Glucocorticoid use in rheumatoid arthritis patients and the onset of pneumonia: a systematic review and meta-analysis
-
Kawther N. Elsouri
, Vania Arboleda
Abstract
Context
Rheumatoid arthritis (RA) is a systemic autoimmune disease that commonly affects joints. Although many treatment options exist, the most common, disease-modifying antirheumatic drugs (DMARDs), have been associated with pulmonary infections. These types of infections (specifically pneumonia) can be detrimental to RA patients. This leads providers to utilize other treatment modalities such as glucocorticoids (GCs). GCs are commonly utilized to treat RA; however, the role of GCs in the onset of pneumonia in RA patients is not fully understood.
Objectives
The goal of this study was to systematically review and statistically analyze pooled data documenting pneumonia as an adverse event in RA patients on DMARDs as a monotherapy vs RA patients on DMARDs and GCs as combination therapy utilizing the Population, Intervention, Comparison, and Outcomes (PICO) framework.
Methods
On August 1, 2021, a search was conducted and completed on six databases: Embase, MEDLINE, Cochrane Central Register of Controlled Trials (CENTRAL), Web of Science, International Pharmaceutical Abstracts (IPA), and ClinicalTrials.gov. A total of 12 researchers were involved with the search and screening of articles (K.E., P.R.; V.A., D.P.C.; C.B., D.C.; T.A., E.S.; S.H., L.B.; K.S., C.S.). Search terms were identified utilizing Medical Subject Headings (MeSH) and Emtree and included “glucocorticoids,” “rheumatoid arthritis,” “pneumonia,” and “respiratory tract infections,” Inclusion criteria included human subjects over the age of 18 with seropositive RA, on a combination of GC (prednisone, methylprednisolone, or prednisolone) with DMARD (methotrexate [MTX], hydroxychloroquine [HCQ], or sulfasalazine [SSZ]) and developed pneumonia of bacterial, viral, or fungal origin. The control groups were on a DMARD monotherapy regimen. Articles were excluded if they were not in English, had less than 20 participants, were case reports or literature reviews, included animal subjects, and did not adhere to the established PICO framework. Five teams of two researchers individually sorted through abstracts of articles based on the inclusion and exclusion criteria. The same teams individually sorted through full-text articles of selected abstracts based on the same criteria. Conflicts between each team were resolved by a separate researcher. Odds ratios were utilized to quantify the effect sizes of combined studies from a random effects model. Chi-square tests and I2 statistics were utilized to analyze heterogeneity.
Results
A total of 3360 articles were identified from all databases, and 416 duplicate articles were removed. Thus, a total of 2944 articles abstracts were screened, of which 2819 articles either did not meet the inclusion criteria or did meet the exclusion criteria. A total of 125 articles were retrieved and assessed for full-text eligibility, of which only three observational articles were included for meta-analysis. Statistical results revealed that patients treated with DMARDs monotherapy are 95% (95% CI: 0.65–0.99) less likely to develop pneumonia compared to patients treated with a DMARD and GCs (p=0.002).
Conclusions
Our data suggest that RA patients have a higher probability of developing pneumonia on combination therapy with GCs, compared to monotherapy with DMARDs. To our knowledge, our findings are the first to systematically review and statistically evaluate the relationship between the use of GCs and show an increased chance of developing pneumonia.
Rheumatoid arthritis (RA) is a chronic autoimmune disease involving inflammatory, localized, or systemic manifestations [1]. Its pathophysiology is characterized by a persistent and symmetrical inflammation of the synovial fluid and membranes that eventually leads to the degradation of the articular joint cartilage, intense pain [2], and severe physical impairment [3] such as severe stiffness, swelling, and joint deformities [4]. Even though the exact etiology of the disease is still unknown, many studies have shown important relationships between environmental triggers and genetic predisposition that lead to the intricate network of inflammatory mediators that underlie the disease’s immunopathogenesis [5]. RA affects approximately 0.6–1% of the US population [4], and the worldwide incidence has been increasing since 1990 [6].
RA is typically managed with the use of drugs such as disease-modifying antirheumatic drugs (DMARDs), nonsteroidal anti-inflammatory drugs (NSAIDs), analgesics, and glucocorticoids (GCs) [7].
DMARDs work by targeting cytokines, cytokine receptors, or cell surface proteins responsible for the inflammatory response mediated by autoreactive lymphocytes infiltrating the synovium [8]. While this mechanism can be useful in suppressing the hyperactive immune system seen in RA patients, it also carries the possibility of increasing the risk for serious infection [9]. In a multicenter retrospective study by Mori et al. [9], Among the 70 RA patients, 85.7% who developed Pneumocystis pneumonia (PCP) were receiving methotrexate (MTX) monotherapy or MTX combined with other DMARDs with a mean duration of onset of 26.6 months. Coexisting RA interstitial lung disease (ILD) was identified as an important risk factor for morbidity due to PCP in RA patients taking DMARDs [9].
GCs affect the inflammatory cascade of the disease on multiple levels, including the inhibition of proliferation and stimulation of macrophages and fibroblasts [10]. They are also involved in the blockade of multiple cytokines and interleukins (such as IL-1, IL6, tumor necrosis factor alpha [TNF-α]) in addition to leukotrienes, among other mediators of inflammation [10]. Furthermore, there is evidence that the use of low-dose GCs during the first 2 years of RA progression may decrease the structural damage caused by the disease [11], most probably due to the inhibition of the factor receptor ligand κβ that activates osteoclasts responsible for joint damage [11]. Because of their important role in managing RA, GCs are commonly utilized as a monotherapy or in combination with DMARDs [12]. Nevertheless, GCs are also associated with serious side effects, including the increased risk of multiple infections [13] in a dose-dependent fashion [14]. Patients prescribed GCs are at a very high risk of developing lower respiratory tract infections (LRTIs) [15], which can result in severe pneumonia, such as that seen in tuberculosis and infection with Pneumocystis carinii [12].
A cohort including 103,386 chronic obstructive pulmonary disease (COPD) patients showed a 37% decrease in the risk of developing pneumonia after the discontinuation of inhaled GCs [16]. In patients with already existing pneumonia, such as influenza pneumonia, the use of GCs increased the probability of secondary pneumonic infections and exacerbations due to the immunosuppression [17]. In the context of rheumatic diseases, infection rates tend to increase with the use of systemic GCs in patients with RA [12], including severe infections such as bacterial pneumonia, in a dose-dependent fashion [12]. It is thought that the probability of these infections may decrease over time in patients with RA relying on biological treatment because the need for prednisone usage decreases over the course of treatment [12].
The most common type of infections occurring in 86,039 seniors with RA were those targeting the lower respiratory tract (with an odds ratio of 4 at low doses and 7.6 at high doses) and therefore constituting a high risk of pneumonia development [18]. RA patients have approximately a 10 to 20% mortality rate due to pulmonary involvement (whether infectious or not) [19], which represents the second most common cause of death in these patients [20]. These serious infections are the result of multiple factors, including immune dysregulation in RA, multi-organ involvement, and immunosuppressive therapy [21]. In some cases, however, the exact pathophysiology of these respiratory manifestations remains not fully understood [22].
Considering the potential associations seen between the use of GCs and the risk of pneumonia, and due to the repeated use of GCs in patients with RA, we aim to review and analyze the studies that examine a relationship between the onset of pneumonia with the use of GCs, specifically in RA patients on DMARDs. To our knowledge, our study is one of the first systematic reviews and meta-analyses aiming to fill this gap in the literature by investigating whether the onset of pneumonia can be directly associated with GC use, regardless of concurrent DMARD use.
Methods
Study design
A systematic review was conducted and completed on August 1, 2021, to evaluate the risk of GC (prednisone, methylprednisolone, and prednisolone) use in RA patients following the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (Figure 1). There was no funding provided, and Institutional Review Board (IRB) and ethical approvals were not required because this was a review of previously published literature that did not include participants’ information.
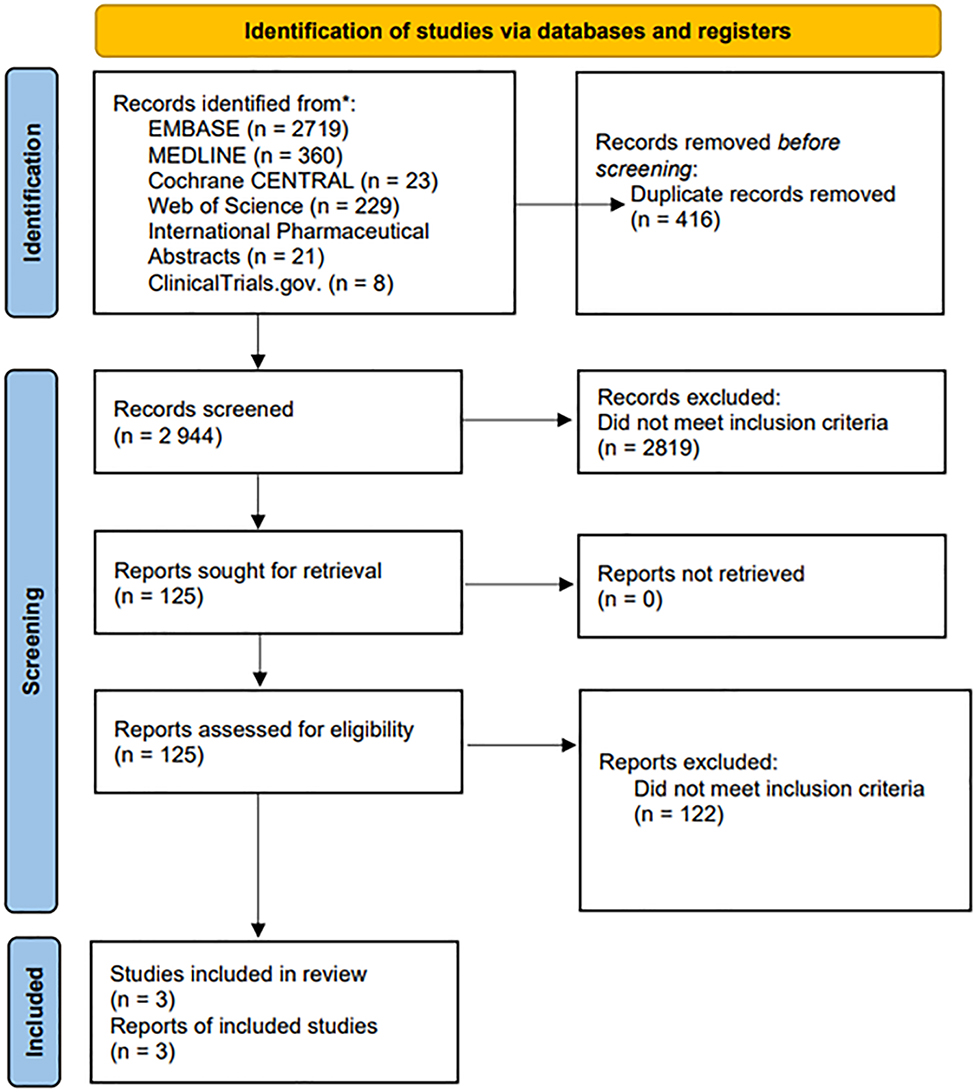
Preferred reporting items for systematic reviews and meta-analyses (PRISMA) flow chart.
Inclusion and exclusion criteria
The criteria to include articles followed the Population, Intervention, Comparison, and Outcomes (PICO) framework. Eligible populations included human subjects over the age of 18 years with a seropositive diagnosis of RA, according to the American College of Rheumatology (ACR)/European League Against Rheumatism (EULAR) 2010 classification. As an intervention, subjects had to be on a combination of a GC (prednisone, methylprednisolone, or prednisolone) while on a DMARD (MTX, hydroxychloroquine [HCQ], or sulfasalazine) and developed pneumonia of bacterial, viral, or fungal origin. As comparisons, the control groups had to be on a DMARD alone without the combination of GCs. The outcomes included the increased risk of developing pneumonia based on the use of GCs to manage their RA. Excluded articles were: articles not in English; articles with less than 20 participants; case reports, literature reviews, or systematic reviews; articles involving animal subjects; and any article/study not adhering to the established PICO framework.
Search strategy and screening
On August 1, 2021, a search was conducted on six databases: Embase, MEDLINE, Cochrane Central Register of Controlled Trials (CENTRAL), Web of Science, International Pharmaceutical Abstracts (IPA), and ClinicalTrials.gov. Keywords and phrases, as well as possible synonyms and abbreviations, were combined with Boolean logic terms (Supplemental Figure 1). Articles were filtered for matches and then exported into Rayyan, a web tool for sorting articles. Five teams of two researchers (VA, DPC; CB, DC; TA, ES; SH, LB; KS, CS) individually sorted through abstracts of articles based on the inclusion and exclusion criteria. Conflicts between each team on the inclusion or exclusion of an abstract were resolved by an independent researcher (KE or PR). Next, the included abstracts were reentered as full-text articles into Rayyan and were evaluated by the five teams based on the inclusion and exclusion criteria. Further conflicts between full-text articles were resolved in the same manner as the abstracts. All researchers conducted a final review of the articles to validate their quality and adherence to the eligibility criteria.
Statistical analysis and summary
In the first stage, data were modeled utilizing the binomial distribution. In the second stage, the normal distribution was utilized after the logit transformation to model the heterogeneity among the studies. Odds ratios were utilized to quantify the effect sizes of combined studies from a random effects model. To evaluate the studies for heterogeneity, chi-square tests and I2 statistics were utilized. A p value of 0.05 was considered statistically significant for the chi-square tests, and I2 values ≥75% was indicative of high heterogeneity. Egger’s test was conducted to examine publication bias. R images (version 4.2.1) were employed for all data analysis. For each included article, authors, year of publication, title, study type, population, relevant results, adverse effects, and limitations were extracted and summarized (Table 1).
Summary of final included articles.
| Study | Study type | Population | DMARD + GC | DMARD alone | Outcomes | Adverse effects | Limitations |
|---|---|---|---|---|---|---|---|
| Housden et al. [23] | Observational retrospective study | United Kingdom -n=1877 RA patients hospitalized between 2004 and 2007 -n=26 RA patients hospitalized for LRTI -Mean age: 69.5 |
n=13 | n=9 (MTX) | None reported |
|
|
| Holland-fischer et al. [24] | Observational prospective cohort study | Denmark n=1220 RA patients diagnosed with pneumonia -n=801 (female) -n=419 (male) -Mean age: 61 |
n=526 | n=143 (MTX) |
|
None reported | |
| Grijalva et al. [25] | Observational retrospective cohort study | United States of America -n=14,586 RA patients -n=192 RA patients hospitalized for pneumonia -Mean age: 57 |
n=100 | n=32 (MTX) n=3 (SSZ) n=27 (HCQ) |
|
None reported |
|
-
DMARD, disease-modifying antirheumatic drug; DNPR, Danish National Patient Register; GC, glucocorticoid; HCQ, hydroxychloroquine; LRTI, lower respiratory tract infection; MTX, methotrexate; RA, rheumatoid arthritis; SSZ, sulfasalazine; TNF-α, tumor necrosis factor alpha.
Results
Systematic review
Our search, utilizing the following search engines, resulted in a total of 3,360 articles: Embase, MEDLINE, Cochrane CENTRAL, Web of Science, IPA, and ClinicalTrial.gov. A total of 416 duplicates were identified and removed before initiating screening. From the remaining records, 2,944 abstracts were ultimately screened, and 2,819 articles did not meet the inclusion criteria or meet the exclusion criteria. The resulting 125 records were sought for retrieval and assessed for eligibility. Of the 125 articles, three were included for final statistical analysis (Table 1).
Housden et al. [23] recreated a study done by internally by the Queen Elizabeth Foundation Hospital Trust in Gateshead, United Kingdom. Utilizing medical records, researchers collected data on patients with RA who were hospitalized for an acute respiratory event between 2004 and 2007 [n=1822] [23]. A total of 26 patients were hospitalized for an LRTI [23]. Two admissions were found to have been for MTX pneumonitis rather than infection and were excluded [23]. The remaining 24 admissions resulted from LRTIs, with all having bacterial pneumonia confirmed on culture or serology [23]. The treatment profile consisted of MTX monotherapy (n=9), MTX and another DMARD (n=5), anti-TNF agent and MTX (n=2), alternative DMARD monotherapy (n=5), and no rheumatic medication (n=3) [23]. Among this population, 54% (n=13) were taking oral steroids in addition to their medication regimens [23]. RA patients on oral steroids had a significantly increased risk of hospitalization due to pneumonia compared to those on DMARDs alone [23].
Holland-Fischer et al. [24] utilized the Danish National Patient Register (DNPR) to collect data on 52,577 Danish patients hospitalized for pneumonia between 1997 and 2011 (n=52,577). Of these hospitalized patients, 1,220 were diagnosed with RA [24]. The treatment profile of these RA patients consisted of MTX monotherapy (n=43), any prednisolone (n=526), any biologics (n=46), other combined DMARDs (conventional synthetic DMARDs [csDMARDs]) as monotherapy (n=63), combination therapy of csDMARDs (n=48), and no RA medication regimen (n=394) [24]. Patients with RA with at least one prescription for prednisolone within 3 months prior to hospitalization had a more than 40% increased 90-day all-cause mortality compared with patients with RA treated with MTX alone [24]. Treatment with biologics did not increase all-cause mortality [24].
Grijalva et al. [25] utilized medical records from TennCare, a health insurance utilized for eligible Medicaid recipients in Tennessee. Researchers collected data on American patients diagnosed with RA and initiating therapy that included a TNF-α antagonist, DMARD, or oral GCs between 1995 and 2005 (n=14,586) [25]. Among this population, 192 were hospitalized for pneumonia [25]. The risk of pneumonia hospitalizations was consistently increased with the initiation of GCs (adjusted hazard ratio [aHR] 2.30, 2.36, and 4.33 for low, medium, and high doses, respectively) [25]. Initiation of leflunomide (LEF), sulfasalazine (SSZ), or HCQ did not increase serious infections, compared with MTX [25]. Both initiation and concurrent GC use were associated with a dose-dependent increase in serious infections [25].
Meta-analysis
Examining model statistics, τ2=4.56 (95% CI: 1.39–44.91], I2=90% (95% CI: 78.8–95.0), H=3.12 (95% CI: 2.17–4.47), and Q=38.82 (p<0.01), demonstrated high heterogeneity and supported the use of a random-effects model. The Egger’s index showed a nonsignificant publication bias (p=0.414). Statistical results reveal that patients treated with DMARDs are 95% (95% CI: 0.65–0.99) less likely to develop pneumonia compared to patients treated with a DMARD and a GC (p=0.002) (Figure 2).
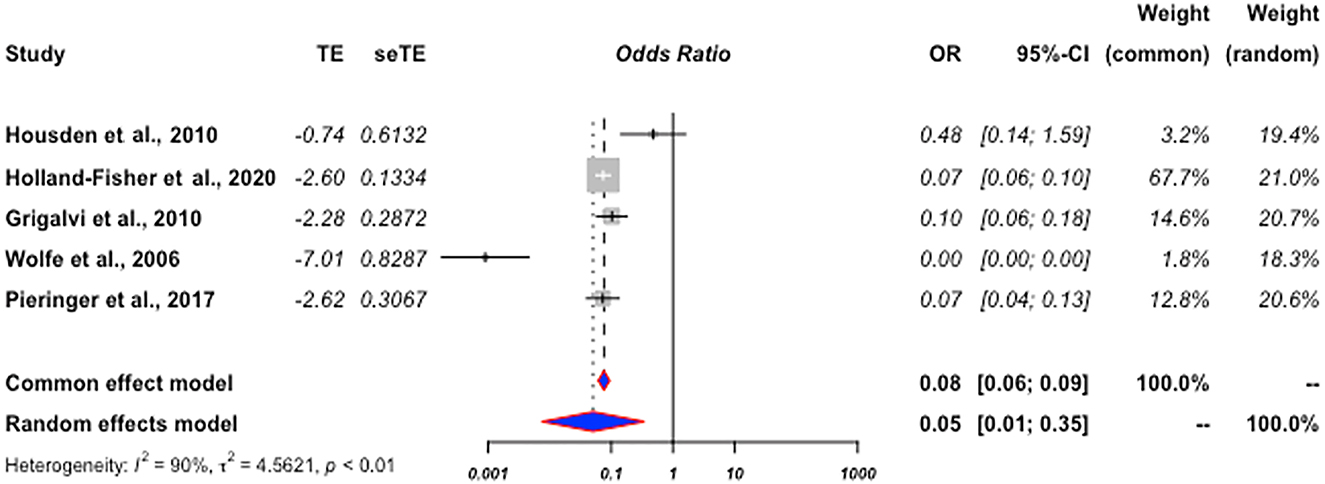
Forrest plot of study outcome 1.
Discussion
Results from our meta-analysis supports our hypothesis that patients with RA who are treated with a DMARD (such as MTX, HCQ, and SSZ) alone are significantly less likely to develop pneumonia compared to patients treated with a DMARD and a GC (such as prednisone, methylprednisolone, and prednisolone). This is noteworthy because ILDs are only second to cardiac disease as a cause of mortality in RA patients [26, 27]. Among the ILDs, which can increase mortality, pneumonia seems to be a common etiology [26]. Despite usual interstitial pneumonia (UIP) being the most common pattern of pneumonia among RA patients with ILD [28], the onset of UIP carries a worse prognosis for RA patients compared to other patterns of RA-ILD [28]. Indeed, some research supports the notion that the outcomes of RA-UIP are similar to interstitial pulmonary fibrosis [28, 29]. While research has traditionally pointed the finger at the use of DMARDs in patients with RA as a cause of the onset of respiratory disease, our findings shift the focus.
Housden et al. [23] suggests that oral steroid therapy may be a major contributory factor in the development of LRTIs. In 22 RA patients hospitalized for pneumonia, 54% were utilizing GCs for the treatment of RA for at least 1 year prior to their admission [23]. The prevalence of oral steroid consumption among admissions was significantly higher than in the RA group as a whole (n=1822; 14%, p=0.021) [23]. Over half of the patients admitted in the study were taking oral steroids, compared to 14% of the RA population in general (p=0.021) [23]. However, the results of Holland-Fischer et al. [24] suggest that rather than DMARD use increasing the risk for pneumonia, the risk lies in disease activity. Patients with RA with at least one prescription for prednisolone within 3 months prior to hospitalization had a more than 40% increased 90-day all-cause mortality compared with patients with RA treated with MTX [24]. While treating RA patients with biologics has been associated with the onset of infection [30], results show that biologics do not significantly increase the mortality rate [24]. This could be due to a multitude of factors. Biologics fall into the class of DMARDs [31]; however, their mechanism of action works by preventing specific aspects of an immune response further along in the process [31], compared to conventional treatment (e.g., MTX), which acts by nonspecifically inhibiting cell-mediated immunity [31]. Biologics are an alternative treatment for RA [31] and have been shown to play a smaller role in the development of ILDs in RA due to this increased specificity [32], in contrast with conventional DMARD agents [32]. Nonetheless, future studies would benefit from analyzing whether the similar pattern of pneumonia onset seen with DMARD combination therapy with GC also occurs with biologic combination therapy with GC.
Grijalva et al. [25] established that the initiation of LEF, SSZ, or HCQ did not increase serious infections, compared with MTX [25]. However, both the initiation of a DMARD and concurrent GC use were associated with a dose-dependent increase in serious infections such as pneumonia [25]. Although these studies were conducted in different parts of the world, they share a common theme: the risk for developing pneumonia in RA patients has consistently been higher in those on combination therapy (DMARD + GC) or GC monotherapy compared to DMARD monotherapy [23], [24], [25]. Additionally, their results suggest that the progression of pneumonia, which can cause irreparable damage to RA patients [28, 29], can be associated with high disease activity. This emphasizes the importance of maintaining RA patients on DMARDs to ensure proper treatment and prevention of disease progression. Common practice tends to stop the use of the DMARD in RA patients at risk for infection, while our findings suggest that this measure could result in a worse prognosis compared to maintaining DMARD treatment [24].
Currently, there is no consensus on how to manage DMARD therapy in the perioperative setting [33]. Although the recent guidelines from the American College of Rheumatology/American Association of Hip and Knee Surgeons recommend continuing conventional DMARDs and holding biologics for one dosing interval before surgery [34], our findings support continuing DMARD therapy and withholding GCs. Ultimately, the cessation of DMARDs may set patients’ progress back by allowing the disease process to continue unchecked and the use of GCs to increase the risk of infection, regardless of the use of a DMARD [24, 34, 35]. Our results should encourage practitioners to be more mindful of discontinuing DMARD therapy and maintaining GCs in RA patients because of the possibility of pneumonia, which carries a significantly high burden of fatality in these patients [28, 29]. Our data do not definitively suggest the use of GCs as an etiology behind the onset of respiratory disease among RA patients, and our data contain natural limitations such as inevitable exhaustion bias from screeners while reading through articles. However, our findings do raise the important question of how to treat the underlying disease without simultaneously triggering the onset of a life-threatening infection. The current literature is limited on the specific adverse effects of treatment for RA. Although a significant amount of research addresses the idea that the effects of disease-managing treatment courses can increase the risk of infection and complicate the course of RA, limited work adequately correlates the onset of pneumonia with the use of GCs with patients already on DMARDs. Due to the specificity of this analysis, we were limited by the number and types of studies we included and by the high heterogeneity (I2=90%) exhibited in included studies. This review and analysis only utilized six search engines, which could have limited the search and possibly skew our statistical findings. Additionally, the performed searches only focused on three DMARDs and GCs commonly utilized for RA. Future studies should analyze the correlation of pneumonia or ILDs with additional DMARDs and GCs, which are also utilized in RA. Furthermore, the exclusion of articles written in English, as done by our review, could downplay the amount of universally present research covering this topic. We believe these factors to be the greatest limitations of this study. However, the data from these analyses appear to provide an interesting linkage between RA treatment and pneumonia. To provide a more salient relationship, future studies should not only focus on analyzing the same factors (RA patients being treated with only a DMARD vs patients treated with a DMARD plus a GC) and the occurrence of pneumonia, but also through an experimental randomized control study within a regulated environment, in order to eliminate heterogeneity.
Conclusions
Our data suggest a concern for pneumonia development in GCs plus DMARD-treated patients; however, our data do not allow us to determine the causality of the relationship. Although there are many published articles suggesting a relationship between GC treatment in rheumatic individuals and the development of infections, we believe that our study is unique in its approach and findings for (1) pneumonia infections specifically, and (2) with the precision that a statistical analysis provides. We believe that our statistical analysis will allow physicians to better devise a holistic treatment plan for rheumatic patients. This approach is central to osteopathic medicine, and will guide physicians to weigh the potential benefits with the statistically significant pneumonic complications.
Acknowledgements
The authors would like to thank Tariq Rahaman, MLIS (Nova Southeastern University College of Osteopathic Medicine, Tampa Bay Regional Campus Library). Mr. Rahaman’s contributions with our literature search and review were vital to the final article produced.
-
Research funding: None reported.
-
Author contributions: All authors provided substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; all authors drafted the article or revised it critically for important intellectual content; all authors gave final approval of the version of the article to be published; and all authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
-
Competing interests: None reported.
References
1. Klarenbeek, NB, Kerstens, PJSM, Huizinga, TWJ, Dijkmans, BAC, Allaart, CF. Recent advances in the management of rheumatoid arthritis. BMJ 2010;341:6942. https://doi.org/10.1136/bmj.c6942.Suche in Google Scholar PubMed
2. Gibofsky, A. Epidemiology, pathophysiology, and diagnosis of rheumatoid arthritis: a synopsis. Am J Manag Care 2014;20:S128–35.Suche in Google Scholar
3. Aletaha, D, Smolen, JS. Diagnosis and management of rheumatoid arthritis: a review. JAMA 2018;320:1360–72. https://doi.org/10.1001/jama.2018.13103.Suche in Google Scholar PubMed
4. van der Woude, D, van der Helm-van Mil, AHM. Update on the epidemiology, risk factors, and disease outcomes of rheumatoid arthritis. Best Pract Res Clin Rheumatol 2018;32:174–87. https://doi.org/10.1016/j.berh.2018.10.005.Suche in Google Scholar PubMed
5. Firestein, GS, Budd, RC, Gabriel, SE, McInnes, IB, O’Dell, JR. Etiology and pathogenesis of rheumatoid arthritis. In: Firestein, GS, Budd, RC, Gabriel, SE, McInnes, IB, O’Dell, JR, editors. Kelley and firestein’s textbook of Rheumatology, 10th ed. Amsterdam, Netherlands: Elsevier; 2017:1115–66 pp.Suche in Google Scholar
6. Safiri, S, Kolahi, A, Hoy, D, Buchbinder, R, Mansournia, M, Bettampadi, D, et al.. Global, regional, and national burden of neck pain in the general population: systematic analysis of the Global Burden of Disease Study 2017. BMJ 2020;368:791–m791. https://doi.org/10.1136/bmj.m791.Suche in Google Scholar PubMed PubMed Central
7. Walsh, DA, McWilliams, DF. Mechanisms, impact and management of pain in rheumatoid arthritis. Nat Rev Rheumatol 2014;10:581–92. https://doi.org/10.1038/nrrheum.2014.64.Suche in Google Scholar PubMed
8. Tamai, H, Nishina, N, Kikuchi, J, Izumi, K, Otomo, K, Yoshimoto, K, et al.. Serum cytokines and bone metabolic markers in patients with rheumatoid arthritis treated with biological disease modifying anti-rheumatic drugs. Clin Rheumatol 2022. https://doi.org/10.1007/s10067-022-06390-x.Suche in Google Scholar PubMed
9. Mori, S, Ueki, Y, Miyamura, T, Ishii, K, Hidaka, T, Yoshitama, T, et al.. Outcomes and risk factors for mortality in pneumocystis pneumonia patients with rheumatoid arthritis: a multicenter retrospective cohort study. Mod Rheumatol 2022;roac088. https://doi.org/10.1093/mr/roac088.Suche in Google Scholar PubMed
10. Macfarlane, E, Seibel, MJ, Zhou, H. Arthritis and the role of endogenous glucocorticoids. Bone Research 2020;8:33. https://doi.org/10.1038/s41413-020-00112-2.Suche in Google Scholar PubMed PubMed Central
11. García-Magallón, B, Silva-Fernández, L, Andreu-Sánchez, JL. Update on the use of steroids in rheumatoid arthritis. Reumatol Clínica 2013;9:297–302. https://doi.org/10.1016/j.reumae.2013.01.015.Suche in Google Scholar
12. Youssef, J, Novosad, SA, Winthrop, KL. Infection risk and safety of corticosteroid use. Rheum Dis Clin N Am 2016;42:157–76. https://doi.org/10.1016/j.rdc.2015.08.004.Suche in Google Scholar PubMed PubMed Central
13. Lim, SY, Bolster, MB. Corticosteroids. In: Cho, TA, Bhattacharyya, S, Helfgott, S, editors. Neurorheumatology. New York City, NY, USA: Springer International Publishing; 2019:261–7 pp.10.1007/978-3-030-16928-2_28Suche in Google Scholar
14. George, MD, Hsu, JY, Hennessy, S, Chen, L, Xie, F, Curtis, J, et al.. Risk of serious infection with low-dose glucocorticoids in patients with rheumatoid arthritis: an instrumental variable analysis. Epidemiology 2022;33:65–74. https://doi.org/10.1097/EDE.0000000000001422.Suche in Google Scholar PubMed PubMed Central
15. Fardet, L, Petersen, I, Nazareth, I. Common infections in patients prescribed systemic glucocorticoids in primary care: a population-based cohort study. PLoS Med 2016;13:e1002024. https://doi.org/10.1371/journal.pmed.1002024.Suche in Google Scholar PubMed PubMed Central
16. Suissa, S, Coulombe, J, Ernst, P. Discontinuation of inhaled corticosteroids in COPD and the risk reduction of pneumonia. Chest 2015;148:1177–83. https://doi.org/10.1378/chest.15-0627.Suche in Google Scholar PubMed
17. Rahmqvist, M, Samuelsson, A, Bastami, MS, Rutberg, H. Direct health care costs and length of hospital stay related to health care-acquired infections in adult patients based on point prevalence measurements. Am J Infect Control 2016;44:500–6. https://doi.org/10.1016/j.ajic.2016.01.035.Suche in Google Scholar PubMed
18. Widdifield, J, Bernatsky, S, Paterson, JM, Gunraj, N, Thorne, JC, Pope, J, et al.. Serious infections in a population-based cohort of 86,039 seniors with rheumatoid arthritis. Arthritis Care Res 2013;65:353–61. https://doi.org/10.1002/acr.21812.Suche in Google Scholar PubMed
19. Urisman, A, Jones, KD. Pulmonary pathology in connective tissue disease. Semin Respir Crit Care Med 2014;35:201–12. https://doi.org/10.1055/s-0034-1371543.Suche in Google Scholar PubMed
20. Pinheiro, FAG, Souza, DCC, Sato, EI. A study of multiple causes of death in rheumatoid arthritis. J Rheumatol 2015;42:2221–8. https://doi.org/10.3899/jrheum.150166.Suche in Google Scholar PubMed
21. Listing, J, Gerhold, K, Zink, A. The risk of infections associated with rheumatoid arthritis, with its comorbidity and treatment. Rheumatology 2013;52:53–61. https://doi.org/10.1093/rheumatology/kes305.Suche in Google Scholar PubMed
22. Alunno, A, Gerli, R, Giacomelli, R, Carubbi, F. Clinical, epidemiological, and histopathological features of respiratory involvement in rheumatoid arthritis. BioMed Res Int 2017;2017:1–8. https://doi.org/10.1155/2017/7915340.Suche in Google Scholar PubMed PubMed Central
23. Housden, MM, Bell, G, Heycock, CR, Hamilton, J, Saravanan, V, Kelly, CA. How to reduce morbidity and mortality from chest infections in rheumatoid arthritis. Clin Med 2010;10:326–9. https://doi.org/10.7861/clinmedicine.10-4-326.Suche in Google Scholar PubMed PubMed Central
24. Holland-Fischer, M, Thomsen, RW, Tarp, U, Nørgaard, M. Prognosis of pneumonia in patients with rheumatoid arthritis: the role of medication and disease activity prior to admission a population-based cohort study. RMD Open 2020;6:e001102. https://doi.org/10.1136/rmdopen-2019-001102.Suche in Google Scholar PubMed PubMed Central
25. Grijalva, CG, Kaltenbach, L, Arbogast, PG, Mitchel, EFJr, Griffin, MR. Initiation of rheumatoid arthritis treatments and the risk of serious infections. Rheumatology 2010;49:82–90. https://doi.org/10.1093/rheumatology/kep325.Suche in Google Scholar PubMed PubMed Central
26. Cavagna, L, Monti, S, Grosso, V, Boffini, N, Scorletti, E, Crepaldi, G, et al.. The multifaceted aspects of interstitial lung disease in rheumatoid arthritis. BioMed Res Int 2013. https://doi.org/10.1155/2013/759760.Suche in Google Scholar PubMed PubMed Central
27. Marigliano, B, Soriano, A, Margiotta, D, Vadacca, M, Afeltra, A. Lung involvement in connective tissue diseases: a comprehensive review and a focus on rheumatoid arthritis. Autoimmun Rev 2013;12:1076–84. https://doi.org/10.1016/j.autrev.2013.05.001.Suche in Google Scholar PubMed
28. Shaw, M, Collins, BF, Ho, LA, Raghu, G. Rheumatoid arthritis-associated lung disease. Eur Respir Rev 2015;24:1–16. https://doi.org/10.1183/09059180.00008014.Suche in Google Scholar PubMed PubMed Central
29. Solomon, JJ, Ryu, JH, Tazelaar, HD, Myers, J, Tuder, R, Cool, C, et al.. Fibrosing interstitial pneumonia predicts survival in patients with rheumatoid arthritis-associated interstitial lung disease (RA-ILD). Respir Med 2013;107:1247–52. https://doi.org/10.1016/j.rmed.2013.05.002.Suche in Google Scholar PubMed
30. Almalag, HM, Alaujan, SS, Alhazzani, HS, Alzamel, L, Tashkandi, R, Alarfaj, H, et al.. Prevalence of adverse reactions to intravenously administered originator biologics in patients with rheumatoid arthritis: a 5-year retrospective study. Saudi Pharmaceut J 2022;30:1044–51. https://doi.org/10.1016/j.jsps.2022.04.008.Suche in Google Scholar PubMed PubMed Central
31. Benjamin, O, Goyal, A, Lappin, SL. Disease modifying anti-rheumatic drugs (DMARD). In: StatPearls [Internet]; 2022. Available from: https://www.ncbi.nlm.nih.gov/books/NBK507863/ [Accessed 26 Oct 2022].Suche in Google Scholar
32. Bes, C. Comprehensive review of current diagnostic and treatment approaches to interstitial lung disease associated with rheumatoid arthritis. Eur J Rheumatol 2018;6:146–9. https://doi.org/10.5152/eurjrheum.2019.19036.Suche in Google Scholar PubMed PubMed Central
33. Krause, ML, Matteson, EL. Perioperative management of the patient with rheumatoid arthritis. World J Orthoped 2014;5:283–91. https://doi.org/10.5312/wjo.v5.i3.283.Suche in Google Scholar PubMed PubMed Central
34. Goodman, SM, Springer, BD, Chen, AF, Davis, M, Fernandez, DR, Figgie, M, et al.. 2022 American college of Rheumatology/American association of Hip and knee Surgeons guideline for the perioperative management of antirheumatic medication in patients with rheumatic diseases undergoing elective total Hip or total knee arthroplasty. Arthritis Care Res 2022;74:1399–408. https://doi.org/10.1002/acr.24893.Suche in Google Scholar PubMed
35. George, MD, Baker, JF. Perioperative management of immunosuppression in patients with rheumatoid arthritis. Curr Opin Rheumatol 2019;31:300–6. https://doi.org/10.1097/BOR.0000000000000589.Suche in Google Scholar PubMed PubMed Central
Supplementary Material
The online version of this article offers supplementary material (https://doi.org/10.1515/jom-2022-0177).
© 2022 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- General
- Review Article
- Glucocorticoid use in rheumatoid arthritis patients and the onset of pneumonia: a systematic review and meta-analysis
- Medical Education
- Original Article
- Barriers to research opportunities among osteopathic medical students
- Musculoskeletal Medicine and Pain
- Case Report
- Structural abnormalities and osteopathic considerations in primary immunodeficiencies
- Neuromusculoskeletal Medicine (OMT)
- Original Article
- Does the osteopathic pedal pump reduce lower limb volume in healthy subjects?
- Neuromusculoskeletal Medicine (OMT)
- Clinical Practice
- Enabling health potential: exploring nonlinear and complex results of osteopathic manual medicine through complex systems theory
- Pediatrics
- Review Article
- Person-centered language and pediatric ADHD research: a cross-sectional examination of stigmatizing language within medical literature
- Clinical Image
- Superficial spreading melanoma within a nevus spilus
Artikel in diesem Heft
- Frontmatter
- General
- Review Article
- Glucocorticoid use in rheumatoid arthritis patients and the onset of pneumonia: a systematic review and meta-analysis
- Medical Education
- Original Article
- Barriers to research opportunities among osteopathic medical students
- Musculoskeletal Medicine and Pain
- Case Report
- Structural abnormalities and osteopathic considerations in primary immunodeficiencies
- Neuromusculoskeletal Medicine (OMT)
- Original Article
- Does the osteopathic pedal pump reduce lower limb volume in healthy subjects?
- Neuromusculoskeletal Medicine (OMT)
- Clinical Practice
- Enabling health potential: exploring nonlinear and complex results of osteopathic manual medicine through complex systems theory
- Pediatrics
- Review Article
- Person-centered language and pediatric ADHD research: a cross-sectional examination of stigmatizing language within medical literature
- Clinical Image
- Superficial spreading melanoma within a nevus spilus

