Abstract
Background
Worldwide, 14.9 million infants (11%) are born preterm each year. Up to 40% of preterm births (PTBs) are associated with genital tract infections. The vaginal pH can reflect changes in the vaginal milieu and, if elevated, indicates an abnormal flora or infection.
Objective
The aim of the study was to investigate whether an increased antenatal vaginal pH >4.5 in pre-labour pregnant women is associated with an increased PTB rate <37 completed weeks gestation.
Search strategy
Key databases included SCOPUS, EMBASE, MEDLINE, PsycInfo and the Cochrane Central Register of Controlled Trials, complemented by hand search, up to January 2017.
Selection criteria
Primary research reporting vaginal pH assessment in pre-labour pregnant women and PTB rate.
Data collection and analysis
Data extraction and appraisal were carried out in a pre-defined standardised manner, applying the Newcastle-Ottawa scale (NOS) and Cochrane risk of bias tool. Analysis included calculation of risk difference (RD) and narrative synthesis. It was decided to abstain from pooling of the studies due to missing information in important moderators.
Main results
Of 986 identified records, 30 were included in the systematic review. The risk of bias was considered mostly high (40%) or moderate (37%). Fifteen studies permitted a calculation of RD. Of these, 14 (93%) indicated a positive association between increased antenatal vaginal pH and PTB (RD range: 0.02–0.75).
Conclusion
An increased antenatal vaginal pH >4.5 may be associated with a higher risk for PTB. It is recommended to conduct a randomised controlled trial (RCT) to investigate the effectiveness of antenatal pH screening to prevent PTB.
Tweetable abstract
Pregnant women with an increased vaginal pH >4.5 may be at higher risk to experience preterm birth.
Introduction
The prevention of preterm birth (PTB) poses a global challenge as PTB affects the short and long-term health of children, adolescents and adults [1], [2], [3]. Worldwide, 14.9 million infants (11%) are born before 37 completed weeks of gestation each year. The prevalence not only varies globally, but also within Europe, where PTB rates range between 5.2% in Iceland and 10.4% in Cyprus [1], [4].
Up to 40% of PTBs are associated with genital tract infections [5], [6], [7]. The vaginal pH can reflect changes in the vaginal milieu and an elevated vaginal pH in pregnancy indicates an abnormal flora or infection [8]. It may therefore serve as an indicator of a local infection at an early stage [9]. Furthermore, an increased vaginal pH ≥5.0 in pregnancy is associated with more caesarean sections, poorer neonatal outcomes and increased admission rates to a neonatal intensive care unit [10].
In numerous studies, bacterial vaginosis (BV) has been discussed as a cause for PTB [11], [12], [13], [14]. However, a Cochrane review showed that an effective eradication of BV during pregnancy did not decrease PTB rates [15].
It was suggested that a routine measurement of the antenatal vaginal pH could reduce the overall PTB rate through a more rapid identification and subsequent treatment of abnormal vaginal flora [9], [16]. In 2000, promising results from a study in Thuringia, Germany, suggested a reduction of PTB rates from 7.7 to 6.8% if women self-assessed their antenatal vaginal pH and sought treatment accordingly [17]. This prompted an implementation of a pH self-assessment programme in five German federal states, which was later established nationwide albeit a lack of rigorous evidence for its benefits [18]. An evaluation of the project, which compared the outcome of women who did or did not request the test kits, found no efficacy in pH self-testing for the prevention of PTB [19]. Overall, observational study findings remain heterogeneous and sources of heterogeneity include different sampling (e.g. convenience [13], [20], [21], [22], [23], [24]), frequency of pH measurement (single measurement [10], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34] vs. multiple measurements [9], [19], [20], [21], [22], [35], [36], [37], [38], [39], [40], [41], [42]) and different pathological pH definitions in the comparison groups (>4.2 to ≥5.0).
It is therefore an unanswered question, whether a routine assessment of the vaginal pH, either by self-examination or by examination through a health professional, can contribute to reduce PTB. Consequently, we conducted a systematic review in order to investigate whether an increased antenatal vaginal pH >4.5 is associated with an increased PTB rate in pre-labour pregnant women of any gestational age.
Methods
The protocol for this systematic review was registered with PROSPERO [43]. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) were applied [44], [45].
Search strategy
The search strategy followed the population-intervention-comparison-outcome framework [46]. No language, publication date or publication status restrictions were imposed. Keywords for the search were in German and English. English terms included the following: pregnancy, antenatal, antepartum, gestation, vaginal pH, vaginal flora, preterm and premature. The search terms were applied to the database SCOPUS and to the platforms PubMed, Web of Science and OVID, which covered, among others, the databases EMBASE, MEDLINE and PsychInfo. In addition, the Cochrane Central Register of Controlled Trials (CENTRAL), the Centre for Review and Dissemination (CRD), the Clinical trials registry and ProQuest Dissertations and Theses Global were searched. Covidence® was used after initial title screening to facilitate abstract and full-text screening. The complete search was conducted in March 2016 and updated in January 2017.
Selection criteria
All original materials reporting data on the vaginal pH of pre-labour pregnant women together with data on the gestational age at birth were included. Both randomised and non-randomised controlled studies were considered. The eligible population consisted of pregnant women of any gestational age before the onset of labour. There were no restrictions on the measurement type for the vaginal pH, which included test strips, swabs, modified examination gloves, modified panty-liners and the use of electronic pH-meters. The complete vaginal area was included, comprising the vaginal vestibule, vaginal canal and anterior and posterior vaginal fornix. All measurement regimes were eligible, comprising single measurements up to serial measurements. The primary outcome measure was PTB, which was defined as birth before 37 completed weeks gestation.
Study selection
After the collection of records, duplicates were identified and removed. The eligibility assessment was performed on the remaining articles independently in a blinded standardised manner by two reviewers (MW and NR). The assessment was carried out in three phases, each preceded by a pilot (Figure 1). First, all records were screened based on the title. Second, the remaining articles were screened based on the title and abstract. In this phase, because information about the measurement of the pH was frequently not obtainable from the abstract, all publications which mentioned the vaginal milieu were moved forward for full-text screening. Third, the full-texts of records were assessed. Studies in languages other than English or German were assessed by native speakers. Conflicts during the second and third screening phase were resolved by consensus and where no consensus could be reached, a third reviewer (SG) was consulted.
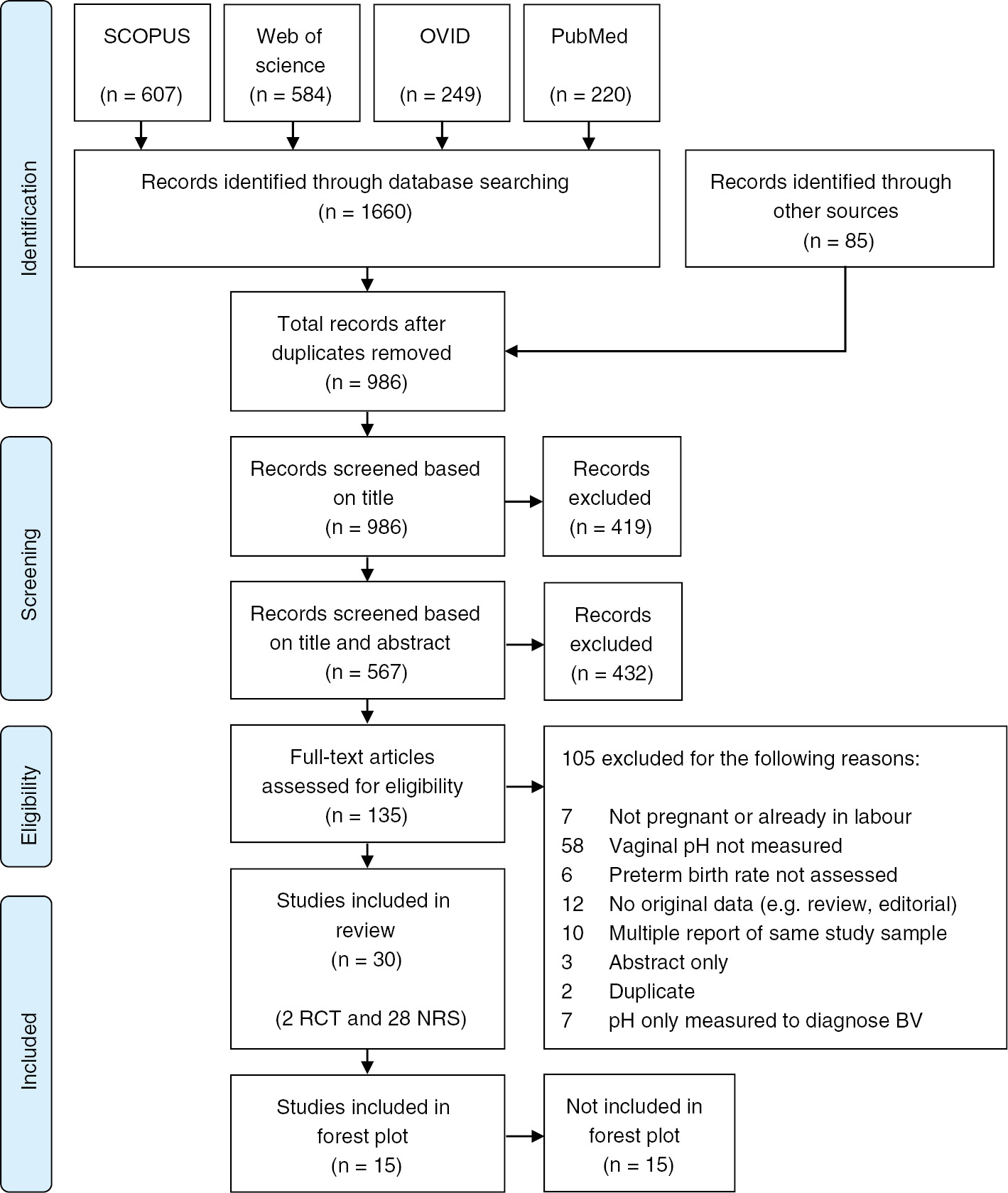
PRISMA flow diagram of included studies (n=30).
Records were excluded if women in the sample were already in labour, if no pH measurement was available, if the PTB rate was not assessed or if the report did not present original material. If the same study was reported in multiple publications, only the most recent or the most complete publication was included. Furthermore, studies were excluded if the only published information was an abstract.
Finally, studies were excluded if the pH was only measured to diagnose BV, as this provided insufficient information to make this study eligible for inclusion.
Assessment of risk of bias
All included studies underwent an individual assessment of their risk of bias, which was carried out by four researchers (MW, AK, LS, SG) in a standardised, blinded manner. Randomised controlled trials (RCTs) were assessed with the Cochrane tool for risk of bias assessment [47]. Non-randomised studies underwent a risk of bias assessment on the basis of the Newcastle-Ottawa scale (NOS) [48], [49]. The scale allows a maximum score of nine stars and was adapted to the study topic after agreement by all authors prior to its application. Conflicts during the assessment of risk of bias were solved by consensus (MW, LS, SG, MG).
Data extraction and analysis
Data were extracted into an Excel® sheet (MW) and was reviewed for correctness (LS, GS) in a standardised manner. Missing data items were requested from the authors. For analysis, the software Stata® version 14.0 was used. The primary outcome was evaluated using the absolute risk difference (RD) where RD >0 implies higher risk for PTB in the increased pH group. A pathological pH was defined as >4.5 [50], [51]. Information on the NOS was included in the forest plot to investigate any possible association between study quality and effects. A total NOS score of <7 was considered as poor study quality. A funnel plot was drawn to investigate the presence of possible small-study effects [52]. It was decided to abstain from pooling the studies due to missing information in important moderators, which would render the evaluation of heterogeneity in a random-effects model impossible [53]. Details of the data extraction procedure, author contact and pre-planned analysis can be found in the published review protocol [43].
Results
The search yielded 1660 records from the search of electronic databases and 85 records from other sources. Seven hundred and fifty nine records were identified as duplicates, leaving 986 records available for screening. Of these, 135 were assessed in full-text and 105 were excluded (Figure 1). Review of the reference lists of included articles revealed no further eligible studies. The screening process identified 30 articles eligible for this systematic review, of which two were RCTs, 27 cohort studies and one case-control study (Figure 1). Fifteen studies did not provide numerical information on either the outcome or exposure (n=4) [35], [38], [40], [42] or both (n=11) [9], [19], [20], [22], [25], [26], [27], [28], [34], [36], [39]. For 15 studies, the RD and 95% confidence interval (CI) were calculated (Figures 2 and 3).
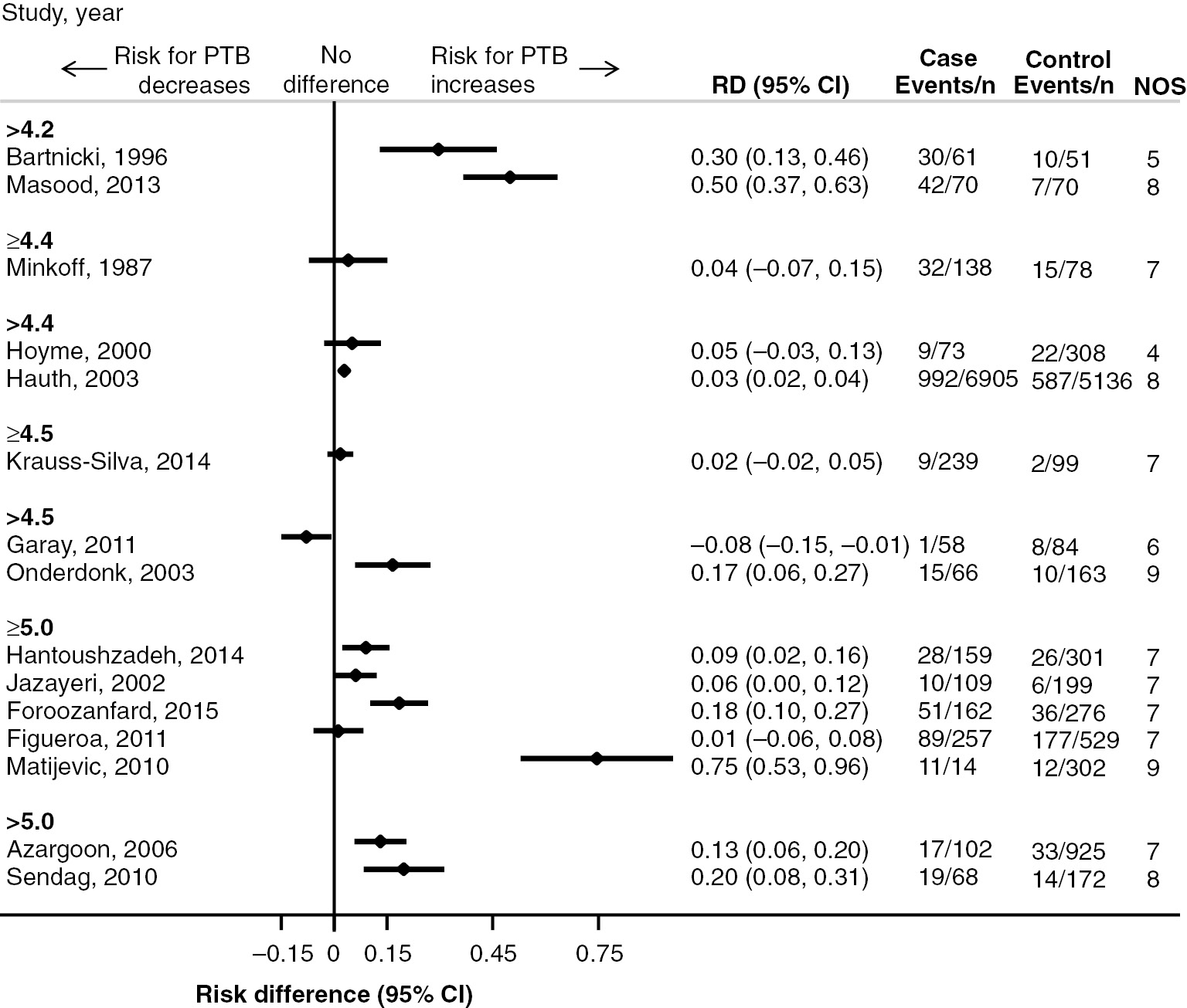
Forest plot with study-specific risk difference (RD) for increased versus normal antenatal vaginal pH and PTB (n=15).

Funnel plot with study-specific risk difference (RD) for increased versus normal antenatal vaginal pH and PTB (n=15).
Initially, all 30 studies included in this systematic review are presented narratively, followed by an illustration of the 15 non-randomised studies with complete numerical information, using a forest plot and funnel plot.
The 30 eligible studies were published between 1987 and 2015. Most frequent geographical origins were the USA (n=8) [24], [26], [29], [31], [32], [38], [40], [54] and Germany (n=7) [9], [19], [22], [33], [34], [36], [37]. Most studies were conducted in high-income countries (n=22) [9], [19], [20], [22], [24], [25], [26], [28], [29], [30], [31], [32], [33], [34], [36], [37], [38], [39], [40], [42], [54], [55] and upper-middle income countries (n=5) [10], [13], [21], [41], [56]. The median age of participants was 27 years (mean range of 22.35–32.35) and the median gestational age range at the study entry was 16–26 weeks. Twelve (40%) studies [9], [19], [22], [25], [27], [30], [33], [36], [37], [38], [40], [42] included women with twin pregnancy and 19 (63%) studies [9], [10], [20], [22], [23], [24], [26], [27], [28], [31], [32], [34], [35], [36], [37], [39], [40], [42], [54] included women with a history of PTB.
The frequency of vaginal pH measurements was mostly single (48%, n=12) [10], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34] followed by two (12%, n=3) [21], [41], [42] or four measurements (4%, n=1) [35] and multiple measurements with unknown frequencies (36%, n=9) [9], [19], [20], [22], [36], [37], [38], [39], [40]. The vaginal pH was measured mostly by one of the researchers (83%, n=25) [10], [13], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [38], [39], [40], [41], [42], [54], [55], [56], whereas in five studies (17%) [9], [19], [35], [36], [37] the participating women measured their vaginal pH by themselves. Most studies distinguished normal from pathological pH (87%, n=26). The definition of pathological pH varied across studies with the majority using a threshold of >4.5 (27%, n=8) [9], [22], [24], [25], [26], [27], [30], [40] or ≥5.0 (20%, n=6) [10], [21], [31], [38], [54], [55]. The methods to assess the vaginal pH were colour sensitive indicator strips (67%, n=16) [10], [13], [21], [23], [24], [25], [26], [28], [29], [31], [32], [34], [35], [41], [54], [56], colour sensitive test gloves (17%, n=4) [9], [19], [37], [55], application of vaginal fluid from a swab to a pH microcomputer (8%, n=2) [20], [39] or a combination of these methods (8%, n=2) [22], [36]. Information on the interval of the measurement method was given by nine (30%) studies and included 0.1 [24], [35], 0.3 [28], [29], [36], [37], [39] and 0.5 [13], [54] pH units. The great majority of the included studies defined PTB as delivery <37 weeks and 0 days (70%, n=21) [9], [10], [13], [19], [21], [24], [25], [26], [27], [29], [31], [32], [33], [35], [36], [37], [38], [39], [40], [41], [55], whilst two used 36 weeks [54], [56] and one used 34 weeks [34] as the cut-off.
Of the 28 included non-randomised studies, 11 cohort studies and one case-control study provided a total score <7 according to NOS, indicating a high individual risk of bias. Four studies provided a particularly low NOS score of 3 [36] or 4 [9], [22], [37]. Both RCTs had a moderate risk of bias (Table 1) [35], [40].
Risk of bias assessment for non-randomised studies based on Newcastle-Ottawa scale (n=28) and for randomised studies based on Cochrane tool for risk of bias assessment (n=2).
| Cohort studies | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| First author and year | Selection | Comparability | Outcome | Total | ||||||
| Exposed | Non-exposed | Ascertainment | Outcome | Multiple pregnancy | History of PTB | Assessment | Follow-up | Lost | ||
| Azargoon, 2006 | * | * | * | * | * | * | * | 7 | ||
| Bartnicki, 1996 | * | * | * | * | * | 5 | ||||
| Bitzer, 2011 | * | * | * | * | * | * | 6 | |||
| Chang, 1997 | * | * | * | * | * | * | 6 | |||
| DeFalco, 2003 | * | * | * | * | * | * | * | 7 | ||
| Faber, 1997 | * | * | * | * | * | 5 | ||||
| Figueroa, 2011 | * | * | * | * | * | * | * | 7 | ||
| Foroozanfard, 2015 | * | * | * | * | * | * | * | 7 | ||
| Garay, 2011 | * | * | * | * | * | * | 6 | |||
| Gleeson, 1989 | * | * | * | * | * | * | * | * | 8 | |
| Hantoushzadeh, 2014 | * | * | * | * | * | * | * | 7 | ||
| Hauth, 2003 | * | * | * | * | * | * | * | * | 8 | |
| Hengst, 1992 | * | * | * | * | 4 | |||||
| Hillier, 1995 | * | * | * | * | * | * | * | * | 8 | |
| Hoyme, 2000 | * | * | * | * | 4 | |||||
| Hoyme, 2010 | * | * | * | * | 4 | |||||
| Jazayeri, 2002 | * | * | * | * | * | * | * | 7 | ||
| Krauss-Silva, 2014 | * | * | * | * | * | * | * | 7 | ||
| Masood, 2013 | * | * | * | * | * | * | * | * | 8 | |
| Matijevic, 2010 | * | * | * | * | * | * | * | * | * | 9 |
| Minkoff, 1987 | * | * | * | * | * | * | * | 7 | ||
| Onderdonk, 2003 | * | * | * | * | * | * | * | * | * | 9 |
| Paternoster, 2002 | * | * | * | * | * | * | * | 7 | ||
| Saling, 1999 | * | * | * | 3 | ||||||
| Schwab, 2015 | * | * | * | * | * | * | 6 | |||
| Sendag, 2010 | * | * | * | * | * | * | * | * | 8 | |
| Simhan, 2003 | * | * | * | * | * | * | 6 | |||
| Case-control study | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| First author and year | Selection | Comparability | Outcome | Total | ||||||
| Case definition | Case selection | Controls definition | Controls selection | Multiple pregnancy | History of PTB | Ascertainment | Comparability | Lost | ||
| Cauci, 2005 | * | * | * | * | * | * | 6 | |||
| RCTs | |||||||
|---|---|---|---|---|---|---|---|
| Sequence generation | Allocation concealment | Blinding for participants and personnel for preterm birth | Blinding for outcome assessors for preterm birth | Incomplete outcome data for preterm birth | Selective outcome reporting | Other sources of bias | |
| Gjedingen, 2000 |  |  |  |  |  |  |  |
| Sungkar, 2012 |  |  |  |  |  |  |  |
Synthesis of results
Fifteen studies provided sufficient information for the calculation of RDs and 95% CIs (Figures 2 and 3). The majority of the 15 non-randomised studies provided evidence of smaller risk for PTB in the group with normal pH (Figure 2). Overall, the studies provided large effects (RD range 0.02–0.75). In the two studies which defined pathological pH as >4.2 the difference in the PTB risk appears particularly large (RD 0.30, 95% CI 0.13–0.46 [33] and RD 0.50, 95% CI 0.37–0.63 [23]). The study by Matijevic et al. (2010) appears as an outlier, with an RD of 0.75 (95% CI 0.53–0.96) [55]. Although this study was assessed as having a low risk of bias (NOS score of 9), it has a particularly imbalanced group size [n (case)=14 vs. n (control)=302]. Four studies failed to provide strong evidence in favour of normal vaginal pH as the interval lines intersect the vertical line of null effect [13], [31], [32], [37]. Only the study by Garay et al. (2011) supports a larger risk for PTB in the group with normal pH (RD −0.08, 95% CI −0.15, −0.01); however, the study has a substantial risk of bias (NOS score of 6) [30].
In the funnel plot, there is an apparent asymmetrical appearance of the studies with a gap in the bottom left corner of the graph that refers to higher risk for PTB in the group with normal pH (Figure 3). This is an indication of a possible small-study effect where small-to-moderate sized studies tend to provide larger effects. In addition, half of the studies are plotted outside the pseudo 95% confidence limits which might be an indication of heterogeneity and possible bias in the study results. At the left side of the funnel plot and at the far right side of the plot, we can distinguish the studies by Garay et al. (2011) (blue plus), Bartnicki et al. (1996) and Masood et al. (2013) (both blue dots) and Matijevic et al. (2010) (red cross), which appear as outliers compared to the rest of the studies [23], [30], [33], [55].
Discussion
Main findings
This is the first systematic review investigating the association between an increased antenatal vaginal pH >4.5 and a PTB rate <37 completed weeks gestation, reporting 30 mainly observational studies including 251.014 women in total. Of the 30 included studies, 15 were presented in a forest plot, including 19.220 women in total. This systematic review shows evidence that an increased antenatal vaginal pH, with the most common definition being >4.5, might be associated with an increased PTB rate.
Other reviews in the field have indicated an association between PTB and infections in general, but did not specifically review the association between antenatal vaginal pH and PTB [7]. A Cochrane review investigating the effectiveness of antenatal screening for genital tract infections for the prevention of PTB presented evidence of moderate quality to support screening for particular pathogens, but the review was based on only one study that did not include pH screening [57], [58]. Therefore, a carefully planned RCT is still required to answer the question, whether a screening programme of the antenatal vaginal pH can help reduce the overall PTB rate by early detection and treatment of infections. Such an RCT should randomise women with and without PTB risk factors to receive in a blind manner either usual antenatal care or antenatal care supplemented by regular pH testing. The present systematic review provides the underlying evidence that the antenatal vaginal pH and the overall PTB rate might be associated.
Strengths and limitations
This systematic review investigated a topic of great importance for care providers and researchers in the field of PTB prevention. One particular strength of this systematic review is the comprehensive search of both academic and grey literature, which was inclusive of all languages, included German and English keywords and identified studies in a range of countries.
Limitations of the present systematic review should be acknowledged. First, the included studies presented a large diversity in the assessment of the vaginal pH, including heterogenous definitions for a pathological pH. Together with a lack of information about the intervals of the measurement method, this impedes accurate comparisons of women with pH ≤4.5 and >4.5. Second, statistical heterogeneity, as manifested by a variation of particularly small and large effects (the latter being a particular characteristic of cohort studies), is eminent across the studies and it may be attributed partially to the weak methodological design of some of the included studies (a high proportion of studies was judged for having a moderate or high risk of bias). Third, in about half of the included studies the quality of reporting precluded the extraction of information on important effect-modifiers, which in turn prevented an in-depth assessment of the statistical heterogeneity in the context of a random-effects model. At the systematic review level, limitations arose from the incomplete retrieval of identified research in spite of extensive effort. Finally, we did not explain the patho-physiological mechanisms of how vaginal pH and PTBs are connected as there are few and partly conflicting theories on this topic and therefore, we decided not to present this debate within our systematic review.
Despite these limitations, this systematic review provides valuable information about relevant studies in the field, which can assist other researchers to reflect on the evidence about the association of antenatal vaginal pH and PTB.
Interpretation
Most women progress through pregnancy with a physiological pH of ≤4.4 but a considerable number of women may present increased vaginal pH values ≥4.7 at multiple occasions (33%) or consistently throughout pregnancy (2.3%) [59]. The acid vaginal pH is largely dependent on the physiological presence of lactobacilli, which produce lactic acid and inhibit the adhesion and spread of pathogens [24], [60]. If an imbalance in the vaginal flora occurs, the number of lactobacilli declines and is replaced by pathological microorganisms. Causes for an increase of the vaginal pH are diverse. They include the invasion or proliferation of pathogens, such as trichomonas vaginalis, mycoplasma hominis or fungal infections [32], [61]. BV is a more general disequilibrium of the vaginal milieu, defined as a vaginal pH >4.5 and characteristic vaginal discharge and odour. BV can be caused by a large number of different bacteria [62]. A Cochrane review found that antibiotic treatment can effectively eradicate BV in pregnancy but did not increase the rate of term births [15]. An increase of the vaginal pH is not necessarily associated with pathological processes and may depend on the ethnicity and individual life style [63], [64], [65].
Numerous risk factors which predispose for a vaginal pH >4.5 are simultaneously independent risk factors for PTB, including, but not limited to, low socio-economic status, minority ethnicity and stress [5], [66], [67]. However, the association between increased vaginal pH and PTB can be largely explained by the mechanism of infection, which links the two variables. It has been established that up to 40% of all PTB are linked to genital tract infections [5], [6], [7]. The exact underlying mechanism and the role of specific pathogens remain largely unclear, but explanatory concepts include decidual activation and the trigger of the foetal immune response [6], [7].
In the studies included in this systematic review, the antenatal vaginal pH was frequently measured only once in the first or early second trimester and it might appear surprising that an increased vaginal pH at such an early stage affects the pregnancy outcome. A possible explanation for this is that pathogens might be already present at conception or very early in pregnancy, but do not cause early labour until the expanding membranes of the embryo seal the endometric cavity [6], [7]. On the other hand, it was argued that the foetal immune response might only be mature enough to contribute to the initiation of labour at around 20 weeks gestation [7]. Overall, there is lack of evidence not only about the mechanism of preterm labour and birth in the presence of infection, but also about the physiological transformation from quiescence of the uterus throughout pregnancy to activation for labour in general [68].
The aim of this systematic review was to explore antenatal vaginal pH as one of the risk factors associated with PTB. This factor was chosen particularly due to the wide implementation of a screening programme in Germany, which encourages women to self-evaluate their vaginal pH regularly in pregnancy, particularly, if their history suggests an increased risk for PTB. The screening programme continues to be supported by health insurance companies and health professionals despite a lack of rigorous evidence for its effectiveness. Although initial studies claimed a possible reduction of overall PTB rates from 7.7 to 6.8% in Thuringia, a later evaluation of the model project failed to support efficiency of the screening programme [17], [19].
The authors are concerned that there is a lack of evidence about potential risks of this screening method, including overtreatment of a potentially physiological process, overuse of antibiotics and unnecessary introduction of uncertainty and fear for women and families. A national screening programme without a positive risk-benefit analysis might suggest to women that their bodies are inherently insufficient and without medical help and constant control would not be able to carry a healthy pregnancy to term [69]. On the other hand, if a screening of the antenatal vaginal pH proves effective for the reduction of PTB rates, then good quality evidence has to be produced to allow other countries to implement this strategy as part of their national guidelines.
Conclusion
This systematic review provides a systematic summary of studies on a topic with high significance to care providers and researchers in the field of PTB prevention. The systematic review indicates that an increased antenatal vaginal pH >4.5 may be associated with an increased risk for PTB. The authors highlight the need for a high-quality prospective trial designed to evaluate the effectiveness and clinical significance of an antenatal pH screening programme.
Acknowledgments
We would like to express our gratitude to the following people, who supported this work in various ways: Prof. Declan Devane (manuscript review and advice on methods and analysis); Dr. Sebastian Voigt-Radloff (manuscript review and advice on analysis and presentation); Nantje Ruescher (screening of references); Anne Kasper (risk of bias assessment of RCTs); Yoana Stancheva (assessment of Bulgarian language article); Erika Sitter (preparation of figures); Marie-Clare Balaam (manuscript review).
Author contributions: All authors contributed important intellectual content to the article. MG, SG and MW conceived and designed this systematic review. The search strategy was developed by MW and reviewed by MG and SG. MW conducted the search and screening was performed by MW, MG and SG. Risk of bias assessment was carried out by MW, SG and MG. Data was extracted by MW and reviewed for correctness by LS. Author requests for missing data were carried out by MW and MG. LS and MW performed the data analysis. The manuscript was written by MW and LS contributed to the results section. All authors reviewed and edited the manuscript and approved the final version of the article for publication. All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
Research funding: None declared.
Employment or leadership: None declared.
Honorarium: None declared.
Competing interests: The funding organization(s) played no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the report for publication.
References
1. Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller AB, Narwal R, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet 2012;379:2162–72.10.1097/01.aoa.0000432360.25014.c3Suche in Google Scholar
2. Saigal S, Doyle LW. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet 2008;371:261–9.10.1016/S0140-6736(08)60136-1Suche in Google Scholar
3. Lackritz EM, Wilson CB, Guttmacher AE, Howse JL, Engmann CM, Rubens CE, et al. A solution pathway for preterm birth: accelerating a priority research agenda. Lancet Glob Health 2013;1:e328–30.10.1016/S2214-109X(13)70120-7Suche in Google Scholar
4. Delnord M, Blondel B, Zeitlin J. What contributes to disparities in the preterm birth rate in European countries? Curr Opin Obstet Gynecol 2015;27:133–42.10.1097/GCO.0000000000000156Suche in Google Scholar
5. Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet 2008;371:75–84.10.1016/S0140-6736(08)60074-4Suche in Google Scholar
6. Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N Engl J Med 2000;342:1500–7.10.1056/NEJM200005183422007Suche in Google Scholar PubMed
7. Nadeau HC, Subramaniam A, Andrews WW. Infection and preterm birth. Semin Fetal Neonatal Med 2016;21:100–5.10.1016/j.siny.2015.12.008Suche in Google Scholar PubMed
8. Saling E, Schreiber M. [The lactobacilli-protection system of pregnant women – Efficient prevention of premature births by early detection of disturbances]. Z Geburtshilfe Neonatol 2005;209:128–34. German.10.1055/s-2005-871305Suche in Google Scholar PubMed
9. Hoyme UB, Huebner J. Prevention of preterm birth is possible by vaginal pH screening, early diagnosis of bacterial vaginosis or abnormal vaginal flora and treatment. Gynecol Obstet Invest 2010;70:286–90.10.1159/000314019Suche in Google Scholar PubMed
10. Hantoushzadeh S, Sheikh M, Javadian P, Shariat M, Amini E, Abdollahi A, et al. Elevated vaginal pH in the absence of current vaginal infection, still a challenging obstetrical problem. J Matern Fetal Neonatal Med 2014;27:582–7.10.3109/14767058.2013.823394Suche in Google Scholar PubMed
11. Foxman B, Wen A, Srinivasan U, Goldberg D, Marrs CF, Owen J, et al. Mycoplasma, bacterial vaginosis-associated bacteria BVAB3, race, and risk of preterm birth in a high-risk cohort. Obstet Gynecol 2014;210:226.e1.10.1016/j.ajog.2013.10.003Suche in Google Scholar PubMed PubMed Central
12. Jakovljević A, Bogavac M, Nikolić A, Milošević Tošic M, Novakovicć Z, Stajić Z. The influence of bacterial vaginosis on gestational week of the completion of delivery and biochemical markers of inflammation in the serum. Vojnosanit Pregl 2014;71:931–5.10.2298/VSP1410931JSuche in Google Scholar
13. Krauss-Silva L, Almada-Horta A, Alves MB, Camacho KG, Moreira MEL, Braga A. Basic vaginal pH, bacterial vaginosis and aerobic vaginitis: prevalence in early pregnancy and risk of spontaneous preterm delivery, a prospective study in a low socioeconomic and multiethnic South American population. BMC Pregnany Childbirth 2014;14:107.10.1186/1471-2393-14-107Suche in Google Scholar PubMed PubMed Central
14. Nelson DB, Hanlon A, Nachamkin I, Haggerty C, Mastrogiannis DS, Liu C, et al. Early pregnancy changes in bacterial vaginosis-associated bacteria and preterm delivery. Paediatr Perinat Epidemiol 2014;28:88–96.10.1111/ppe.12106Suche in Google Scholar PubMed PubMed Central
15. Brocklehurst P, Gordon A, Heatley E, Milan SJ. Antibiotics for treating bacterial vaginosis in pregnancy. Cochrane Database Syst Rev 2013;CD000262. doi: 10.1002/14651858.CD000262.pub4.10.1002/14651858.CD000262.pub4Suche in Google Scholar PubMed
16. Saling E, Schreiber M, Al-Taie T. A simple, efficient and inexpensive program for preventing prematurity. J Perinat Med 2001;29:199–211.10.1515/JPM.2001.029Suche in Google Scholar PubMed
17. Hoyme UB, Möller U, Saling E. [Results and potential consequences of the thuringia prematurity preventional campaign 2000]. Geburtshilfe Frauenheilkd 2002;62:257–63. German.10.1055/s-2002-25221Suche in Google Scholar
18. Siegmund-Schultze E, Hoyme UB, Bitzer E, Wenzlaff P. [pH self assessment to reduce the risk of preterm birth – a pilot study becomes a model project]. Geburtshilfe Frauenheilkd 2005;65:80–3. German.10.1055/s-2004-830486Suche in Google Scholar
19. Bitzer EM, Schneider A, Wenzlaff P, Hoyme UB, Siegmund-Schultze E. Self-testing of vaginal pH to prevent preterm delivery: a controlled trial. Dtsch Arztebl 2011;108:81–6.Suche in Google Scholar
20. Chang JC, Hsu TY, Hsieh CH, Hsu YR, Tai MC, Chen LF. Vaginal and cervical pH measurements in normal pregnancy and preterm labor. J Matern Fetal Investig 1997;7:193–6.Suche in Google Scholar
21. Foroozanfard F, Tabasi Z, Mesdaghinia E, Sehat M, Mahdian M. Cervical length versus vaginal PH in the second trimester as preterm birth predictor. Pak J Med Sci 2015;31:374–8.10.12669/pjms.312.6310Suche in Google Scholar PubMed PubMed Central
22. Hengst P, Uhlig B, Bollmann R, Kokott T. Usefulness of vaginal pH determination for preventing premature births. Z Geburtshilfe Perinatol 1992;196:238–41.Suche in Google Scholar
23. Masood H, Ashraf S, Siddique H, Masood M, Masood MS. Role of PH and leukocyte count in prediction of preterm labour. Med Forum Monthly 2013;24:2–4.Suche in Google Scholar
24. Onderdonk AB, Lee M-L, Lieberman E, Delaney ML, Tuomala RE. Quantitative microbiologic models for preterm delivery. J Clin Microbiol 2003;41:1073–9.10.1128/JCM.41.3.1073-1079.2003Suche in Google Scholar PubMed PubMed Central
25. Paternoster DM, Stella A, Gerace P, Manganelli F, Plebani M, Snijders D, et al. Biochemical markers for the prediction of spontaneous pre-term birth. Int J Gynecol Obstet 2002;79:123–9.10.1016/S0020-7292(02)00243-6Suche in Google Scholar
26. Hillier SL, Nugent RP, Eschenbach DA, Krohn MA, Gibbs RS, Martin DH, et al. Association between bacterial vaginosis and preterm delivery of a low-birth-weight infant. N Engl J Med 1995;333:1737–42.10.1056/NEJM199512283332604Suche in Google Scholar
27. Schwab FD, Zettler EK, Moh A, Schotzau A, Gross U, Gunthert AR. Predictive factors for preterm delivery under rural conditions in post-tsunami Banda Aceh. J Perinat Med 2016;44:511–5.10.1515/jpm-2015-0004Suche in Google Scholar
28. Cauci S, McGregor J, Thorsen P, Grove J, Guaschino S. Combination of vaginal pH with vaginal sialidase and prolidase activities for prediction of low birth weight and preterm birth. Am J Obstet Gynecol 2005;192:489–96.10.1016/j.ajog.2004.07.023Suche in Google Scholar
29. Hauth JC, MacPherson C, Carey JC, Klebanoff MA, Hillier SL, Ernest JM, et al. Early pregnancy threshold vaginal pH and Gram stain scores predictive of subsequent preterm birth in asymptomatic women. Am J Obstet Gynecol 2003;188:831–5.10.1067/mob.2003.184Suche in Google Scholar
30. Garay G, Fraca M, Martínez I, da Silva A, López-Valverde M, Esteban V, et al. Utility of vaginal pH determination in the diagnosis of vulvovaginitis and its association with obstetric pathology. Prog Obstet Ginecol 2011;54:568–74.10.1016/j.pog.2011.07.001Suche in Google Scholar
31. Figueroa D, Mancuso MS, Szychowski JM, Paden MM, Owen J. Does midtrimester Nugent score or high vaginal pH predict gestational age at delivery in women at risk for recurrent preterm birth? Am J Obstet Gynecol 2011;204:46.e1–4.10.1016/j.ajog.2010.08.029Suche in Google Scholar
32. Minkoff H, Grunebaum A, Feldman J, Cummings M, McCormack WM. Relationship of vaginal pH and papanicolaou smear results to vaginal flora and pregnancy outcome. Int J Gynecol Obstet 1987;25:17–23.10.1016/0020-7292(87)90179-2Suche in Google Scholar
33. Bartnicki J, Casal D, Kreaden US, Saling E, Vetter K. Fetal fibronectin in vaginal specimens predicts preterm delivery and very-low-birth-weight infants. Am J Obstet Gynecol 1996;174:971–4.10.1016/S0002-9378(96)70335-6Suche in Google Scholar
34. Faber R, Stepan H, Springer C, Viehweg B. [Assessment of the clinical relevance of the vaginal flora for preterm birth with discriminance analysis]. Zentralbl Gynakol 1997;119(Suppl 1): 28–32. German.Suche in Google Scholar
35. Sungkar A, Purwosunu Y, Aziz MF, Pratomo H, Sutrisna B, Sekizawa A. Influence of early self-diagnosis and treatment of bacterial vaginosis on preterm birth rate. Int J Gynecol Obstet 2012;117:264–7.10.1016/j.ijgo.2012.01.007Suche in Google Scholar PubMed
36. Saling E, Al-Taie T, Luethje J. [Prematurity-prevention program. Cooperation between the doctor, midwife and patient]. Gynakologe 1999;32:39–45. German.10.1007/PL00003171Suche in Google Scholar
37. Hoyme UB, Grosch A, Roemer VM, Saling E. [Bacterial vaginosis as risk factor]. Gynakologe 2000;33:331–5. German.10.1007/s001290050557Suche in Google Scholar
38. Simhan HN, Caritis SN, Krohn MA, Hillier SL. Elevated vaginal pH and neutrophils are associated strongly with early spontaneous preterm birth. Am J Obstet Gynecol 2003;189:1150–4.10.1067/S0002-9378(03)00582-9Suche in Google Scholar
39. Gleeson RP, Elder AM, Turner MJ, Rutherford AJ, Elder MG. Vaginal pH in pregnancy in women delivered at and before term. Br J Obstet Gynaecol 1989;96:183–7.10.1111/j.1471-0528.1989.tb01659.xSuche in Google Scholar
40. Gjerdingen D, Fontaine P, Bixby M, Santilli J, Welsh J. The impact of regular vaginal pH screening on the diagnosis of bacterial vaginosis in pregnancy. J Fam Pract 2000;49:39–43.Suche in Google Scholar
41. Azargoon A, Darvishzadeh S. Association of bacterial vaginosis, trichomonas vaginalis, and vaginal acidity with outcome of pregnancy. Arch Iran Med 2006;9:213–7.Suche in Google Scholar
42. De Falco CL, Volpicelli T, Battista L, D’Angiolella ML, D’Ettore A, Vitelli A, et al. Variations of vaginals pH in pregnancy: clinical applications. G Ital Ostet Ginecol 2003;25:33–6.Suche in Google Scholar
43. Weckend MJ, Grylka-Baeschlin S, Ruescher N, Spineli L, Gross MM. Association between increased versus normal antenatal vaginal pH and preterm birth: protocol for a systematic review. PROSPERO 2016;CRD42016042377. http://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42016042377.Suche in Google Scholar
44. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 2009;6:e1000100.10.1371/journal.pmed.1000100Suche in Google Scholar
45. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Reprint – preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Phys Ther 2009;89:873–80.10.1093/ptj/89.9.873Suche in Google Scholar
46. Schardt C, Adams MB, Owens T, Keitz S, Fontelo P. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med Inform Decis Mak 2007;7:16.10.1186/1472-6947-7-16Suche in Google Scholar
47. Higgins JPT, Altman DG, Sterne JAC, editors. Chapter 8: assessing risk of bias in included studies. In: Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from: www.handbook.cochrane.org.Suche in Google Scholar
48. Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. [Internet]. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. [cited 2017 Apr 7] Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm.Suche in Google Scholar
49. Reeves BC, Deeks JJ, Higgins JPT, Wells GA. Chapter 13: Including non-randomized studies. In: Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from: www.handbook.cochrane.org.Suche in Google Scholar
50. Amsel R, Totten PA, Spiegel CA, Chen KC, Eschenbach D, Holmes KK. Nonspecific vaginitis. Diagnostic criteria and microbial and epidemiologic associations. Am J Med 1983;74:14–22.10.1016/0002-9343(83)91112-9Suche in Google Scholar
51. Linhares IM, Summers PR, Larsen B, Giraldo PC, Witkin SS. Contemporary perspectives on vaginal pH and lactobacilli. Am J Obstet Gynecol 2011;204:120.e1–5.10.1016/j.ajog.2010.07.010Suche in Google Scholar PubMed
52. Anzures-Cabrera J, Higgins JPT. Graphical displays for meta-analysis: an overview with suggestions for practice. Res Synth Methods 2010;1:66–80.10.1002/jrsm.6Suche in Google Scholar PubMed
53. Ioannidis JP, Patsopoulos NA, Rothstein HR. Reasons or excuses for avoiding meta-analysis in forest plots. Br Med J 2008;336:1413–5.10.1136/bmj.a117Suche in Google Scholar PubMed PubMed Central
54. Jazayeri A, Arnold H, Jazayeri MK, Spellacy WN. A prospective study of vaginal pH as a predictor of preterm delivery. J Matern Fetal Neonatal Med 2002;11:30–3.10.1080/jmf.11.1.30.33Suche in Google Scholar PubMed
55. Matijevic R, Grgic O, Knezevic M. Vaginal pH versus cervical length in the mid-trimester as screening predictors of preterm labor in a low-risk population. Int J Gynaecol Obstet 2010;111:41–4.10.1016/j.ijgo.2010.05.011Suche in Google Scholar PubMed
56. Sendag F, Kazandi M, Akercan F, Kazandi AC, Karadadas N, Sagol S. Vaginal fluid pH, cervicovaginitis and cervical length in pregnancy. Clin Exp Obstet Gynecol 2010;37:127–30.Suche in Google Scholar
57. Sangkomkamhang US, Lumbiganon P, Prasertcharoensuk W, Laopaiboon M. Antenatal lower genital tract infection screening and treatment programs for preventing preterm delivery. Cochrane Database Syst Rev 2015;2:CD006178.10.1002/14651858.CD006178.pub2Suche in Google Scholar PubMed
58. Kiss H, Petricevic L, Husslein P. Prospective randomised controlled trial of an infection screening programme to reduce the rate of preterm delivery. Br Med J 2004;329:371.10.1136/bmj.38169.519653.EBSuche in Google Scholar PubMed PubMed Central
59. Saling E, Fuhr N, Placht A, Schumacher E. Erste Ergebnisse der „Selbst-Vorsorge-Aktion von Schwangeren“ zur Frühgeburtenvermeidung. Arch Gynecol Obstet 1995;257: 178–85. German.10.1007/BF02264816Suche in Google Scholar PubMed
60. Saling E, Schreiber M. Most efficient screening for prevention of premature birth. Gynaecol Perinatol 2003;12(Suppl 1):105–13.Suche in Google Scholar
61. Hoyme UB. [Diagnosis of bacterial genital infection in pregnancy]. Gynakol Prax 2013;37:239–51. German.Suche in Google Scholar
62. Africa CWJ, Nel J, Stemmet M. Anaerobes and bacterial vaginosis in pregnancy: virulence factors contributing to vaginal colonisation. Int J Environ Res Public Health 2014;11:6979–7000.10.3390/ijerph110706979Suche in Google Scholar PubMed PubMed Central
63. Fiscella K, Klebanoff MA. Are racial differences in vaginal pH explained by vaginal flora? Am J Obstet Gynecol 2004;191:747–50.10.1016/j.ajog.2004.03.032Suche in Google Scholar PubMed
64. Stevens-Simon C, Jamison J, Mcgregor JA, Douglas JM. Racial variation in vaginal pH among healthy sexually active adolescents. Sex Transm Dis 1994;21:168–72.10.1097/00007435-199405000-00007Suche in Google Scholar PubMed
65. Nicole W. A question for women’s health: chemicals in feminine hygiene products and personal Lubricants. Environ Health Perspect 2014;122:A71–5.10.1289/ehp.122-A70Suche in Google Scholar PubMed PubMed Central
66. Wen A, Srinivasan U, Goldberg D, Owen J, Marrs C, Misra D, et al. Selected vaginal bacteria and risk of preterm birth: an ecological perspective. J Infect Dis 2014;209:1087–94.10.1093/infdis/jit632Suche in Google Scholar
67. Witkin SS, Ledger WJ. Complexities of the uniquely human vagina. Sci Transl Med 2012;4:132fs11.10.1126/scitranslmed.3003944Suche in Google Scholar
68. Rubens CE, Sadovsky Y, Muglia L, Gravett MG, Lackritz E, Gravett C. Prevention of preterm birth: harnessing science to address the global epidemic. Sci Transl Med 2014;6:262sr5.10.1126/scitranslmed.3009871Suche in Google Scholar
69. Miller S, Abalos E, Chamillard M, Ciapponi A, Colaci D, Comande D, et al. Beyond too little, too late and too much, too soon: a pathway towards evidence-based, respectful maternity care worldwide. Lancet 2016;388:2176–92.10.1016/S0140-6736(16)31472-6Suche in Google Scholar
Correction statement
Correction added after ahead-of-print publication on 8 September 2018: The link in reference [43] was updated.
©2019 Walter de Gruyter GmbH, Berlin/Boston
Artikel in diesem Heft
- Frontmatter
- Editorial
- Ultrasound Doppler waveform assessment: the story continues
- Review
- Association between increased antenatal vaginal pH and preterm birth rate: a systematic review
- Mini Review
- Update on uterine tachysystole
- Research Articles – Obstetrics
- First trimester prediction of gestational diabetes mellitus using plasma biomarkers: a case-control study
- Emergency peripartal hysterectomy – a single-center analysis of the last 13 years at a tertiary perinatal care unit
- Efficacy and safety of misoprostol vaginal insert vs. oral misoprostol for induction of labor
- Vitamin A and β-carotene in pregnant and breastfeeding post-bariatric women in an urban population
- Effect of dual tocolysis with fenoterol and atosiban in human myometrium
- Antecedents of red cell transfusion in a large contemporary obstetric cohort
- Effect of n-3 long-chain polyunsaturated fatty acids supplementation in healthy mothers on DHA and EPA profiles in maternal and umbilical blood: a randomized controlled trial
- Research Articles – Fetus
- Effect of psychotropic drugs on fetal behavior in the third trimester of pregnancy
- Prognostic value of the aortic isthmus Doppler assessment on late onset fetal growth restriction
- Doppler evaluation of the fetal pulmonary artery pressure
- Mechanisms of death in structurally normal stillbirths
- The diagnostic value of a detailed first trimester anomaly scan in fetuses with increased nuchal translucency thickness
- Research Articles – Newborn
- Small for gestational age and extremely low birth weight infant outcomes
- Does heart rate variability improve prediction of failed extubation in preterm infants?
Artikel in diesem Heft
- Frontmatter
- Editorial
- Ultrasound Doppler waveform assessment: the story continues
- Review
- Association between increased antenatal vaginal pH and preterm birth rate: a systematic review
- Mini Review
- Update on uterine tachysystole
- Research Articles – Obstetrics
- First trimester prediction of gestational diabetes mellitus using plasma biomarkers: a case-control study
- Emergency peripartal hysterectomy – a single-center analysis of the last 13 years at a tertiary perinatal care unit
- Efficacy and safety of misoprostol vaginal insert vs. oral misoprostol for induction of labor
- Vitamin A and β-carotene in pregnant and breastfeeding post-bariatric women in an urban population
- Effect of dual tocolysis with fenoterol and atosiban in human myometrium
- Antecedents of red cell transfusion in a large contemporary obstetric cohort
- Effect of n-3 long-chain polyunsaturated fatty acids supplementation in healthy mothers on DHA and EPA profiles in maternal and umbilical blood: a randomized controlled trial
- Research Articles – Fetus
- Effect of psychotropic drugs on fetal behavior in the third trimester of pregnancy
- Prognostic value of the aortic isthmus Doppler assessment on late onset fetal growth restriction
- Doppler evaluation of the fetal pulmonary artery pressure
- Mechanisms of death in structurally normal stillbirths
- The diagnostic value of a detailed first trimester anomaly scan in fetuses with increased nuchal translucency thickness
- Research Articles – Newborn
- Small for gestational age and extremely low birth weight infant outcomes
- Does heart rate variability improve prediction of failed extubation in preterm infants?


