of vaginal fluid was performed and the concentration of IL-6vag was measured in these samples. Maternal serum concentration of IL-6 (IL-6blood) and CRP (CRPblood), as well as maternal WBC was analyzed daily as part of the routine monitoring procedure. Timing as well as the mode of delivery was up to the decision of attending physician based on the guidelines (status August 2010 [36]) and independent from study participation. The German guidelines applicable at the time of the study advised prolongation before 34.0/7 weeks and active termination of pregnancy after 34.0/7 weeks according to ultrasound in early pregnancy or last menstruation period. The combinations of essential parameters according to German guidelines were employed to diagnose clinical infection: fever (38 °C axillary), maternal-fetal tachycardia (>100 bpm/>150 bpm), uterine tenderness, foul-smelling amniotic fluid, increasing uterine contraction and maternal blood tests (C-reactive protein and white cell count). After delivery, the neonates were treated based on current guidelines and local protocols. Neonatal outcome was analyzed based on documentation of clinical status and IL-6 concentration in the initial umbilical cord blood analysis. A histological examination of placenta and umbilical cord was performed and signs of inflammation were documented according to Redline criteria [37], [38]. If the inflammatory process affects the chorion and amnion, this is termed acute chorioamnionitis as a sign of the maternal inflammatory response. When the inflammatory process involves the umbilical cord, this is referred to as funisitis, the histological counterpart of the fetal inflammatory response syndrome [39]. Histological diagnosis was reevaluated by a senior pathologist blinded to clinical outcome as well as previous diagnoses. Depending on neonatal outcomes and histological findings, the cases were allocated to an inflammation and a control group. The IL-6vag concentration as well as the maternal blood parameters were evaluated and compared. We excluded cases with protocol violations/insufficient data or without successful measurements 48 h before delivery from final analysis (see Figure 1).
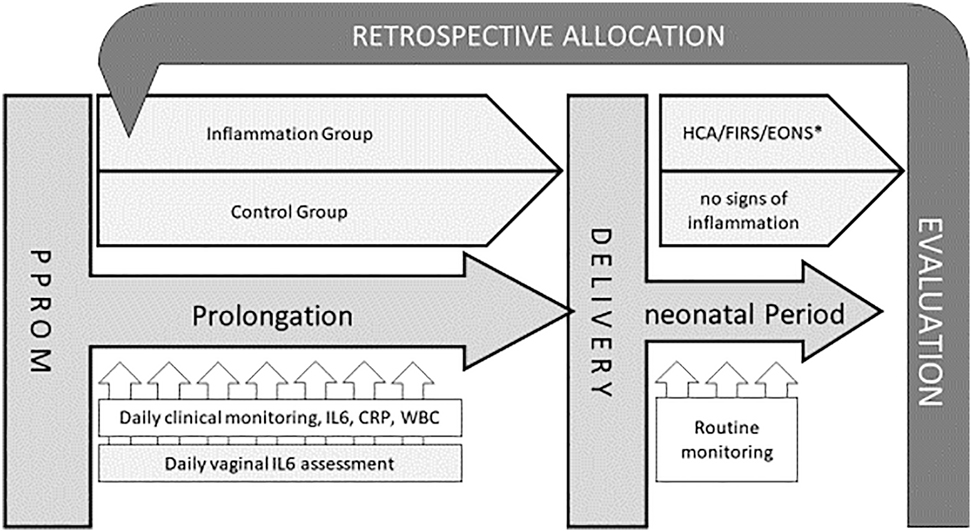
Study design.
∗HCA, histological chorioamnionitis (Redline maternal stage 2 or higher, fetal any stage); FIRS, fetal inflammatory response (cord blood IL-6>60 pg/mL); EONS, early onset neonatal sepsis (clinical signs of sepsis within first 72 h of life).
Vaginal fluid sampling and IL-6vag measurement
We invented an in-house diagnostic product (petty patent no. 20 2017 006 181/IPC A61B 10/00) for vaginal fluid sampling by combining a commercial device for saliva sampling (Salivette Cortisol code blau®, Sarstedt AG & Co, Nümbrecht, Germany) with a surgical thread (MARLIN® violet, made from polyglycolic acid, 1 USP, CATGUT GmbH, Markneukirchen, Germany). The commercial sampling kit consists of a synthetic fiber sponge with a diameter of approx. 10 mm and a length of 38 mm. Saliva collections is performed by placing the sponge in the patients mouth for 1–2 min. After removal the saliva sample is collected by centrifugation in a special single use system. The system features by an established safety in clinical use, especially in context of mucosa contact and a high recovery rate of sample volume. In original use, the average sample volume was 1.1 mL. For vaginal use, we connected the sponge with a surgical thread to simplify removal (see Supplemental Figure 1). The device was sterilized before application.
For vaginal fluid sampling, the device was applied in the lower third of the vagina by medical staff or by the patient after instruction. The device stayed there for approximately 30–45 min. After this time it was removed. The specimen was separated from the sponge by centrifugation (2000 × g for 15 min) using the commercial kit provided for saliva sampling (see Supplemental Figure 1). The IL-6 concentration was determined immediately after sampling or the specimens were stored at −20 °C before further processing. Interleukine-6 concentration was determined using an electrochemiluminescence immunoassay (ECLIA)-kit (Cobas Elecsys IL-6, Roche Diagnostics, Rotkreuz, Switzerland) with a minimum sample volume of 30 µL and a measuring range from 1.5 to 5,000 pg/mL [40]. Samples with an IL-6 concentration above 5,000 pg/mL were diluted 1:10 until concentration was within measuring range. The attending physicians were blinded to clinical outcome as well as previous diagnoses.
Defining the inflammation and the control group
Clinical data from maternal as well as neonatal records were evaluated systematically. A case was allocated to the inflammation group if at least one of the following criteria was met:
Clinical signs of neonatal sepsis diagnosed by the attending physician according to the NEO-KISS criteria [41] within 72 h after delivery.
Cases which didn’t meet any of these criteria were considered as controls. If data was not available for one or more of the criteria (i.e., no histological examination performed), the case was removed from analysis. In twin pregnancies, the case was allocated to inflammation group if at least one child met any of the criteria.
NEO-KISS criteria for neonatal sepsis are: Need for antibiotic treatment for at least five days without any other apparent infection on other site OR positive blood culture for coagulase negative staphylococcus together with elevated inflammatory markers OR blood culture of any other species together with at least two of: temperature >38.0 or <36.5 °C or temperature instability, tachycardia or bradycardia, apnoea, extended recapillarisation time, metabolic acidosis, hyperglycaemia, other signs of bloodstream infection [45].
Statistical analysis
The primary outcome was the presence or absence of histological chorioamnionitis (HCA) and/or early onset neonatal sepsis (EONS) and the IL-6vag concentration measured in the last vaginal fluid sample before delivery. We calculated means and medians of the variable (IL-6vag) at last measurements before delivery in the inflammation and the control group. We performed a sample size estimation based on data from our own pilot study data and on data from Kacerovsky et al. [46]. To evaluate the primary outcome measurement (IL-6vag concentration measured in the last vaginal fluid sample before delivery) at a significance level of 0.05 and a power of 80% in a two-tailed t test based on an expected difference of 214 pg/mL (σ=200), 15 participants per group were required. Due to an expected dropout rate of up to 45%, the goal was to include at least 27 participants each in the inflammation and in the control group.
Using Youden’s-Index (J) the optimal cut-off value for vaginal fluid interleukin 6 (IL-6vag) was calculated to predict an intrauterine inflammation [47]. This index indicates the performance at a given cutoff and is the sum of sensitivity and specificity minus one.
We also conducted an analysis of the values grouped for days before delivery. A day was defined as the time interval of 24 h from the moment of delivery. To compare this outcome with the routine diagnostics, a similar analysis was done for maternal CRPblood and IL-6blood concentration as well as WBC. To assess the test performance and to compare the study parameter with the routine-diagnostics (maternal CRPblood- and IL6blood – concentration as well as WBC), we conducted a receiver operating characteristic (ROC) analysis and compared the area under curve (AUC) of the variables and the routine diagnostics in our study population.
Statistical analysis was performed using IBM SPSS 25. In metric variables, we performed a Kolmogorov-Smirnov-test for normal distribution and, if appropriate, a t-test for differences between the means. Otherwise we performed the Mann-Whitney-U-test. The p-values (except for primary outcome measurement) are interpreted in an exploratory manner without correction for multiple comparisons.
Results
Study population
In total 57 patients were enrolled in this study (Study center (Sc) 1: n=26, Sc2: 16, Sc3: 8, Sc4: 7). Thirty seven cases completed sampling and follow-up successfully without protocol violations and were included for final analysis (details see Figure 2). Twenty cases met inflammation criteria as described above (inflammation group), while 17 cases were defined as controls. Histological chorioamnionitis (HCA) was the most frequent sign of inflammation with 14 positive cases, while early onset neonatal sepsis (EONS) occurred in 7 cases (Supplemental Table 1). Sixty five percentage (n=13) of cases were presented with only one criterion, 35% (n=7) showed a combination of at least two criteria (details see Supplemental Table 1).
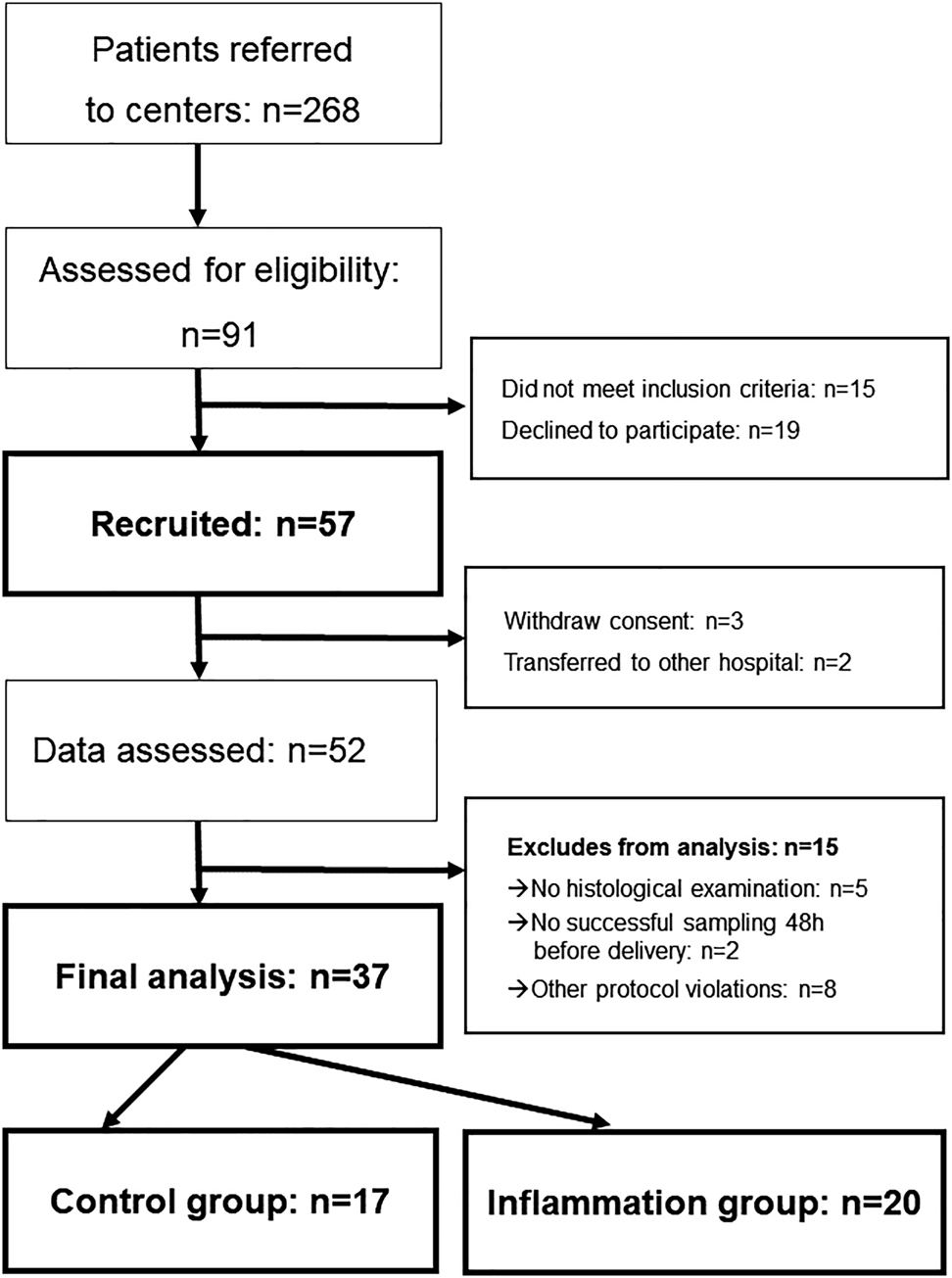
Patient recruitment flow diagram.
Baseline-characteristics as well as perinatal risk factors are presented in Table 1. The inflammation group showed a higher median in previous pregnancies (n=3 vs. n=2, p=0.01), and a higher rate of clinically suspected chorioamnionitis as indication for delivery according to patient records (55 vs. 24%, p=0.05). In cases with suspected inflammation, deliveries were triggered by elevated maternal inflammatory markers, fetal tachycardia, maternal tachycardia, abdominal pain or persistent contractions in combination with rising inflammatory markers.
Baseline characteristics (Data are median or % (n) unless otherwise specified).
| Parameter | Control (n=17) | Inflammation (n=20) | p-Value |
|---|---|---|---|
| Maternal age, years | 31 | 31 | 0.48 |
| Number of previous pregnancies | 2 | 3 | 0.01 |
| History of miscarriage or preterm delivery | 12% (2) | 5% (1) | 0.45 |
| Diabetes | 12% (2) | 15% (3) | 0.77 |
| Smoker | 29% (5) | 25% (5) | 0.76 |
| BMI before pregnancy, kg/m2 | 25.9 | 22.2 | 0.39 |
| Gestational age at delivery, days/weeks | 232/33 | 210/30 | 0.19 |
| Gestational age at PPROM, days | 213 | 197 | 0.13 |
| Latency PPROM – delivery, days | 6 | 9 | 0.29 |
| Group B streptococcus positive | 6% (1) | 10% (2) | 0.65 |
| Administration of antenatal steroids | 94% (16) | 85% (17) | 0.37 |
| Antenatal antibiotic prophylaxisa | 94% (16) | 89% (17) | 0.61 |
| Tocolysis | 64% (11) | 50% (10) | 0.37 |
| AFI in normal range | 35% (6) | 15% (3) | 0.15 |
| Twin pregnancies | 24% (4) | 0% (0) | 0.22 |
| Indication for delivery | |||
| Suspected inflammation | 24% (4) | 55% (11) | 0.05 |
| Gestational age | 18% (3) | 10% (2) | 0.50 |
| Others | 52% (9) | 35% (7) | 0.27 |
| Medical induction of labor | 29% (5) | 15% (3) | 0.29 |
| Mode of delivery | |||
| Planned caesarean | 12% (2) | 0% (0) | 0.12 |
| Vaginal delivery | 41% (7) | 35% (7) | 0.70 |
| Unplanned caesarean or instrumental vaginal delivery | 47% (8) | 65% (13) | 0.27 |
aStandard antibiotic prophylaxis regime was not given in four cases. In these cases, interval from diagnose to delivery was short so the patients received only a single shot antibiotic treatment.
The neonatal outcome of the two groups is presented in Supplemental Table 2: there is a lower birth weight (1490 vs. 1892 g, p=0.05), longer treatment in the neonatal ICU (16 vs. 11 d, p=0.045) as well as longer duration of antibiotic treatment (5 vs. 0 d, p<0.001) to see in the inflammation group compared with the controls. However, the gestational age at delivery in the inflammation group was 210 vs. 232 days in the control group. The rate of female newborns was higher in the inflammation-group than in the control-group (65 vs. 24%, p=0.005).
Feasibility
The invented device was easy to use in the clinical routine. No serious adverse events were recorded. Two women reported temporary vaginal discomfort, one woman discontinued participation for that reason. Successful ad hoc measurement of IL-6 in the 172 vaginal fluid samples within patients in the main study center reached 86%.
Findings
The median concentration of IL-6vag in the last vaginal fluid sample before delivery was substantially higher in the Inflammation group than in controls (17,085 vs. 1,888 pg/mL; p=0.01; => primary outcome measure (see Figure 3).
![Figure 3: Boxplot of the last IL-6vag concentration [pg/ml] before delivery.](/document/doi/10.1515/jpm-2020-0406/asset/graphic/j_jpm-2020-0406_fig_003.jpg)
Boxplot of the last IL-6vag concentration [pg/ml] before delivery.
We calculated an optimal cut-off to predict an intrauterine inflammation at 6,417 pg/mL using the Youden’s index. For this cut-off, IL-6vag showed a sensitivity of 65% (95%CI [44.1–85.9]) and a specificity of 76.5% (95%CI [56.3–96.6]). A threshold of 1,150 pg/mL showed a sensitivity of 95% (95%CI [85.4–100.0]) with a specifity of 35.3% (95%CI [12.6–58.0]).
In order to evaluate the changes in vaginal IL-6 (IL-6vag) over time, measurements were recorded and grouped according to the number of the days between sampling and delivery (whereby one day equals 24 h before the moment of delivery). In this analysis the difference in the median IL-6vag concentration remained relevant up to two days before delivery (data presented in Figure 4).
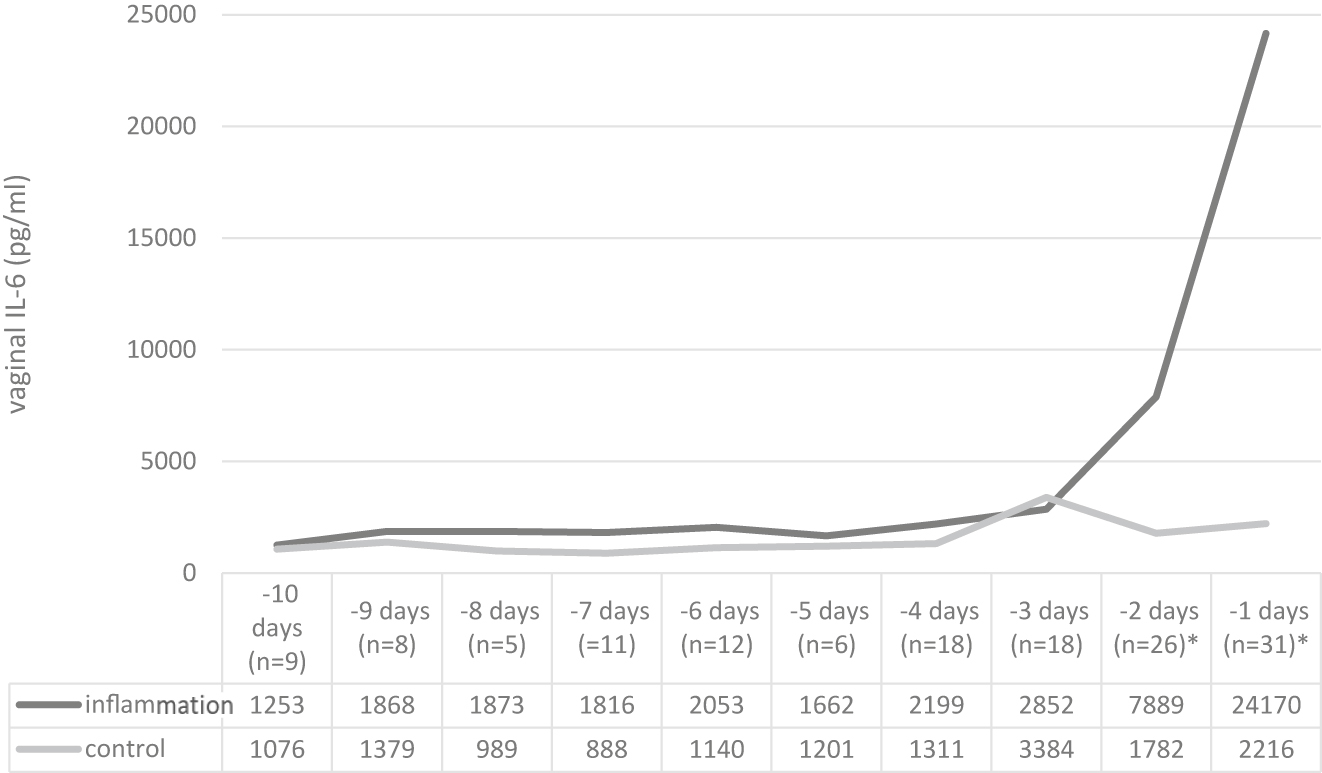
Median IL-6vag concentration values grouped for days before delivery (p<0.05 marked with asterisk∗).
To illustrate the test performances of IL-6vag, we calculated the receiver operating characteristic to predict an inflammation (see Figure 5). The area under the curve was 0.731 one day before delivery (95%CI [0.553–0.909], p=0.01) and 0.788 two days before delivery (95%CI [0.609–0.966], p<0.01).
![Figure 5: Left: IL-6vag ROC one day before delivery; (AUC IL-6vag-01: 0.731 [CI 0.553–0.909; p=0.011]) – Right: IL-6vag ROC two days before delivery (AUC IL-6vag-02: 0.788 [CI 0.609–0.966; p<0.01]).](/document/doi/10.1515/jpm-2020-0406/asset/graphic/j_jpm-2020-0406_fig_005.jpg)
Left: IL-6vag ROC one day before delivery; (AUC IL-6vag-01: 0.731 [CI 0.553–0.909; p=0.011]) – Right: IL-6vag ROC two days before delivery (AUC IL-6vag-02: 0.788 [CI 0.609–0.966; p<0.01]).
To compare the IL-6vag concentration with the maternal parameters assessed in routine monitoring, we conducted a comparative analysis for maternal white blood cell count (WBC), maternal serum-concentration of C-reactive protein (CRPblood) and Interleukine-6 concentration (IL-6blood). In the last measurement before delivery, CRPblood and IL-6blood showed a difference in median concentrations between the inflammation and control groups. However, the absolute difference remained small (median CRPblood 10.9 vs. 5.1 mg/L, p=0.03; median IL-6blood 9.7 vs. 3.7 pg/mL, p=0.02) and the difference was significantly only within 24 h before delivery in our timeline analysis. The maternal WBC did not show significant differences in any analysis (see Figure 6).
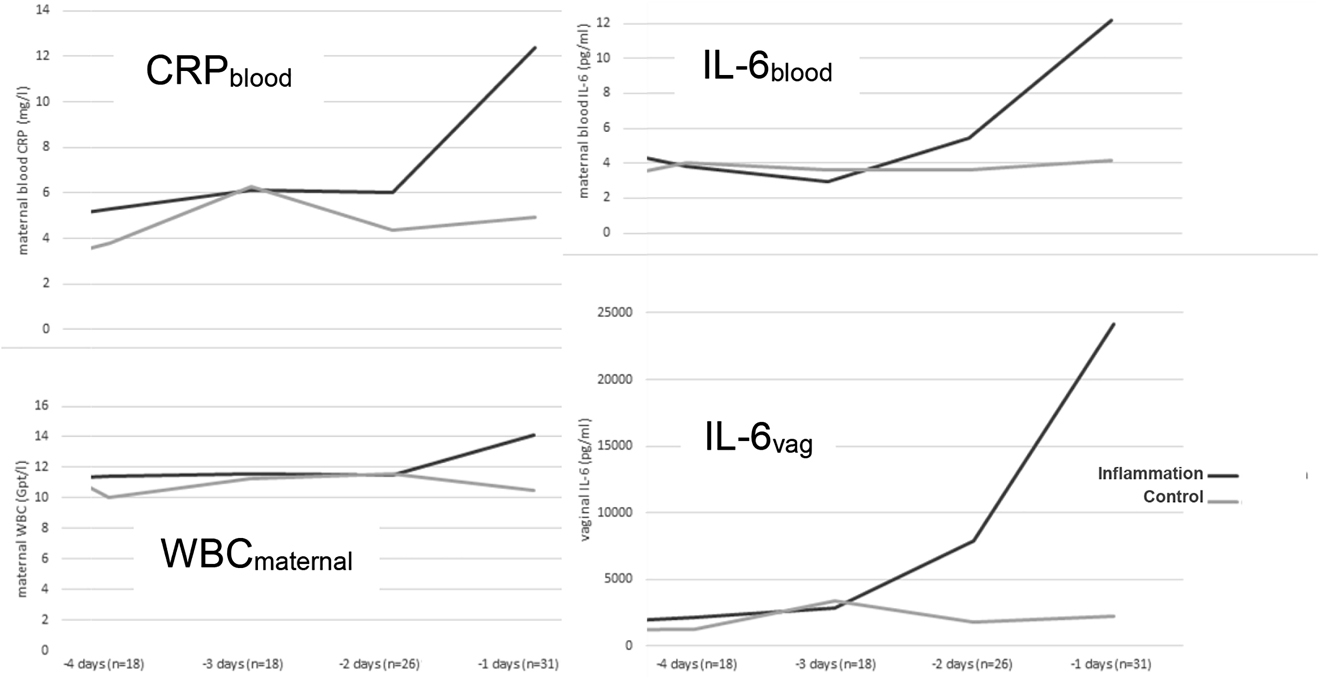
IL-6vag concentration and maternal blood values grouped for days before delivery.
To compare the test performance in the last two days before delivery, we used the receiver operating curve and calculated AUC for all cases with complete values (cases with missing values for any of the parameter were excluded from this analysis). On the last day before delivery maternal CRP showed the best AUC (0.829) while IL-6blood and IL-6vag had an AUC of 0.761 each. Two days before delivery, IL-6vag remained the only parameter with a sufficient ROC with an AUC of 0.877 (see Table 2).
IL-6vag and maternal blood routine parameters.
| Day 1 before delivery | Day 2 before delivery | |||||||
|---|---|---|---|---|---|---|---|---|
| AUC | p-Value | 95% CI | AUC | p-Value | 95% CI | |||
| IL-6vag | 0.761 | 0.012 | 0.558 | 0.964 | 0.877 | <0.001 | 0.670 | 10.083 |
| WBCblood | 0.803 | 0.004 | 0.596 | 1.000 | 0.568 | 0.634 | 0.288 | 0.847 |
| CRPblood | 0.829 | <0.001 | 0.650 | 1.000 | 0.630 | 0.335 | 0.366 | 0.893 |
| IL-6blood | 0.761 | 0.016 | 0.548 | 0.973 | 0.704 | 0.119 | 0.447 | 0.960 |
ROC-AUC, p-value, 95% confidence interval (CI), IL-6vag, WBCblood, CRPblood, IL-6blood.
Discussion
In PPROM routine diagnostic parameters show only a weak correlation with adverse neonatal outcome and risk for inflammation [19], [21], [48]. The reason is probably the ‘compartimentation’ of the feto-maternal unit [49]. An inflammation of the fetus and/or the amniotic space frequently occurs without any concomitant sign of maternal inflammation and therefore is unnoticed by the physician. On the other hand, the mother could suffer from an independent medical condition like pyelonephritis, which leaves the fetus unharmed but mimics an intrauterine inflammation. Therefore effort was made to identify parameters which could determine an inflammation of the ‘core compartments’ (amniotic cavity and fetus) i.e., by performing cardiotocography or amniocentesis [24], [26], [49], [50]. Fetal heart rate remains the only of these parameters suitable for daily monitoring while amniocentesis is only performed in unclear situations or within study protocols. Vaginal fluid is considered to be a potential specimen to detect an intrauterine inflammation since it is at least partly of intraamniotic origin.
Several studies have investigated the potential of vaginal parameters to predict intrauterine infection: Combs and colleagues did a research on 414 pregnancies with preterm labor and intact membranes. The test performance of 43 parameters in vaginal fluid was examined to predict an intrauterine infection/inflammation (positive AF-culture/bacterial PCR and/or AF-IL-6>11 ng/mL) [26]. Of all parameters, IL-6 had the best test performance with a ROC-AUC of 0.848. Park and colleagues assessed IL-6, IL-8, and WBC in cervicovaginal fluid of 85 pregnancies with preterm labor and intact membranes. All parameters were associated with intrauterine inflammation (defined as Amniotic fluid IL-6>2.6 ng/mL and/or positive culture) wile IL-6 showed the best performance with a AUC of 0.85 in ROC-analysis [25]. The same group found a comparable test performance (AUC 0.84) of vaginal IL-6 to predict intrauterine infection in a cohort of 86 women with PPROM [27]. The group Jacobsson, Kacerovsky and colleagues found similar results in other studies with a comparable design [31], [51]. Interleukin-6 is a well-established marker of inflammation, which is produced by several cell types, amongst others by decidual tissues [52], [53], [54]. An intrauterine inflammation would result in a rise of local IL-6 concentration.
In our study we focus on clinical and laboratory signs of inflammation rather than microbial amniotic fluid testing since microbial culture has a high rate of false negative results [55], [56] and detection of bacterial nucleic acid alone might be of small prognostic value since it is also possible in the upper genital tract of healthy women as well as normal pregnancies [57], [58].
In most study designs, the vaginal fluid sampling was conducted during the initial examination and therefore as a one point measurement with a variable interval to delivery [28], [29]. Furthermore, many different vaginal fluid sampling techniques were used to predict inflammation or infection after PPROM. Most studies used swabs to collect vaginal fluid during gynecological examination (i.e., Ryu 2013, Jacobsson 2005, Kacerovsky 2015, Musilova 2016, Lucovink 2011 [29], [32], [51], [59], [60]). Other studies used an aspiration tool (i.e., syringe) for sample collection (i.e., Dorfeuille 2016; Kuyumcuoglu 2010; Torbé 2005 [28], [61], [62]). Both techniques share the disadvantage that sampling implies a gynecological examination, which would lead to stress and manipulation as a daily procedure. Most studies that use a swab or an aspiration technique have only a single point vaginal fluid assessment in their study protocol, mostly simultaneous to amniocentesis or within the first clinical examination of the patient. This study design leaves one major question unattended: monitoring requires regular assessment.
Taking the highly dynamic process of intra-amniotic inflammation and/or infection into account, this could explain the heterogeneity in the available study results. Due to the daily measurement, we were able to evaluate the change in vaginal IL-6vag over time, as only few other studies did. In 2016 Kunze et al. performed a remarkable study measuring IL-6 and tumor necrosis factor α in vaginal fluid samples obtained by squeezing specimens from sanitary pads used by women with PPROM [33]. The primary outcome was a combination of clinical or histological signs of neonatal/placental infection/inflammation as well as elevated IL-6 levels in cord blood. In this setting, a daily measurement was possible. The group with an adverse outcome showed a significantly higher level of the examined parameter in the last 48 h before delivery. However, a timeline analysis (especially in the interval three days immediately before delivery) was not performed. Another notable method, that could allow the daily monitoring of intrauterine inflammation markers, was published by Lee, Yoon and colleagues in 2014. They invented a cervical fluid collector (Yoon’s AF Collector™) a device that can be placed on the cervix uteri to obtain cervical fluid without further gynecological examination. Based on the determination of interleukin-8 (IL-8) in fluid retrieved by a transcervical amniotic fluid collector, the IL-8-point-of-care test was predictive of intraamniotic inflammation [63], [64].
In our prospective case-control diagnostic accuracy study, we describe a new technique for vaginal fluid sampling. We were able to prove its feasibility in daily clinical routine. A method has been established which allows the daily analysis of the IL-6vag after PPROM. With this procedure we show for the first time the IL-6vag concentration after PPROM over time until delivery. Apparently unaffected by other maternal factors (e.g., maternal respiratory tract infection or pyelonephritis), the IL-6vag remains stable in the low range over the prolongation period (Figure 4).
We were able to show a significant difference in IL-6vag between pregnancies with signs of fetal inflammation and/or early onset sepsis and controls. This difference remained significant up to two days before delivery. This is remarkable, since routine diagnostics like maternal CRPblood were significant in measurements only within 24 h before delivery or showed no significance at all. This supports the hypothesis that the parameters of the ‘inner compartments’ (amniotic cavity and fetus) predicts an intrauterine inflammation faster and more reliably than the parameters in the maternal blood [49]. This gain in time could provide a tremendous advantage in determining an appropriate time for delivery and therefore reduce inflammation or infection related neonatal complications like the development of an EONS.
The predictive values of ‘established’ maternal inflammatory parameters for neonatal sepsis remain unsatisfying. In a 2014 metanalysis by Su et al. [19], maternal serum WBC, CRP and IL-6 showed a sensitivity of 0.47, 0.53, and 0.76 with a specificity of 0.76, 0.76, and 0.86 respectively. Cord blood IL-6 showed a much better prediction with a sensitivity of 0.91 and a specificity of 0.9 although this parameter is not suitable for daily monitoring.
In our prospective case-control diagnostic accuracy study, the ROC-analysis showed better test performance in IL-6vag compared with maternal routine parameters already two days before delivery: AUC=0.88, indicating moderate to good accuracy. Hence, a possible perspective for clinical practice might be the measurement of IL-6vag-concentration as part of the daily routine followed by an amniocentesis only in uncertain situations (i.e., if IL-6vag is exceeding the threshold of 1,150 pg/mL=95%-sensitivity-cut-off).
The current study has some limitations. Since we used a combination of three different outcome-criteria as an endpoint – of which only one indicates a severe neonatal inflammation/infection – our study is too small to explore the potential of IL-6vag in the prediction of adverse neonatal outcome. A second limitation is that our study focused on clinical and laboratory signs of inflammation rather than microbial amniotic fluid testing. Therefore, the comparability of the results with other studies is limited. A third limitation is that the study was carried out without funding and therefore a relatively high number of protocol violations occurred. Hence, 15 cases had to be excluded from the final analysis. Therefore only 37 cases could be included in the final analysis.
The sampling technique described in our study requires the daily placement of a synthetic fiber sponge in the lower third of the vagina for 30–45 min. Although we believe the grade of manipulation is comparable with normal intimate hygiene and is considerably smaller than other devices [64], vaginal manipulation could increase the risk of fetal infection. In our cohort the rate of clinical EONS was approximately 18%. Other studies report a frequency of 14% [8] or 16% [65] while current guidelines estimate the incidence of postnatal infections after PPROM of 15–25% [66]. Nevertheless, this problem has to be addressed in larger studies.
We believe, however, that our results justify the performance of an appropriately powered prospective, randomized, clinical trial. This study should have a clinical endpoint (which indicates a severe neonatal inflammation/infection), standardized measurement procedure (see above), and a control group without considering the parameter IL-6vag. Such a study protocol would clarify whether the adverse effects of daily vaginal examination (increasing risk of infection) outweigh the possible advantages.
In this respect, our study is another puzzle piece on the path toward delivery after PPROM right on time.
Conclusions
This prospective case-control diagnostic accuracy study established a new technique for vaginal fluid sampling, which offers for the first time the possibility of assessing the IL-6vag concentration noninvasively in daily monitoring. The median concentration of IL-6vag in the last vaginal fluid sample before delivery was significantly higher in the inflammation group than in the control group. In contrast to all maternal routine parameters (WBC, CRPblood, IL-6blood), the difference in the median IL-6vag concentration (inflammation vs. control group) was significant up to two days before the delivery. This gain in time could provide a tremendous advantage in determining an appropriate time for delivery. Hence measurement of IL-6vag daily may improve the clinical management of patients with PPROM.
Acknowledgments
We thank David Petroff for proof-reading the manuscript.
Research funding: None declared.
Author contributions: Gregor Seliger Conceptualization: Lead, Investigation: Lead, Methodology: Lead, Validation: Lead, Writing – original draft: Equal. Michael Bergner Conceptualization: Supporting, Data curation: Lead, Formal analysis: Supporting, Software: Lead, Validation: Equal, Visualization: Lead, Writing – original draft: Equal. Roland Haase Conceptualization: Supporting, Data curation: Equal, Methodology: Supporting, Supervision: Supporting, Validation: Supporting, Writing – review & editing: Supporting. Holger Stepan Conceptualization: Supporting, Project administration: Supporting, Resources: Supporting, Supervision: Supporting, Writing – review & editing: Supporting. Ekkehard Schleuβner Conceptualization: Supporting, Methodology: Supporting, Resources: Supporting, Supervision: Supporting, Validation: Supporting, Writing – review & editing: Supporting. Janine Zöllkau Data curation: Supporting, Formal analysis: Supporting, Methodology: Supporting, Validation: Supporting, Writing – review & editing: Supporting. Sven Seeger Conceptualization: Supporting, Data curation: Supporting, Investigation: Supporting, Resources: Supporting, Writing – review & editing: Supporting. F. Bernhard Kraus Conceptualization: Supporting, Data curation: Supporting, Resources: Supporting, Software: Supporting, Validation: Supporting, Writing – review & editing: Supporting. G.G. Ruth Hiller Conceptualization: Supporting, Formal analysis: Supporting, Methodology: Supporting, Resources: Supporting, Validation: Supporting, Writing – review & editing: Supporting. Andreas Wienke Conceptualization: Supporting, Formal analysis: Supporting, Investigation: Supporting, Methodology: Supporting, Software: Supporting, Supervision: Supporting Validation: Supporting, Visualization: Supporting, Writing – original draft: Supporting. Michael Tchirikov Conceptualization: Supporting, Funding acquisition: Supporting, Resources: Supporting, Supervision: Supporting, Validation: Supporting, Writing – review & editing: Supporting. All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
Competing interests Authors state no conflict of interest.
Informed consent: Informed consent from all participants (pregnant women and parents/legally authorized representative of the neonates) was obtained from all individual participants included in the study.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study design was approved by the institutional ethics review board committee of the University of Halle (Saale) (2015-121), the University of Leipzig (339/16-lk) and the University of Jena (5203-06/17) and the medical association of Saxony-Anhalt (51/16).
References
1. Mercer, B. Preterm premature rupture of the membranes. Obstet Gynecol 2003;101:178–93.10.1016/S0029-7844(02)02366-9Search in Google Scholar
2. Mercer, BM, Goldenberg, RL, Meis, PJ, Moawad, AH, Shellhaas, C, Das, A, et al.. The Preterm Prediction Study: prediction of preterm premature rupture of membranes through clinical findings and ancillary testing – the combination of short cervical length, history of preterm birth, and a positive result of fetal fibronectin testing is highly associated with preterm delivery after preterm premature rupture of membranes. Am J Obstet Gynecol 2000;183:738–45.10.1067/mob.2000.106766Search in Google Scholar
3. Miller, HC, Jekel, JF. Epidemiology of spontaneous premature rupture of membranes: factors in pre-term births. Yale J Biol Med 1989;62:241–51.Search in Google Scholar
4. Sae-Lin, P, Wanitpongpan, P. Incidence and risk factors of preterm premature rupture of membranes in singleton pregnancies at Siriraj Hospital. J Obstet Gynaecol Res 2019;45:573–7.10.1111/jog.13886Search in Google Scholar
5. Moutquin, J-M. Classification and heterogeneity of preterm birth. BJOG 2003;110(20 Suppl):30–3.10.1016/S1470-0328(03)00021-1Search in Google Scholar
6. Kenyon, S, Boulvain, M, Neilson, JP. Antibiotics for preterm rupture of membranes. Cochrane Database Syst Rev 2010;8:CD001058.10.1002/14651858.CD001058.pub2Search in Google Scholar
7. Bond, DM, Middleton, P, Levett, KM, van der Ham, DP, Crowther, CA, Buchanan, SL, et al.. Planned early birth versus expectant management for women with preterm prelabour rupture of membranes prior to 37 weeks’ gestation for improving pregnancy outcome. Cochrane Database Syst Rev 2017;3:CD004735. https://doi.org/10.1002/14651858.CD004735.pub4.Search in Google Scholar
8. Drassinower, D, Friedman, AM, Obican, SG, Levin, H, Gyamfi-Bannerman, C. Prolonged latency of preterm prelabour rupture of membranes and neurodevelopmental outcomes: a secondary analysis. BJOG 2016;123:1629–35.10.1111/1471-0528.14133Search in Google Scholar
9. Arora, P, Bagga, R, Kalra, J, Kumar, P, Radhika, S, Gautam, V. Mean gestation at delivery and histological chorioamnionitis correlates with early-onset neonatal sepsis following expectant management in pPROM. J Obstet Gynaecol 2014;35:235–40.10.3109/01443615.2014.958143Search in Google Scholar
10. Liu, L, Oza, S, Hogan, D, Perin, J, Rudan, I, Lawn, JE, et al.. Global, regional, and national causes of child mortality in 2000–13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet 2015;385:430–40.10.1016/S0140-6736(14)61698-6Search in Google Scholar
11. Liu, L, Oza, S, Hogan, D, Chu, Y, Perin, J, Zhu, J, et al.. Global, regional, and national causes of under-5 mortality in 2000–15: an updated systematic analysis with implications for the Sustainable Development Goals. Lancet 2016;388:3027–35.10.1016/S0140-6736(16)31593-8Search in Google Scholar
12. Tchirikov, M, Schlabritz-Loutsevitch, N, Maher, J, Buchmann, J, Naberezhnev, Y, Winarno, AS, et al.. Mid-trimester preterm premature rupture of membranes (PPROM): etiology, diagnosis, classification, international recommendations of treatment options and outcome. J Perinat Med 2018;46:465–88.10.1515/jpm-2017-0027Search in Google Scholar PubMed
13. Tchirikov, M, Bapayeva, G, Zhumadilov, ZS, Dridi, Y, Harnisch, R, Herrmann, A. Treatment of PPROM with anhydramnion in humans: first experience with different amniotic fluid substitutes for continuous amnioinfusion through a subcutaneously implanted port system. J Perinat Med 2013;41:657–63.10.1515/jpm-2012-0296Search in Google Scholar PubMed
14. Tchirikov, M, Arnold, C, Oshovskyy, V, Heinrich, U-R, Thäle, V. Three years’ experience of using a 29-gauge atraumatic needle for amniocentesis. J Perinat Med 2012;40:413–7.10.1515/jpm-2011-0224Search in Google Scholar PubMed
15. American College of Obstetricians and Gynecologists. Practice bulletin No. 172: premature rupture of membranes. Obstet Gynecol 2016;128:e165–77.10.1097/AOG.0000000000001712Search in Google Scholar PubMed
16. DGGG, OEGGG, SGGG. Prevention and therapy of preterm labour. Guideline of the DGGG, OEGGG and SGGG; 2019. Available from: https://www.awmf.org/leitlinien/detail/ll/015-025.html.Search in Google Scholar
17. Thomson, AJ. Care of women presenting with suspected preterm prelabour rupture of membranes from 24+0 Weeks of gestation: green-top guideline No. 73. BJOG 2019;126:e152–66.10.1111/1471-0528.15803Search in Google Scholar PubMed
18. Romero, R, Chaemsaithong, P, Korzeniewski, SJ, Kusanovic, JP, Docheva, N, Martinez-Varea, A, et al.. Clinical chorioamnionitis at term III: how well do clinical criteria perform in the identification of proven intra-amniotic infection? J Perinat Med 2016;44:23–32.10.1515/jpm-2015-0044Search in Google Scholar PubMed PubMed Central
19. Su, H, Chang, S-S, Han, C-M, Wu, K-Y, Li, M-C, Huang, C-Y, et al.. Inflammatory markers in cord blood or maternal serum for early detection of neonatal sepsis-a systemic review and meta-analysis. J Perinatol 2014;34:268–74.10.1038/jp.2013.186Search in Google Scholar PubMed
20. Stepan, M, Cobo, T, Musilova, I, Hornychova, H, Jacobsson, B, Kacerovsky, M, et al.. Maternal serum C-reactive protein in women with preterm prelabor rupture of membranes. PloS One 2016;11:e0150217.10.1371/journal.pone.0150217Search in Google Scholar PubMed PubMed Central
21. Higgins, RD, Saade, G, Polin, RA, Grobman, WA, Buhimschi, IA, Watterberg, K, et al.. Evaluation and management of women and newborns with a maternal diagnosis of chorioamnionitis: summary of a workshop. Obstet Gynecol 2016;127:426–36.10.1097/AOG.0000000000001246Search in Google Scholar PubMed PubMed Central
22. Cobo, T, Kacerovsky, M, Palacio, M, Hornychova, H, Hougaard, DM, Skogstrand, K, et al.. A prediction model of histological chorioamnionitis and funisitis in preterm prelabor rupture of membranes: analyses of multiple proteins in the amniotic fluid. J Matern Fetal Neonatal Med 2012;25:1995–2001.10.3109/14767058.2012.666592Search in Google Scholar PubMed
23. Combs, CA, Gravett, M, Garite, TJ, Hickok, DE, Lapidus, J, Porreco, R, et al.. Amniotic fluid infection, inflammation, and colonization in preterm labor with intact membranes. Am J Obstet Gynecol 2014;210:125.e1–15.10.1016/j.ajog.2013.11.032Search in Google Scholar PubMed
24. Romero, R, Miranda, J, Chaemsaithong, P, Chaiworapongsa, T, Kusanovic, JP, Dong, Z, et al.. Sterile and microbial-associated intra-amniotic inflammation in preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med 2015;28:1394–409.10.3109/14767058.2014.958463Search in Google Scholar PubMed PubMed Central
25. Park, JW, Park, KH, Lee, SY. Noninvasive prediction of intra-amniotic infection and/or inflammation in women with preterm labor: various cytokines in cervicovaginal fluid. Reprod Sci 2013;20:262–8.10.1177/1933719112451794Search in Google Scholar PubMed
26. Combs, CA, Garite, TJ, Lapidus, JA, Lapointe, JP, Gravett, M, Rael, J, et al.. Detection of microbial invasion of the amniotic cavity by analysis of cervicovaginal proteins in women with preterm labor and intact membranes. Am J Obstet Gynecol 2015;212:482.e1–12.10.1016/j.ajog.2015.02.007Search in Google Scholar PubMed
27. Jun, JK, Yoon, BH, Romero, R, Kim, M, Moon, JB, Ki, SH, et al.. Interleukin 6 determinations in cervical fluid have diagnostic and prognostic value in preterm premature rupture of membranes. Am J Obstet Gynecol 2000;183:868–73.10.1067/mob.2000.109034Search in Google Scholar PubMed
28. Dorfeuille, N, Morin, V, Tetu, A, Demers, S, Laforest, G, Gouin, K, et al.. Vaginal fluid inflammatory biomarkers and the risk of adverse neonatal outcomes in women with PPROM. Am J Perinatol 2016;33:1003–7.10.1055/s-0036-1582130Search in Google Scholar PubMed
29. Kacerovsky, M, Musilova, I, Jacobsson, B, Drahosova, M, Hornychova, H, Janku, P, et al.. Cervical fluid IL-6 and IL-8 levels in pregnancies complicated by preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med 2015;28:134–40.10.3109/14767058.2014.908179Search in Google Scholar PubMed
30. Kayem, G, Goffinet, F, Batteux, F, Jarreau, PH, Weill, B, Cabrol, D. Detection of interleukin-6 in vaginal secretions of women with preterm premature rupture of membranes and its association with neonatal infection: a rapid immunochromatographic test. Am J Obstet Gynecol 2005;192:140–5.10.1016/j.ajog.2004.07.015Search in Google Scholar PubMed
31. Musilova, I, Andrys, C, Drahosova, M, Soucek, O, Pliskova, L, Jacobsson, B, et al.. Cervical fluid interleukin 6 and intra-amniotic complications of preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med 2017;31:827–36.10.1080/14767058.2017.1297792Search in Google Scholar PubMed
32. Ryu, A, Park, KH, Oh, KJ, Lee, SY, Jeong, EH, Park, JW. Predictive value of combined cervicovaginal cytokines and gestational age at sampling for intra-amniotic infection in preterm premature rupture of membranes. Acta Obstet Gynecol Scand 2013;92:517–24.10.1111/aogs.12073Search in Google Scholar PubMed
33. Kunze, M, Klar, M, Morfeld, CA, Thorns, B, Schild, RL, Markfeld-Erol, F, et al.. Cytokines in noninvasively obtained amniotic fluid as predictors of fetal inflammatory response syndrome. Am J Obstet Gynecol 2016;215:96.e1–8. https://doi.org/10.1016/j.ajog.2016.01.181.Search in Google Scholar
34. Kent, A, Kirtley, S. Insights from outside BJOG. BJOG 2016;123:1051–5.10.1111/1471-0528.14124Search in Google Scholar
35. Bossuyt, PM, Reitsma, JB, Bruns, DE, Gatsonis, CA, Glasziou, PP, Irwig, L, et al.. STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies. Clin Chem 2015;61:1446–52.10.1373/clinchem.2015.246280Search in Google Scholar PubMed
36. DGGG. Vorgehen beim vorzeitigen Blasensprung; 2010. Available from: https://www.dggg.de/fileadmin/documents/leitlinien/archiviert/federfuehrend/015029_Empfehlungen_zum_Vorgehen_beim_vorzeitigen_Blasensprung/015029_2010.pdf.Search in Google Scholar
37. Khong, TY, Mooney, EE, Ariel, I, Balmus, NCM, Boyd, TK, Brundler, M-A, et al.. Sampling and definitions of placental lesions: Amsterdam placental workshop group consensus statement. Arch Pathol Lab Med 2016;140:698–713. https://doi.org/10.5858/arpa.2015-0225-CC.Search in Google Scholar
38. Redline, RW, Faye-Petersen, O, Heller, D, Qureshi, F, Savell, V, Vogler, C. Amniotic infection syndrome: nosology and reproducibility of placental reaction patterns. Pediatr Dev Pathol 2003;6:435–48.10.1007/s10024-003-7070-ySearch in Google Scholar PubMed
39. Kim, CJ, Romero, R, Chaemsaithong, P, Chaiyasit, N, Yoon, BH, Kim, YM. Acute chorioamnionitis and funisitis: definition, pathologic features, and clinical significance. Am J Obstet Gynecol 2015;213:52.10.1016/j.ajog.2015.08.040Search in Google Scholar PubMed PubMed Central
40. La Roche Ltd. Elecsys® IL-6. Available from: https://diagnostics.roche.com/ch/de/products/params/elecsys-il-6.html [Accessed 4 July 2019].Search in Google Scholar
41. Nationales Referenzzentrum für Surveillance von nosokomialen Infektionen. Surveillance von nosokomialen Infektionen, multiresistenten Erregern und Antibiotika-Anwendungen bei Frühgeborenen mit einem Geburtsgewicht unter 1.500 g: Protokoll. Berlin: Institut für Hygiene und Umweltmedizin Charité – Universitätsmedizin Berlin; 2016.Search in Google Scholar
42. Arnon, S, Litmanovitz, I. Diagnostic tests in neonatal sepsis. Curr Opin Infect Dis 2008;21:223–7.10.1097/QCO.0b013e3282fa15ddSearch in Google Scholar PubMed
43. Resch, B, Gusenleitner, W, Müller, WD. Procalcitonin and interleukin-6 in the diagnosis of early-onset sepsis of the neonate. Acta Paediatr 2003;92:243–5.10.1111/j.1651-2227.2003.tb00534.xSearch in Google Scholar PubMed
44. Verboon-Maciolek, MA, Thijsen, SFT, Hemels, MAC, Menses, M, van Loon, AM, Krediet, TG, et al.. Inflammatory mediators for the diagnosis and treatment of sepsis in early infancy. Pediatr Res 2006;59:457.10.1203/01.pdr.0000200808.35368.57Search in Google Scholar PubMed
45. Geffers, C, Baerwolff, S, Schwab, F, Gastmeier, P. Incidence of healthcare-associated infections in high-risk neonates: results from the German surveillance system for very-low-birthweight infants. J Hosp Infect 2008;68:214–21.10.1016/j.jhin.2008.01.016Search in Google Scholar PubMed
46. Kacerovsky, M, Musilova, I, Hornychova, H, Kutova, R, Pliskova, L, Kostal, M, et al.. Bedside assessment of amniotic fluid interleukin-6 in preterm prelabor rupture of membranes. Am J Obstet Gynecol 2014;211:385.e1–9.10.1016/j.ajog.2014.03.069Search in Google Scholar PubMed
47. Youden, WJ. Index for rating diagnostic tests. Cancer 1950;3:32–5.10.1002/1097-0142(1950)3:1<32::AID-CNCR2820030106>3.0.CO;2-3Search in Google Scholar
48. Serdar Kutuk, M, Bastug, O, Ozdemir, A, Adnan Ozturk, M, Tuncay Ozgun, M, Basbug, M, et al.. Relationship between maternal c-reactive protein level and neonatal outcome in patients with preterm premature rupture of membranes treated with Ampicillin and Azithromycin. J Obstet Gynaecol 2016;36:772–7.10.3109/01443615.2016.1162772Search in Google Scholar
49. Dulay, AT, Buhimschi, IA, Zhao, G, Bahtiyar, MO, Thung, SF, Cackovic, M, et al.. Compartmentalization of acute phase reactants Interleukin-6, C-Reactive Protein and Procalcitonin as biomarkers of intra-amniotic infection and chorioamnionitis. Cytokine 2015;76:236–43.10.1016/j.cyto.2015.04.014Search in Google Scholar
50. Cobo, T, Kacerovsky, M, Holst, R-M, Hougaard, DM, Skogstrand, K, Wennerholm, U-B, et al.. Intra-amniotic inflammation predicts microbial invasion of the amniotic cavity but not spontaneous preterm delivery in preterm prelabor membrane rupture. Acta Obstet Gynecol Scand 2012;91:930–5.10.1111/j.1600-0412.2012.01427.xSearch in Google Scholar
51. Musilova, I, Bestvina, T, Hudeckova, M, Michalec, I, Cobo, T, Jacobsson, B, et al.. Vaginal fluid interleukin-6 concentrations as a point-of-care test is of value in women with preterm prelabor rupture of membranes. Am J Obstet Gynecol 2016;215:619.e1–12.10.1016/j.ajog.2016.07.001Search in Google Scholar
52. Hunter, CA, Jones, SA. IL-6 as a keystone cytokine in health and disease. Nat Immunol 2015;16:448–57.10.1038/ni.3153Search in Google Scholar
53. Dudley, DJ, Trautman, MS, Araneo, BA, Edwin, SS, Mitchell, MD. Decidual cell biosynthesis of interleukin-6: regulation by inflammatory cytokines. J Clin Endocrinol Metab 1992;74:884–9.10.1210/jcem.74.4.1548355Search in Google Scholar
54. Lockwood, CJ, Murk, WK, Kayisli, UA, Buchwalder, LF, Huang, SJ, Arcuri, F, et al.. Regulation of interleukin-6 expression in human decidual cells and its potential role in chorioamnionitis. Am J Pathol 2010;177:1755–64.10.2353/ajpath.2010.090781Search in Google Scholar
55. Redline, RW. The clinical implications of placental diagnoses. Semin Perinatol 2015;39:2–8. https://doi.org/10.1053/j.semperi.2014.10.002.Search in Google Scholar
56. Cobo, T, Kacerovsky, M, Jacobsson, B. Noninvasive sampling of the intrauterine environment in women with preterm labor and intact membranes. Fetal Diagn Ther 2017;43:241–9.10.1159/000480232Search in Google Scholar
57. Chen, C, Song, X, Wei, W, Zhong, H, Dai, J, Lan, Z, et al.. The microbiota continuum along the female reproductive tract and its relation to uterine-related diseases. Nat Commun 2017;8:875.10.1038/s41467-017-00901-0Search in Google Scholar PubMed PubMed Central
58. Fortner, KB, Grotegut, CA, Ransom, CE, Bentley, RC, Feng, L, Lan, L, et al.. Bacteria localization and chorion thinning among preterm premature rupture of membranes. PloS One 2014;9:e83338.10.1371/journal.pone.0083338Search in Google Scholar PubMed PubMed Central
59. Jacobsson, B, Mattsby-Baltzer, I, Hagberg, H. Interleukin-6 and interleukin-8 in cervical and amniotic fluid: relationship to microbial invasion of the chorioamniotic membranes. BJOG 2005;112:719–24.10.1111/j.1471-0528.2005.00536.xSearch in Google Scholar PubMed
60. Lucovnik, M, Kornhauser-Cerar, L, Premru-Srsen, T, Gmeiner-Stopar, T, Derganc, M. Neutrophil defensins but not interleukin-6 in vaginal fluid after preterm premature rupture of membranes predict fetal/neonatal inflammation and infant neurological impairment. Acta Obstet Gynecol Scand 2011;90:908–16.10.1111/j.1600-0412.2011.01177.xSearch in Google Scholar PubMed
61. Kuyumcuoglu, U, Kangal, K, Guzel, AI, Celik, Y. Clinical significance of procalcitonin in cervicovaginal secretions of women with preterm rupture of membranes. Clin Exp Obstet Gynecol 2010;37:319–21.Search in Google Scholar
62. Torbé, A, Czajka, R. Are vaginal fluid procalcitonin levels useful for the prediction of subclinial infection in patients with preterm premature rupture of membranes? J Obstet Gynaecol Res 2005;31:464–70.10.1111/j.1447-0756.2005.00321.xSearch in Google Scholar PubMed
63. Lee, SM, Romero, R, Park, JS, Chaemsaithong, P, Jun, JK, Yoon, BH. A transcervical amniotic fluid collector: a new medical device for the assessment of amniotic fluid in patients with ruptured membranes. J Perinat Med 2015;43:381–9.10.1515/jpm-2014-0276Search in Google Scholar PubMed PubMed Central
64. Oh, KJ, Lee, J, Romero, R, Park, HS, Hong, J-S, Yoon, BH. A new rapid bedside test to diagnose and monitor intraamniotic inflammation in preterm PROM using transcervically collected fluid. Am J Obstet Gynecol 2020;223:423.e1–15.10.1016/j.ajog.2020.02.037Search in Google Scholar PubMed
65. Stepan, M, Cobo, T, Maly, J, Navratilova, M, Musilova, I, Hornychova, H, et al.. Neonatal outcomes in subgroups of women with preterm prelabor rupture of membranes before 34 weeks. J Matern Fetal Neonatal Med 2016;29:2373–7.10.3109/14767058.2015.1086329Search in Google Scholar PubMed
66. American College of Obstetricians and Gynecologists. Prelabor rupture of membranes: ACOG practice bulletin, number 217. Obstet Gynecol 2020;135:e80–97.10.1097/AOG.0000000000003700Search in Google Scholar PubMed
Supplementary Material
The online version of this article offers supplementary material (https://doi.org/10.1515/jpm-2020-0406).
© 2021 Gregor Seliger et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Review
- Neonatal lupus erythematosus – practical guidelines
- Original Articles – Obstetrics
- Optimal timing to screen for asymptomatic bacteriuria during pregnancy: first vs. second trimester
- Amniotic fluid embolism – implementation of international diagnosis criteria and subsequent pregnancy recurrence risk
- COL1A1, COL4A3, TIMP2 and TGFB1 polymorphisms in cervical insufficiency
- Pregnancy and neonatal outcomes of twin pregnancies – the role of maternal age
- Comparison of maternal third trimester hemodynamics between singleton pregnancy and twin pregnancy
- Daily monitoring of vaginal interleukin 6 as a predictor of intraamniotic inflammation after preterm premature rupture of membranes – a new method of sampling studied in a prospective multicenter trial
- Association between the number of pulls and adverse neonatal/maternal outcomes in vacuum-assisted delivery
- Original Articles – Fetus
- The effect of nuchal umbilical cord on fetal cardiac and cerebral circulation-cross-sectional study
- Recognition of facial expression of fetuses by artificial intelligence (AI)
- Correlation of first-trimester thymus size with chromosomal anomalies
- Fetal intracranial structures: differences in size according to sex
- Original Articles – Neonates
- Antenatal care and perinatal outcomes of asylum seeking women and their infants
- Maturation of the cardiac autonomic regulation system, as function of gestational age in a cohort of low risk preterm infants born between 28 and 32 weeks of gestation
- Short Communication
- The impact of transfers from neonatal intensive care to paediatric intensive care
- Letter to the Editor
- Differential microRNA expression in placentas of small-for-gestational age neonates with and without exposure to poor maternal gestational weight gain
Articles in the same Issue
- Frontmatter
- Review
- Neonatal lupus erythematosus – practical guidelines
- Original Articles – Obstetrics
- Optimal timing to screen for asymptomatic bacteriuria during pregnancy: first vs. second trimester
- Amniotic fluid embolism – implementation of international diagnosis criteria and subsequent pregnancy recurrence risk
- COL1A1, COL4A3, TIMP2 and TGFB1 polymorphisms in cervical insufficiency
- Pregnancy and neonatal outcomes of twin pregnancies – the role of maternal age
- Comparison of maternal third trimester hemodynamics between singleton pregnancy and twin pregnancy
- Daily monitoring of vaginal interleukin 6 as a predictor of intraamniotic inflammation after preterm premature rupture of membranes – a new method of sampling studied in a prospective multicenter trial
- Association between the number of pulls and adverse neonatal/maternal outcomes in vacuum-assisted delivery
- Original Articles – Fetus
- The effect of nuchal umbilical cord on fetal cardiac and cerebral circulation-cross-sectional study
- Recognition of facial expression of fetuses by artificial intelligence (AI)
- Correlation of first-trimester thymus size with chromosomal anomalies
- Fetal intracranial structures: differences in size according to sex
- Original Articles – Neonates
- Antenatal care and perinatal outcomes of asylum seeking women and their infants
- Maturation of the cardiac autonomic regulation system, as function of gestational age in a cohort of low risk preterm infants born between 28 and 32 weeks of gestation
- Short Communication
- The impact of transfers from neonatal intensive care to paediatric intensive care
- Letter to the Editor
- Differential microRNA expression in placentas of small-for-gestational age neonates with and without exposure to poor maternal gestational weight gain


