Abstract
Objectives
Congenital hypothyroidism (CHT) is a treatable cause of intellectual disability. Late diagnosis and delayed initiation of treatment leads to irreversible neurodevelopmental and intellectual disability. Thus, newborn screening is crucial. However, 71 % of babies are born in an area with no established newborn screening program and Kenya is not an exception. We aimed to determine the incidence of CHT, developmental outcomes of patients in the absence of newborn screening.
Methods
A retrospective data of subjects who met the inclusion criteria, newborn and infants from 3 days to 2 years whose thyroid function test (TFT) was undertaken during well baby visit or clinical suspicion of CHT were collected. Laboratory reference range for age was used to interpret the result and TSH>10 Uiu/mL after 6 weeks of life is considered abnormal according to ESPE guideline. Developmental outcome of children was collected from patient file documented by primary physician and parental concern.
Results
Of 1,426 children met inclusion criteria, 90 had elevated TSH. Out of which 70 repeat TFT showed normal TSH and free T4. The incidence of abnormal TSH across the different age groups was 2.4 , 7.2 and 10.5 % for ages 0–29 days, 1–11 months, and 1–2 years, respectively with p-value of =0.0002. While 20 cases with CHT identified with incidence of 14 per 1,000 children (1.4 %; 95 % CI: 0.9–2.1 %). Out this 12 (60 %) had poor developmental outcomes. Down’s syndrome was the common associated condition 9/20 (45 %). All cases were primary CHT.
Conclusions
This study shows high incidence of CHT in a small cohort of patients over 5-year period with poor development outcome.
Introduction
Congenital hypothyroidism (CHT) is the common congenital endocrine disorder defined as thyroid hormone deficiency present at birth and is common cause of preventable intellectual disability [1]. Early detection and treatment of CHT through neonatal screening prevents irreversible neurodevelopmental delay and optimizes developmental outcomes.
Despite the existence of newborn screening for CHT over five decades in developed countries, approximately 71 % of babies worldwide are born in an area with unestablished newborn screening program [2]. As a result, most babies with CHT worldwide are not detected and treated early, leading to a significant economic burden of intellectual disability due to untreated CHT, which remains a major public health challenge in developing countries.
A majority (95 %) of patients with CHT are asymptomatic at birth due to some residual thyroid function. In addition, partial transplacental passage of maternal thyroid hormone offers temporary protection especially for the first days of life [3]. Only 5 % of newborns with severe CHT present with clinical signs and symptoms soon after birth leading to late diagnosis, where the critical time to salvage the brain has lapsed. This makes newborn screening a crucial way for an early diagnosis [2].
A child presenting with CHT lasting 4–6 weeks of life may present with poor feeding, constipation, lethargy or excessive sleeping and hoarse cry. The physical examination may reveal failure to thrive, large anterior and posterior fontanels, dry skin, prolonged jaundice, mottling, umbilical hernia, macroglossia and coarsening of the face [4]. Untreated CHT results in neurological and developmental deficits, including spasticity, disturbances of gait and coordination, and intellectual disability. CHT is a major cause of preventable mental retardation if not detected soon after birth [5].
CHT can occur due to insufficient thyroid gland development (dysgenesis) or an inborn error in thyroid hormone biosynthesis (dyshormonogenesis) or transport defect [4]. It is classified as permanent, requiring lifelong treatment or transient, requiring treatment just for a defined period of time, most commonly resolving within the first three years of life [6]. Transient CHT can be caused by secondary factors such as iodine deficiency or excess, transplacental passage of maternal antibodies or drugs, prematurity, non-thyroidal disease, neonatal use of dopamine and some others [7].
Globally, the incidence of congenital hypothyroidism (CHT) varies between 1:2,000 and 1:4,000 live births [8]. Most recent studies have reported increase in the incidence of CHT due to increased diagnosis of cases with normally located gland in situ CHT [9]. The highest incidence is being reported from various locations in the Middle East due to high rate of consanguinity with ever highest observed in Iran 1:314 [10]. However, there are no published CHT incidence data in Africa. This lack of data could be attributed to the region’s low or no screening rates and lack of full government support for newborn screening due to competing health priorities [11]. In the late 1970s, there were several pilot studies for congenital hypothyroidism in South Africa. Despite successful pilot studies indicating cost effectiveness, little progress is achieved to date [12]. Previously, attempts to incorporate CHT in newborn screening with the sickle cell screening program in Nigeria failed due to a lack of political commitment and funding challenges [13]. Conversely, a one-year cross sectional multi-center study in Addis Ababa, Ethiopia, found a 3.6 % prevalence of transient CHT and none for permanent type among 4,206 consecutive newborn subjects [14].
The global challenge of newborn screening persists due to insufficient support in programming, finance, and politics. Hence, determining the incidence of CHT and development outcome in the absence of newborn screening could significantly impact the integration of CHT screening into newborn routine care.
Subjects and methods
The study employed retrospective descriptive study design at Gertrude’s Children’s Hospital (GCH) sought to determine incidence and developmental outcomes of children with CHT. GCH is a not-for-profit hospital located in Nairobi, Kenya. The hospital attends an average of 300,000 outpatients and 9,000 inpatients paediatric patients annually at its 100-bed facility with an extensive outpatient clinic.
Since there is no well-established newborn screening government supported program for CHT in Kenya, GCH has adopted a protocol for CHT screening for newborns and infants as part of their well-baby review. CHT screening is done using TSH/TSH and FT4 on all infants from 3 days of age to 2 years old. The laboratory uses enzyme-linked fluorescent immunoassay (ELFA) on the bioMérieux Vidas platform. The laboratory reference range for TSH and FT4 for gender and age group is used and with TSH>10 Uiu/mL after 6 weeks of life considered abnormal according to the ESPE guidelines.
Subjects included in the study were infants starting from 3 days of life up to 2 year whose thyroid function test was done as part of their regular check-up or clinical suspicion during a visit to GCH from year 2015 to 2020. Subjects were thyroid function test done while acutely sick were excluded from the study to avoid sick euthyroid syndrome. Since incidence of acquired hypothyroidism in neonate and children less than 3 year is very rare, all cases identified were presumed to be congenital. Anti-thyroid antibodies were not taken in any of the children diagnosed with CHT in this study.
Data for developmental outcomes as described as developmental delay, mainly speech by primary physicians, were collected in those children with CHT who had attained 3 years of age and above at the time of the study.
Statistics
After project approval from the research Ethics Review Committee of GCH Nairobi Kenya, data collection commenced.
Variables include dependent, congenital hypothyroidism and independent being age at screening, thyroid function test, gender, twin, consanguinity, and development outcome.
All data collected were entered into Microsoft Excel sheet and imported into SPSS version 25 for statistical analysis. Continuous variables were summarised using measures of central tendency with corresponding measures of variability, whereas the categorical data were summarised using frequency counts and percentages. A chi-square test was applied to compare the study group (CH type) with the gender and age group of the children. Binary logistic regression was applied to determine the magnitude of associations between the outcome of interest and explanatory variables (gender and age group). Odds ratios and corresponding 95 % confidence interval are reported with p-value <0.05 considered statistically significant.
Results
A total of 1,426 children met the inclusion criteria were included in this analysis with an estimated median age of 12 weeks (IQR: 5–43) and with 53 % being male. Of all the TSH tests performed in the study group, 98 (6.9 %) of them had abnormal TSH test results. No consanguinity and twins were identified in the study population as shown in Table 1.
Demographic characteristics of the study population (2015–2020).
| Demographic characteristics | Number | % |
|---|---|---|
| Gender | ||
| Female | 633 | 47 |
| Male | 759 | 53 |
| Age | ||
| 3–29 days | 292 | 20.42 |
| 1–11 months | 858 | 60.21 |
| 1–2 years | 276 | 19.37 |
| Family history of thyroid illness | ||
| Yes | 2 | 0.14 |
| No | 1,424 | 98.86 |
| TSH | ||
| Abnormal | 98 | 6.9 |
| Normal | 1,328 | 93.1 |
The median FT4 for ages 3–29 days was estimated to be 1.47 ng/dL (IQR: 1.23, 1.77), and that of TSH was estimated to be 2.75 Iu/mL (IQR: 1.77, 4.46). Summary measures of FT4 and TSH and the age of the children are presented in Table 2.
Summary of TSH (µIU/mL) and ft4 (ng/dL).
| Characteristics | 3–29 days, n=292 | 1–11 months, n=858 | 1–2 years n=276 |
|---|---|---|---|
| TSH, median (IQR) | 2.75 (1.774, 46) | 2.72 (1.86, 4.02) | 2.61 (1.69, 3.78) |
| FT4, median (IQR) | 1.47 (1.23, 1.77) | 1.25 (1.09, 1.43) | 1.22 (1.05, 1.47) |
The prevalence of abnormal TSH across the different age groups was 10.5 , 7.2, and 2.4 % for the children aged 1–2 years, 1–11 months, and 3–29 days, respectively. There was a significant association with the probability of finding abnormal TSH value when TFT was done between age of 1–11 months of age of the children and the test results (p=0.0002), as shown in Table 3.
Cross-tabulations of TSH result with gender and age groups.
| Variable | Abnormal n (%) | Normal n (%) | Total n | p-Value |
|---|---|---|---|---|
| Gender | ||||
| Females | 44 (6.6) | 623 (93.4) | 667 | 0.7533 |
| Males | 54 (7.1) | 705 (92.9) | 759 | |
| Age-group | ||||
| 3–29 days | 7 (2.4) | 285 (97.6) | 292 | |
| 1–11 months | 62 (7.2) | 796 (92.8) | 858 | 0.0002 |
| 1–2 years | 29 (10.5) | 247 (89.5) | 276 |
Incidence of congenital hypothyroidism in a 5-year cohort of screened participants
There were 20/1,426 (1.4 %; 95 % CI: 0.9–2.1 %) of the children diagnosed with CHT and put on replacement therapy, of whom 12 (60 %) were males. Of the children with CHT, 16 (80.0 %) were aged 1–11 months, 3 (15.0 %) were aged 1–2 years, and 1 (5 %) was aged 3–29 days. The proportion of children with developmental delay was 12 (60.0 %). Only 3 (15.0 %) had normal development and 5 (25.0 %) were lost to follow-up as shown in Table 4. Out of the 20 cases with CHT, 9 (45 %) had Down’s syndrome, while the rest had no associated major abnormality detected.
Demographic and clinical characteristics of the children with congenital hypothyroidism (n=20).
| Characteristics | Number | % |
|---|---|---|
| Gender | ||
| Females | 8 | 40 |
| Males | 12 | 60 |
| Age-group | ||
| 3–29 days | 1 | 5 |
| 1–11 months | 16 | 80 |
| 1–2 years | 3 | 15 |
| Current outcome | ||
| Development delay | 12 | 60 |
| Normal development | 3 | 15 |
| Lost-to-follow-up | 5 | 25 |
| Type of CHT | ||
| Transient | 3 | 15 |
| Permanent | 17 | 85 |
| Associated conditions | ||
| Down’s syndrome | 9 | 45 |
| No obvious associated condition | 11 | 55 |
The transient nature of congenital hypothyroidism was determined through clinical and laboratory normal thyroid profile after one month of discontinuation of treatment when the child was 3 years.
Flow chart below describes diagnosis and treatment decision of infants with abnormal TSH (Figure 1).
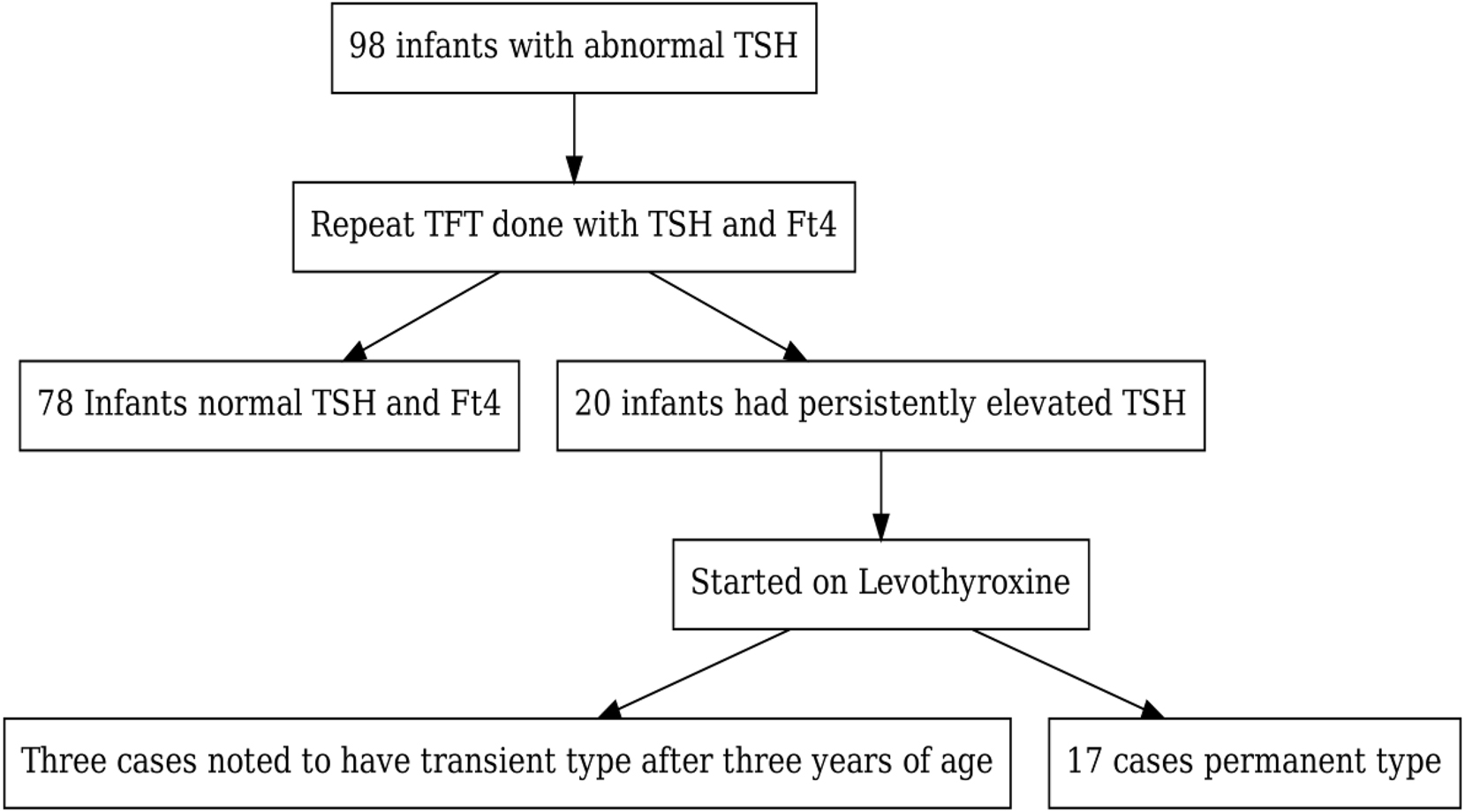
Flow chart of infants with abnormal TSH results and follow up outcomes.
Of female and male patients with CHT, 3 (37.5 %) and 9 (62.5 %) had development delays, respectively. According to the age groups, 10 (62.5 %) of children aged 1–11 months and 2 (60.0 %) of the children aged 1–2 years had developmental delays. There was, however, no significant association between the developmental outcome with gender (p-value=0.2351) and age groups (p-value=0.3302) of the children as shown in Table 5.
Demographic characteristics stratified by development outcomes.
| Characteristics | Current outcome | p-Value | ||
|---|---|---|---|---|
| Development delay n=12 (%) | Normal development n=3 (%) | Lost-to-follow-up n=5 (%) | ||
| Gender | ||||
| Females | 3 (37.5) | 2 (25.0) | 3 (37.5) | |
| Males | 9 (75.0) | 1 (8.3) | 2 (16.7) | 0.2351 |
| Age-group | ||||
| 3–29 days | 0 (0.0) | 1 (100.0) | 0 (0.0) | |
| 1–11 months | 10 (62.5) | 2 (12.5) | 4 (25.0) | 0.3302 |
| 1–2 years | 2 (66.6) | 0 (0.0) | 1 (33.3) | |
The majority (72.2 %) of CHT cases were diagnosed between 1 and 11 months, with fewer cases in other age groups. Most of these children had permanent CHT (65 %), while a smaller percentage were either lost to follow-up or had transient CHT. Regarding gender, 60 % were male and 40 % female, with more males in the permanent CHT category. The mean TSH value was 34.0 µIU/mL (±32.2) overall, higher in permanent CHT cases (44.6 ± 35.8), while the mean FT4 was 1.0 ng/dL (±0.5) overall, lower in permanent CHT cases (0.9 ± 0.5) as shown in Tables 6 and 7. The mean TSH and FT4 values for CHT patients with developmental delay and normal development are detailed in Table 7.
Mean and median TSH and Ft4 value at diagnosis.
| Type of CHT | ||||
|---|---|---|---|---|
| Characteristic | Overall, n=20 | Lost from follow up | Permanent | Transient |
| Age group, n (%) | ||||
| 0–29 days | 1 (0.0) | 0 (0.0) | 0 (0.0) | 1 (33.3) |
| 1–11 months | 16 (72.2) | 2 (66.7) | 10 (66.7) | 2 (66.6) |
| 1–2 years | 3 (27.8) | 1 (33.3) | 3 (33.3) | 0 (0.0) |
| Gender, n (%) | ||||
| Female | 8 (40.0) | 1 (25.0) | 5 (38.5) | 2 (66.7) |
| Male | 12 (60.0) | 3 (75.0) | 8 (61.5) | 1 (33.3) |
| TSH, mean (SD) | 34.0 (32.2) | 14.9 (1.3) | 44.6 (35.8) | 13.2 (8.2) |
| FT4, mean (SD) | 1.0 (0.5) | 1.5 (0.3) | 0.9 (0.5) | 1.3 (0.2) |
Characteristics of CHT with developmental outcome stratified by TSH and FT4.
| Developmental outcome | |||
|---|---|---|---|
| Characteristic | Overall, n=11 | Developmental delay, n=8 | Normal development |
| Age group, n (%) | |||
| 0–29 days | 1 (9.1) | 0 (0) | 1 (33.0) |
| 1–11 months | 7 (63.6) | 5 (62.5) | 2 (66.0) |
| 1–2 years | 3 (27.3) | 3 (37.5) | 0 (0.0) |
| Gender, n (%) | |||
| Female | 5 (45.5) | 3 (37.5) | 2 (66.7) |
| Male | 6 (54.5) | 5 (62.5) | 1 (33.3) |
| TSH, mean (SD) | 46.8 (38.5) | 59.4 (37.9) | 13.2 (8.2) |
| FT4, mean (SD) | 1.0 (0.6) | 0.8 (0.7) | 1.3 (0.2) |
Discussion
The main aim of this study was to determine the incidence and developmental outcomes of patients with CHT in the absence of newborn screening program. According to this facility-based study, the incidence of CHT in GCH over a 5-year study period (2015–2020) is 20/1,420, translating to 14 per 1,000. All cases were primary hypothyroidism; secondary/tertiary forms were not picked during the study period.
This incidence rate is much higher than the worldwide incidence of CHT, which varies between 1:2,000 and 1:4,000 live births, according to a study by Simpser and Rapaport [15]. A possible explanation for the high incidence could be attributed to several factors. Firstly, our study encompassed a broader age range, spanning from three days to 2 years old, whereas most studies typically rely on data from newborn screening alone. Secondly, there may be some degree of selection bias, as a significant proportion of diagnosed CHT cases in our cohort are associated with Down syndrome. Moreover, it’s plausible that screening was more frequently conducted among children presenting with symptoms and developmental/cognitive delays. Additionally, the study found that despite the hospital having guidelines and protocols for CHT screening in place for over five years, and a substantial paediatric population patient flow, particularly in the outpatient department, only a few infants undergo screening during routine check-ups. This finding is the tip of the iceberg, suggesting that many patients could be missed or left undiagnosed. It underscores the importance of implementing a systematic approach to raise awareness among primary physicians about CHT screening and devising a method to assess the effectiveness of the procedure.
The study also investigated the developmental outcome of patients who were diagnosed and put on thyroxine for CHT: most patients had developmental delays, mainly speech and behavioral abnormalities like concentration and some intellectual disability. The study identified two siblings with CHT: the first was identified at the age of one year, and the younger sibling at three months. A comparison of the development outcome between the siblings indicates that the firstborn has difficulty in language development, with challenges in obeying orders and concentration. In the meantime, the younger one has shown faster development speaks and understands better than the firstborn. This finding demonstrates that the earlier CHT is diagnosed and treated will improve long term developmental outcome.
Study conducted in 2011 with the aim of examining the neurodevelopmental outcome of children aged 1–5 years with CH after patients were divided into two groups, including early treated group less than 3 months of age (59 people) and late treated group 3–9 months of age (21 people). Among 59 early treated group 6 (10.1 %), 8 (13.5 %), 4 (6.7 %) had delayed gross motor, fine motor, and cognition respectively [16].Whereas among 21 late treated group 12 (57 %), 15 (71.4 %), 14 (66.6 %) children had delay in gross motor, fine motor, and cognition, respectively. Whereas among 21 late treated group, speech delay was noticed among 16 (76.1 %) children. The study concludes that CHT is a serious condition of newborn babies, which leads to permanent mental and physical retardation if not identified within first few weeks of life. The later the treatment is started the poorer the outcome will be [17].
The possible explanation for the poor development outcome among children with CHT in this study is not different from the above studies: it is late diagnosis and therapy and initiation of thyroxine. The median age of patients diagnosed in this study is 12 weeks with 80 % of patients diagnosed between one month and 11 months. This highlights the need for newborn screening and community sensitization to decrease the impacts of developmental delay, both at household and national levels.
Although the high incidence of CHT in our study could be explained by the wide age range included in our study, further studies are highly recommended.
Limitation of the study
The primary limitation of our research lies as it is a facility-based study conducted in a country where there is no standardized newborn screening program for CHT, compounded by its retrospective nature. While the data were sourced from arguably the largest pediatrics hospital in Kenya, where newborns as young as one week old attend well-baby pediatric reviews, it may not offer a comprehensive representation of Kenyan children diagnosed with CHT.
Conclusions and recommendation
In conclusion, this study demonstrated the incidence and impact of late diagnosis of congenital CHT on developmental outcomes, showing that a significant proportion of affected children experience developmental delays due to delayed identification, exacerbated by the absence of early screening protocols. Therefore, implementing newborn screening for CHT in developing countries is crucial for preventing intellectual disability caused by late diagnoses. However, challenges such as the lack of central laboratories and unreliable patient addresses for result notifications hinder the effective implementation of CHT newborn screening programs in these regions.
Point-of-care testing can effectively circumvent these challenges, as the quick turnaround time for results enables prompt intervention and follow-up [18]. Integrating point-of-care testing for TFT during initial physician reviews and immunization visits can further expedite early detection and treatment [19]. Empowering mothers during antenatal care follow-ups about the importance of CHT screening for their newborns is crucial [20]. Raising awareness and providing education on newborn screening among midwives, nurses, physicians, and community health workers can enhance knowledge and promote early detection.
Additionally, leveraging telemedicine for remote consultations and result interpretation by specialists can extend the reach and effectiveness of newborn screening programs in resource-limited settings. These multifaceted strategies aim to reduce the burden of intellectual disability associated with untreated CHT in newborns, ensuring better long-term outcomes for children with CHT in developing countries.
This study could be used as a guide for policymakers and the Ministry of Health to incorporate the CHT screening program as part of newborn care to improve their comprehensive well-being, with good developmental outcomes and towards creating productive future children. The newborn screening program once started can also generate data for further study.
Funding source: European Society for Paediatric Endocrinology
Acknowledgments
I extend my sincere appreciation to Prof. Jan Lebl, Consultant Paediatric Endocrinologist, and Supervisor on my ESPE Clinical Fellowship Motol University Hospital in Prague for the invaluable support in reviewing the manuscript and mentorship throughout this endeavor. My husband and family for the continous support.
-
Research ethics: The local Institutional Review Board deemed the study exempt from review.
-
Informed consent: Not applicable.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Use of Large Language Models, AI and Machine Learning Tools: None declared.
-
Conflict of interest: The authors state no conflict of interest.
-
Research funding: European Society for Pediatric Endocrinology (ESPE).
-
Data availability: Not applicable.
References
1. Kollati, Y, Akella, RRD, Naushad, SM, Thalla, M, Reddy, GB, Dirisala, VR. The rs1991517 polymorphism is a genetic risk factor for congenital hypothyroidism. 3 Biotech 2020;10:285. https://doi.org/10.1007/s13205-020-02273-7.Search in Google Scholar PubMed PubMed Central
2. Ford, G, LaFranchi, SH. Screening for congenital hypothyroidism: a worldwide view of strategies. Best Pract Res Clin Endocrinol Metab 2014;28:175–87. https://doi.org/10.1016/j.beem.2013.05.008.Search in Google Scholar PubMed
3. Eng, L, Lam, L. Thyroid function during the fetal and neonatal periods. Neo Rev 2020;21:e30–6. https://doi.org/10.1542/neo.21-1-e30.Search in Google Scholar PubMed
4. Rastogi, MV, LaFranchi, SH. Congenital hypothyroidism. Orphanet J Rare Dis 2010;5:17. https://doi.org/10.1186/1750-1172-5-17.Search in Google Scholar PubMed PubMed Central
5. Selva, KA, Harper, A, Downs, A, Blasco, P, LaFranchi, S. Neurodevelopmental outcomes in congenital hypothyroidism: comparison of initial T4 dose and time to reach target T4 and TSH. J Pediatr 2005;147:775–80. https://doi.org/10.1016/j.jpeds.2005.07.024.Search in Google Scholar PubMed
6. Gaudino, R, Garel, C, Czernichow, P, Léger, J. Proportion of various types of thyroid disorders among newborns with congenital hypothyroidism and normally located gland: a regional cohort study. Clin Endocrinol 2005:444–8. https://doi.org/10.1111/j.1365-2265.2005.02239.x.Search in Google Scholar PubMed
7. Van Trotsenburg, P, Stoupa, A, Léger, J, Rohrer, T, Peters, C, Fugazzola, L, et al.. Congenital hypothyroidism: a 2020–2021 consensus guidelines update – an ENDO-European reference network initiative endorsed by the European society for pediatric endocrinology and the European society for endocrinology. Thyroid 2021;31:387–419. https://doi.org/10.1089/thy.2020.0333.Search in Google Scholar PubMed PubMed Central
8. Zdraveska, N, Kocova, M, Nicholas, AK, Anastasovska, V, Schoenmakers, N. Genetics of gland-in-situ or hypoplastic congenital hypothyroidism in Macedonia. Front Endocrinol 2020;11:413. https://doi.org/10.3389/fendo.2020.00413.Search in Google Scholar PubMed PubMed Central
9. Peters, C, Van Trotsenburg, A, Schoenmakers, N. Diagnosis of endocrine disease: congenital hypothyroidism: update and perspectives. Eur J Endocrinol 2018;179:R297–317. https://doi.org/10.1530/eje-18-0383.Search in Google Scholar PubMed
10. Habib, A, Shojazadeh, A, Molayemat, M, Habib, A, Jeddi, M, Arabsolghar, R, et al.. Prevalence and predictive factors of transient and permanent congenital hypothyroidism in Fars Province, Iran. BMC Pediatr 2021;21:264. https://doi.org/10.1186/s12887-021-02729-6.Search in Google Scholar PubMed PubMed Central
11. Padilla, CD, Krotoski, D, Therrell, BL. Newborn screening progress in developing countries – overcoming internal barriers. Semin Perinatol 2010;34:145–55. https://doi.org/10.1053/j.semperi.2009.12.007.Search in Google Scholar PubMed
12. Malherbe, HL, Bonham, J, Carrihill, M, Chetty, K, Conradie, EH, Dercksen, M, et al.. Newborn screening in South Africa: the past, present, and plans for the future. Rare Dis Orphan Drugs J 2024;3:7. https://doi.org/10.20517/rdodj.2023.49.Search in Google Scholar
13. Yarhere, IE, Jaja, T, Briggs, D, Iughetti, L. Newborn screening in Nigeria: associating the screening of congenital hypothyroidism and sickle cell disease can be a winning choice? Acta Biomed 2019;90:316–20. https://doi.org/10.23750/abm.v90i2.8485.Search in Google Scholar PubMed PubMed Central
14. Feleke, Y, Enquoselassie, F, Deneke, F, Abdulkadir, J, Hawariat, GW, Tilahun, M, et al.. Neonatal congenital hypothyroidism screening in Addis Ababa, Ethiopia. East Afr Med J 2000;77:377–81.Search in Google Scholar
15. Simpser, T, Rapaport, R. Update on some aspects of neonatal thyroid disease. J Clin Res Pediatr Endocrinol 2010;2:95–9. https://doi.org/10.4274/jcrpe.v2i3.95.Search in Google Scholar PubMed PubMed Central
16. Gulshan, A, Tahmina, B, Fouzia, M, Mizanur, R. Neurodevelopmental outcome of congenital hypothyroidism in children between 1–5 years of age. Bangladesh J Med Sci 2011;10:245. https://doi.org/10.3329/bjms.v10i4.9495.Search in Google Scholar
17. Ehsani, R, Alijanpour, M, Salehiomran, M, Kheirkhah, F, Moslemi, L, Aghajanpour, F. Evaluation of the developmental outcome in children with congenital hypothyroidism. Caspian J Intern Med 2021;12:315–22. https://doi.org/10.22088/cjim.12.3.315.Search in Google Scholar PubMed PubMed Central
18. Alvarez, OA, Hustace, T, Voltaire, M, Mantero, A, Liberus, U, Saint Fleur, R. Newborn screening for sickle cell disease using point-of-care testing in low-income setting. Pediatrics 2019;144. https://doi.org/10.1542/peds.2018-4105.Search in Google Scholar PubMed
19. Grob, F, Odame, I, Van Vliet, G. Worldwide newborn screening and early immunizations: aligning advances in preventive pediatrics. J Pediatr 2024;264:113732. https://doi.org/10.1016/j.jpeds.2023.113732.Search in Google Scholar PubMed
20. Majid, H, Jafri, L, Farooqui, AJ, Ahmed, S, Nisa Khan, ZU, Khan, AH. Factors associated with awareness of literate mothers about newborn screening: a cross-sectional study from a low-middle-income country. J Pakistan Med Assoc 2023;73:1805–10. https://doi.org/10.47391/jpma.7004.Search in Google Scholar PubMed
© 2024 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Review
- Osteogenesis imperfecta: shifting paradigms in pathophysiology and care in children
- Opinion Paper
- CRH receptor antagonist crinecerfont – a promising new treatment option for patients with congenital adrenal hyperplasia due to 21-hydroxylase deficiency
- Original Articles
- Age and sex mark clinical differences in the presentation of pediatric type 1 diabetes mellitus
- Geographic information system mapping and predictors of glycemic control in children and youth with type 1 diabetes: a study from Western India
- Body composition assessment measured via bioelectrical impedance analysis in euthyroid children with newly diagnosed Hashimoto’s thyroiditis
- Outcomes of newborns screened for congenital hypothyroidism in Turkey – a single center experience
- High yield of congenital hypothyroidism among infants attending Children Hospital, Nairobi, Kenya. Facility based study in the absence of newborn screening
- Immune checkpoint inhibitors and endocrinopathies in pediatric brain tumor patients
- Assessment of quality of life in families affected by maple syrup urine disease: a cross sectional study
- Case Reports
- Reninoma: an unusual cause of growth failure
- Persistent hypoglycemia in congenital syphilis: hyperinsulinemic hypoglycemia with a focal pancreatic lesion
- Diagnostic challenges in pediatric Cushing’s disease associated with chronic renal failure: a report of three patients
- A novel de novo missense OTC mutation in an Iranian girl: a case report
Articles in the same Issue
- Frontmatter
- Review
- Osteogenesis imperfecta: shifting paradigms in pathophysiology and care in children
- Opinion Paper
- CRH receptor antagonist crinecerfont – a promising new treatment option for patients with congenital adrenal hyperplasia due to 21-hydroxylase deficiency
- Original Articles
- Age and sex mark clinical differences in the presentation of pediatric type 1 diabetes mellitus
- Geographic information system mapping and predictors of glycemic control in children and youth with type 1 diabetes: a study from Western India
- Body composition assessment measured via bioelectrical impedance analysis in euthyroid children with newly diagnosed Hashimoto’s thyroiditis
- Outcomes of newborns screened for congenital hypothyroidism in Turkey – a single center experience
- High yield of congenital hypothyroidism among infants attending Children Hospital, Nairobi, Kenya. Facility based study in the absence of newborn screening
- Immune checkpoint inhibitors and endocrinopathies in pediatric brain tumor patients
- Assessment of quality of life in families affected by maple syrup urine disease: a cross sectional study
- Case Reports
- Reninoma: an unusual cause of growth failure
- Persistent hypoglycemia in congenital syphilis: hyperinsulinemic hypoglycemia with a focal pancreatic lesion
- Diagnostic challenges in pediatric Cushing’s disease associated with chronic renal failure: a report of three patients
- A novel de novo missense OTC mutation in an Iranian girl: a case report


