Abstract
Structural skeletal abnormalities are associated with primary immunodeficient (PID) patients. These abnormalities have not been well studied in PID with reference to osteopathic medicine tenets. Osteopathic structural examinations of PID patients with respect to these tenets and the diagnosis of somatic dysfunctions preventing the free flow of lymph fluids back into the circulation and the disruption of the skeletal microenvironment may have an impact on the status of the immune system in patients with a PID. A standardized evaluation was conducted in a patient with a phosphatidylinositol 3-kinase regulatory subunit 1 (PIK3R1) mutation who presented with skeletal abnormalities. A literature review was also conducted to determine the breadth of other PIDs with structural irregularities. Osteopathic structural clinical examinations (OSCEs) were performed by an osteopathic medical student, fellow, and attending after receiving informed consent from the patient. The findings were collected regionally noting severity, tissue texture changes, asymmetry, altered range of motion (ROM), and tenderness according to DO-Touch.NET physical examination and treatment form. A literature review was conducted utilizing various search engines and the textbook, Stiehm’s Immune Deficiencies, 4th edition. The significant findings found from the patient were right sidebending rotation cranial strain pattern with decreased left temporal bone motion, temporomandibular joint crepitus, and right deviation upon mandibular opening. The thoracolumbar region revealed tissue tenderness and restricted psoas ROM. Bilateral sacroiliac joint tenderness, right superior sheering, and anterior innominate rotation, along with left-on-left sacral flexion, were associated with valgus knees. The literature search showed multiple other PIDs outside of PIK3R1 that have associated skeletal and structural abnormalities. Irregular skeletal features found in immunodeficient patients may have an additive defect on the immunological responses due to somatic dysfunction impinging on the lymphatic flow to the central circulation. Other different immunodeficient patients suffer from boney structural abnormalities, which may lead to further immune hindrance caused by impingement of flow as well as bone marrow microenvironment impact on the peripheral immunological output. We present the first osteopathic examination with detailed findings of somatic dysfunction in a patient with PID. Future studies on PID patients should require more attention to structure and function, as found by a thorough osteopathic examination in order to unrestrict preformed cellular and humoral components back into the peripheral circulation.
The interrelation of structure and function is an important tenet of osteopathy that applies to the central and peripheral immune system [1]. These interrelationships may convey a functional relevance to the immune system due to its effect on movement of lymph and its effects on the microenvironments of bone marrow [2].
The boney structural abnormalities in patients with immune deficiencies may be an additive effect on their immunological responses. Gharib and Gupta [3] have previously described 96 primary immunodeficiencies associated with these abnormalities. A comprehensive evaluation of structural boney lesions in primary immunodeficiencies (PIDs) in reference to an osteopathic examination has not been done previously. We have described the first concise osteopathic examination of an immunodeficient patient with a phosphatidylinositol 3-kinase regulatory subunit 1 (PIK3R1) mutation and a review of musculoskeletal abnormalities found in more common PID.
Case report
We present a 28-year-old White female with a PID associated with B cell abnormalities and PIK3R1 heterozygous gene mutation (c.1425 + 1G>A). She presented to the clinic in June 2019. Her anterior craniofacial structural anomalies had been previously described [4] and became more pronounced with age: triangular facies, ocular depression, lipodystrophy, and hypoplastic nasal alae (Figure 1). Informed consent was obtained on paper by author Marija Rowane, and an osteopathic structural clinical examinations (OSCEs) was independently performed by an attending, fellow, and osteopathic medical student, without disclosing findings until concluding each regional evaluation [5]. The use of three evaluators was utilized in order to mitigate any errors in the evaluation of the patient’s osteopathic examination. Severity, tissue texture changes, asymmetry, altered range of motion (ROM), and tenderness were organized under anatomical regions in the DO-Touch.NET Physical Examination and Treatment Form (Table 1) [5, 6]. Discrepancies were re-evaluated to confirm the diagnoses, and the table displays the consensus of the evaluators.
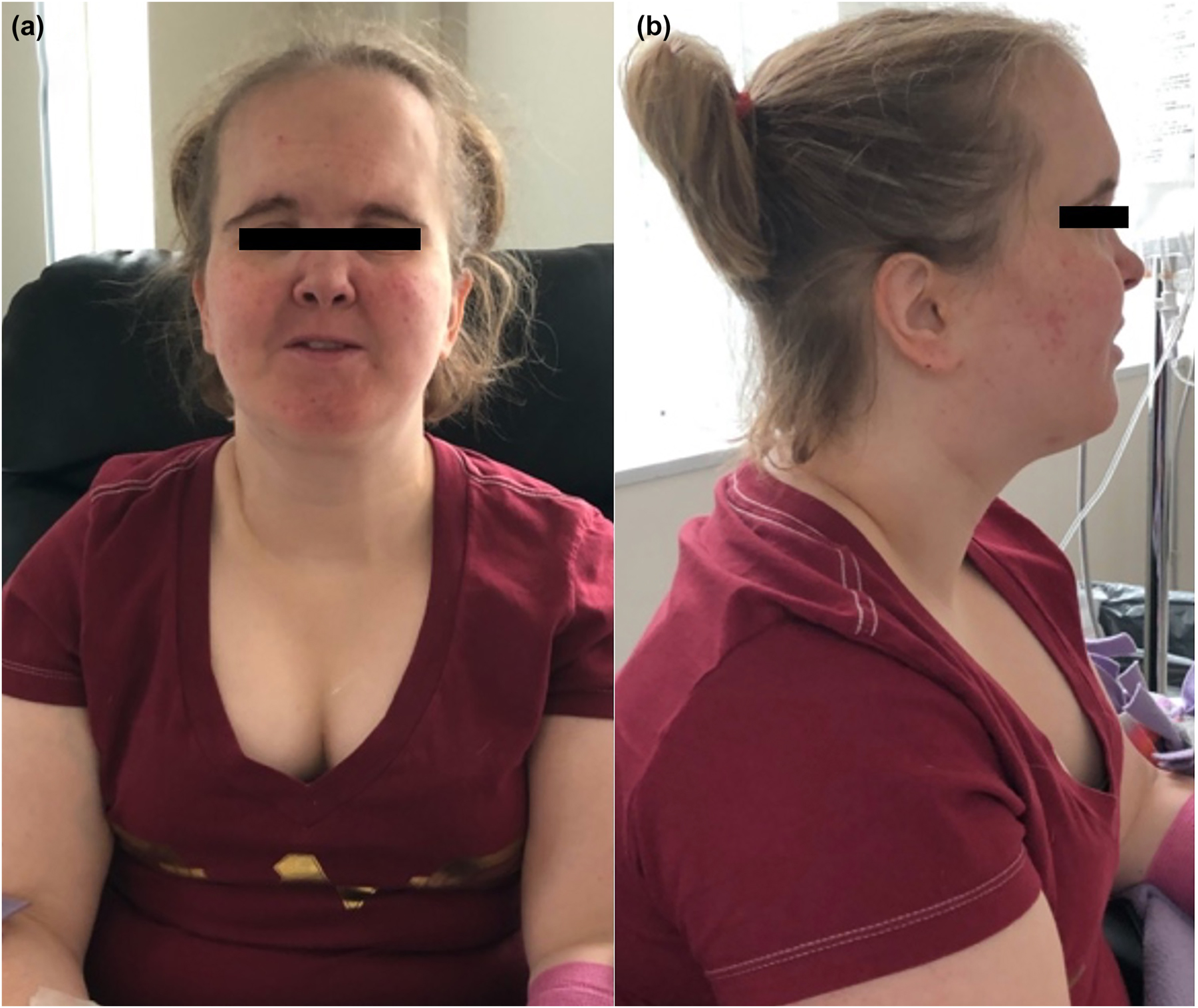
Craniofacial presentations of a 28-year-old female patient with phosphatidylinositol 3-kinase regulatory subunit 1 (PIK3R1) mutation. (A) Anterior view. (B) Lateral view.
Osteopathic structural clinical examination (OSCE) consensus findings of somatic dysfunction in phosphatidylinositol 3-kinase regulatory subunit 1 (PIK3R1) mutation primary immunodeficiency.
| Region | Side (R/L)/vertebra(e) | Severity | TART | Notes | |||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | Tt | As | Rr | Te | |||
| Cranium/face | X | X | X | Cranial strain pattern: R sidebending rotation (↑ extension, ↓ flexion; L temporal bone motion “locked”, ↑ motion on R) | |||||
| CRI 12 cycles/min | |||||||||
| TMJ bilateral crepitus (R>L), R mandibular deviation | |||||||||
| Neck | X | X | X | X | X | C1 RRSL, C2 NRLSR, C3 RRSR (−) spurling maneuver | |||
| Thorax | T1–4 | X | X | X | X | X | T3 FRRSR rotational ease | ||
| Prominent L paravertebral musculature | |||||||||
| Mild kyphosis | |||||||||
| T5–9 | X | X | X | X | X | T5 RLSL | |||
| T7 transition of prominent paravertebral musculature from L (upper thoracic region) to R | |||||||||
| T10–12 | X | X | X | X | X | T12 L hypertonicity | |||
| Ribs | R | X | X | X | X | Expiratory ribs (inspiratory restriction)a | |||
| CTJ T1 RRSR | |||||||||
| L | X | X | X | X | Inspiratory ribs (expiratory restriction) | ||||
| CTJ L rib 1 S (mid-clavicular) | |||||||||
| Rib 10 P | |||||||||
| Lumbar | R | X | X | X | X | X | Lumbosacral region RRSL; (+) ASIS Compression; L5 hypertonicity | ||
| L | X | X | X | X | X | Thoracolumbar region RLSL | |||
| Sacrum | X | X | X | X | X | L-on-L sacral torsion [(+) R SFTSE (R PSIS S/F), R A/D sacral sulcus, L P/S inferior lateral angle] | |||
| Bilateral sacroiliac joint tenderness | |||||||||
| Pelvis/innominate | R | X | X | X | X | (+) R SFT ST ; superior sheer and anterior innominate rotation (PSIS S/ASIS A)a | |||
| L | X | X | X | X | Inferior sheer and posterior innominate rotation (PSIS I/ASIS S)a | ||||
| Shoulder | R | X | |||||||
| L | X | X | X | X | X | ||||
| Upper extremity | R | X | X | X | Arm length shorter (styloid process) with superior sheer, R clavicular ease with I motion | ||||
| L | X | X | X | Arm length longer (styloid process) with inferior sheer, L clavicular ease with S motion | |||||
| Hand/wrist | R | X | X | X | |||||
| L | X | X | X | ||||||
| Proximal lower extremity | R | X | X | X | (−) Radiculopathy/Lasegue’s test; (−) Patrick FABER; SLT (65°); long restrictors Rr (psoas>piriformis); dorsiflexion elicits pain | ||||
| L | X | X | X | (−) Radiculopathy/Lasegue test; (−) Patrick FABRE; SLT (75°); long restrictor (psoas) mild-moderate Rr | |||||
| Distal lower extremity | R | X | X | X | X | X | Leg length shorter (M malleolus); dorsiflexion elicits less pain | ||
| Pes planus (M longitudinal arc Rr, pronounced supination) | |||||||||
| L | Leg length longer (M malleolus) | ||||||||
| Knee/calf/shin | R | X | X | X | Valgus knees, Rr | ||||
| L | X | X | X | Valgus knees, Rr L>R (25°) | |||||
| Gait/posture | X | X | Short stature | ||||||
| Hypotonality (exaggerated when sedentary) | |||||||||
| Antalgic gait (subtle limp) | |||||||||
-
Adapted categorization from Degenhardt et al.’s DO-Touch.NET Physical Examination and Treatment Form. aThe patient expressed mild tenderness to palpation (TTP) in these areas. R, right; L, left; TART, Tt, tissue texture changes; As, asymmetry; Rr, restricted range of motion; Te, tenderness; ↑, increased; ↓, decreased; CRI, cranial rhythmic impulse; TMJ, temporomandibular joint; C, cervical; R, rotated; S, sidebent; N, neutral; (−), negative; T, Thoracic vertebral level(s); F, flexed; CTJ, cervicothoracic junction; S, superior; P, posterior; (+), positive; ASIS, anterior superior iliac spine; SFTSE, Seated Flexion Test; PSIS, posterior superior iliac spine; A, anterior; D, deep; SFTST, Standing Flexion Test; I, inferior; FABER, Flexion, ABduction, External Rotation; SLT, Straight Leg Test.
An extensive literature search was performed by author MC and reviewed by mentor RH utilizing the standard immunological text from Stiehm’s Immune Deficiencies textbook published in 2022 (Editors: Kathleen E. Sullivan and E. Richard Stiehm) by reviewing for musculoskeletal issues. An additional literature review was conducted utilizing PubMed, Google Scholar, OSTMED.DR, AOA Osteopathic Research Database, American Academy of Osteopathy Journal – Index, Osteopathic Research Web, CORE, iSEEK, BASE, and MEDLINE. The search terms that were utilized included “osteopathy”, “musculoskeletal”, “immune”, “skeletal dysplasia”, “skeletal”, and “PID”, and by searching the specific PIDs found within the previously mentioned Stiehm’s Immune Deficiencies textbook.
The most significant findings of the osteopathic examination included a right sidebending rotation cranial strain pattern with decreased left temporal bone motion, temporomandibular joint crepitus, and right deviation upon mandibular opening. The thoracolumbar region revealed tissue tenderness and restricted psoas ROM. Bilateral sacroiliac joint tenderness, right superior sheering, anterior innominate rotation, and left-on-left sacral flexion were associated with valgus knees.
Discussion
The skeletal frame to which the muscles, nerves, vessels, and connective tissue suspend serves as the fulcrum for the movement of the structure and bodily fluids. Movement allows for the fluids of the lymph system containing predetermined immunoglobulins and T cells to flow to an inflammatory or homeostatic site of the body for repair, infection fighting, or maintenance [7]. Although this may suggest that the bone is only an observer in the process, it also provides a microenvironment within which the immune precursors may develop and prepare for departure into the nonosseus structure. This science has been characterized as osteoimmunology [2].
Although the understanding of osteoimmunology may reflect the immune system’s effect on bone disorders, it also focuses on the effects of the bone structure on immune development. Aberrations of the structure of the bone may impact the output of effector cells of both innate and adaptive immune systems such as what are found in many primary immunodeficiencies such as DiGeorge syndrome [8], ADA deficiency [9, 10], signal transducer and activator of transcription 3 (STAT3) and 5b mutations [11, 12], cartilage-hair hypoplasia [13], and PIK3R1 mutations (described previously). Although the aberrancies of the skeletal structure do not play a role in the isolated molecular mutations found in such immune issues, these aberrancies may contribute to the overall immunological responses. A review of the structural abnormalities have not been performed in the literature, possibly due to the rarity of these disorders.
A mutation in PIK3R1 causes broad immune deficiencies, such as low serum immunoglobulin G (IgG) and A (IgA) levels with elevated IgM that progressed to an absence of IgM over the course of intravenous immunoglobulin replacement therapy [4]. The characteristic skeletal abnormalities associated with this syndrome include short stature, triangular facies, hypoplastic nasal alae, and other anterior facial bone abnormalities [9].
Combined immune deficiencies have been associated with structural abnormalities. Adenosine deaminase severe combined immunodeficiency (ADA-SCID) is a genetic defect causing immune deficiency due to the accumulation of purines within the lymphocytes [14]. The aggregation of these molecules can manifest as lymphopenia and recurrent infections. This multiorgan disorder commonly presents with skeletal abnormalities and defects. These are due to improper osteoblast activity leading to reduced bone volume and formation [14]. This has manifested previously as flared ribs, leading to a rachitic rosary [15], large low-set ears, and small bones [8].
Isolated T cell defects have been associated with structural abnormalities. DiGeorge syndrome is a genetic disorder caused by a 22q11.2 microdeletion [16]. One of the hallmarks of this disease is a hypoplastic or absent thymus, which leads to immune deficiencies caused by abnormal T cell development [16, 17]. The characteristic skeletal defects of these patients involve the cranium, jaw, palate, and other facial defects [18]. These structural irregularities can be the root of somatic dysfunctions, and osteopathic consideration should be given to these patients to enhance their bodily function.
B cell defects caused by STAT3 mutations in Hyper-IgE have also been associated with skeletal issues. Hyper-IgE syndrome can be caused by numerous mutations, but the autosomal dominant STAT3 mutation is the most prevalent [19]. The normal triad of symptoms is eczema rashes, cold skin abscesses, and recurrent sinopulmonary infections [19]. There are many nonimmunologic clinical features of this disease including characteristic facies, hyperextensibility, and scoliosis [19].
Generalized bone growth defects has been associated with immunological aberrancies. Cartilage-hair hypoplasia (CHH) is a rare disorder caused by a mutation in the RNA component of the mitochondrial RNA processing endonuclease (RMRP) RNA gene causing immune deficiencies in both cellular and humoral immunity [20]. In addition to immune dysregulation, CHH presents with growth defects, scoliosis, lumbar lordosis, thin hair, and cranial growth abnormalities [21].
Other immunodeficiencies associated with skeletal abnormalities are described elsewhere, but individual discussion is beyond the scope of this article [3]. Skeletal features found in immunodeficient patients, such as the one describe with PIK3R1 mutation, suggest that there may be additive effects on PID immunological responses.
Conclusions
Although manipulation of the skeleton has been shown not to influence the immune system, its ability to act as a fulcrum or motion, to allow flow of effector cells through lymph and its internal microenvironment, for immune development points to the importance of a full osteopathic structural examination in patients with PIDs [22]. We have described the first concise osteopathic examination of an immunodeficient patient with a PIK3R1 mutation and a review of musculoskeletal abnormalities found in more common PIDs. Our study and review of the literature does not completely identify all of the PIDs that have an associated skeletal disease due to the lack of attention to the skeletal system in these disorders.
Acknowledgments
The authors thank Brain Degenhardt, DO (Director of DO-Touch.NET and A.T. Still Research Institute of A.T. Still University) for his protocol recommendations on evaluating the patient with the DO-Touch.NET Physical Examination and Treatment Form.
-
Research funding: None reported.
-
Author contribution: All authors provided substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; M.A.C. drafted the article or revised it critically for important intellectual content; R.W.H. authors gave final approval of the version of the article to be published; and all authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
-
Competing interests: None reported.
-
Informed consent: Written and verbal informed consent was obtained from the patient for the Osteopathic Structural Clinical Examination and educational presentation of deidentified patient information and images.
References
1. Seffinger, MA. Foundations of osteopathic medicine. Philosophy, science, clinical applications and research. Philadelphia, PA: Wolters Kluwer; 2018.Search in Google Scholar
2. Guder, C, Gravius, S, Burger, C, Wirtz, DC, Schildberg, FA. Osteoimmunology: a current update of the interplay between bone and the immune system. Front Immunol 2020;11:57. https://doi.org/10.3389/fimmu.2020.00058.Search in Google Scholar PubMed PubMed Central
3. Gharib, A, Gupta, S. Skeletal and joint manifestations of primary immunodeficiency diseases. SOJ Immunology 2016;4:1–13. https://doi.org/10.15226/2372-0948/4/1/00145.Search in Google Scholar
4. Sanan, N, Hostoffer, RW. Posterior cranial structural abnormality (arnold chiari malformation) associated with PIK3R1 mutation [abstract]. San Francisco, CA: American Academy of Allergy, Asthma and Immunology Annual Meeting; 2018. Available from: https://www.uhhospitals.org/-/media/Files/Medical-Education/Pediatric-and-Adults-Allergy-Immunology-Fellowship/a-pik3r1-mutation-associated-with-a-posterior-cranial-structural-abnormality.pdf.Search in Google Scholar
5. Rowane, MP, Evans, P. Basic musculoskeletal manipulation skills: the 15-minute office encounter, 2nd ed. Indianapolis, IN: American Academy of Osteopathy; 2019.Search in Google Scholar
6. Degenhardt, BF, Johnson, JC, Gross, SR, Hagan, C, Lund, G, Curry, WJ, et al.. Preliminary findings on the use of osteopathic manipulative treatment: outcomes during the formation of the practice-based research network, DO-Touch.NET. J Am Osteopath Assoc 2014;114:154–70. https://doi.org/10.7556/jaoa.2014.033.Search in Google Scholar PubMed
7. Chikly, BJ. Manual Techniques addressing the lymphatic system: origins and development. J Am Osteopath Assoc 2005;105:457–64. https://doi.org/10.7556/jaoa.2005.105.10.457.Search in Google Scholar
8. Ratech, H, Greco, MA, Gallo, G, Rimoin, DL, Kamino, H, Hirschhorn, R. Pathologic findings in adenosine deaminase-deficient severe combined immunodeficiency. I. Kidney, adrenal, and chondro-osseus tissue alterations. Am J Pathol 1985;120:157–69.Search in Google Scholar
9. Sun, L, Zhang, Q, Li, Q, Tang, Y, Wang, Y, Li, X, et al.. A novel PIK3R1 mutation of SHORT syndrome in a Chienes female with diffuse thyroid disease: a case report and review of literature. BMC Med Genet 2020;215. https://doi.org/10.1186/s12881-020-01146-3.Search in Google Scholar PubMed PubMed Central
10. Minagawa, H, Miyamura, T, Hashii, Y, Kanegane, H, Ozono, K. Skeletal dysplasia in adenosine deaminase deficiency. Pediatr Int 2022;64:e15214. https://doi.org/10.1111/ped.15214.Search in Google Scholar PubMed
11. Tsilifis, C, Freeman, AF, Gennery, AR. STAT3 hyper-IgE syndrome – an update and unanswered questions. J Clin Immunol 2021;41:864–80. https://doi.org/10.1007/s10875-021-01051-1.Search in Google Scholar PubMed PubMed Central
12. Hwa, V. STAT5B deficiency: impacts on human growth and immunity. Growth Hormone IGF Res 2016;28:16–20. https://doi.org/10.1016/j.ghir.2015.12.006.Search in Google Scholar PubMed PubMed Central
13. Riley, PJr, Weiner, DS, Leighley, B, Jonah, D, Morton, DH, Strauss, KA, et al.. Cartilage hair hypoplasia: characteristics and orthopaedic manifestations. J Child Orthop 2015;9:145–52. https://doi.org/10.1007/s11832-015-0646-z.Search in Google Scholar PubMed PubMed Central
14. Sauer, AV, Mrak, E, Hernandez, RJ, Zacchi, E, Cavani, F, Casiraghi, M, et al.. ADA-deficient SCID is associated with a specific microenvironment and bone phenotype characterized by RANKL/OPG imbalance and osteoblast insufficiency. Blood 2009;114:3216–26. https://doi.org/10.1182/blood-2009-03-209221.Search in Google Scholar PubMed
15. Holland, SM, Rosenzweig, SD, Schumacher, RF, Notarangelo, LD. Chapter 78: immunodeficiencies. In: Cohen, J, Powderly, WG, Opal, SM, editors. Infectious diseases, 4th ed. Saunders, Elsevier; 2017:705–22 pp.10.1016/B978-0-7020-6285-8.00078-2Search in Google Scholar
16. Funato, N, Nakamura, M, Richardson, JA, Srivastava, D, Yangisawa, H. Loss of tbx1 induced bone phenotypes similar to cleidocranial dysplasia. Hum Mol Genet 2015;24:424–35. https://doi.org/10.1093/hmg/ddu458.Search in Google Scholar PubMed
17. Lackey, AE, Muzio, MR. DiGeorge syndrome. Treasure Island (FL): StatPearls Publishing; 2022.Search in Google Scholar
18. Funato, N. Craniofacial phenotypes and genetics of DiGeorge syndrome. J Dev Biol 2022;10:18. https://doi.org/10.3390/jdb10020018.Search in Google Scholar PubMed PubMed Central
19. Sowerwine, KJ, Holland, SM, Freeman, AF. Hyper-IgE syndrome update. Ann N Y Acad Sci 2012;1250:25–32. https://doi.org/10.1111/j.1749-6632.2011.06387.x.Search in Google Scholar PubMed PubMed Central
20. Rohr, J, Alvarews, C, Niebur, HB, Patel, S, Velasco, D, Pirruccello, S. Partial recapitulation of fetal thymic T-cell constitution postnatally in a patient with cartilage hair hypoplasia-anauxetic dysplasia spectrum disorder: a case report. Cytometry B Clin Cytometry 2021;102. https://doi.org/10.1002/cyto.b.22009.Search in Google Scholar PubMed
21. Arponen, H, Evälahti, M, Mäkitie, O. Craniofacial and craniocervical features in cartilage-hair hypoplasia: a radiological study of 17 patients and 34 controls. Front Endocrinol 2021;12:741548. https://doi.org/10.3389/fendo.2021.741548.Search in Google Scholar PubMed PubMed Central
22. Kawchuk, G, Goertz, CM, Axen, I, Descarreaux, M. The effect of spinal adjustment/manipulation on immunity and the immune system: a rapid review of relevant literature. World Federation of Chiropractic; 2020.Search in Google Scholar
© 2023 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- General
- Review Article
- Glucocorticoid use in rheumatoid arthritis patients and the onset of pneumonia: a systematic review and meta-analysis
- Medical Education
- Original Article
- Barriers to research opportunities among osteopathic medical students
- Musculoskeletal Medicine and Pain
- Case Report
- Structural abnormalities and osteopathic considerations in primary immunodeficiencies
- Neuromusculoskeletal Medicine (OMT)
- Original Article
- Does the osteopathic pedal pump reduce lower limb volume in healthy subjects?
- Neuromusculoskeletal Medicine (OMT)
- Clinical Practice
- Enabling health potential: exploring nonlinear and complex results of osteopathic manual medicine through complex systems theory
- Pediatrics
- Review Article
- Person-centered language and pediatric ADHD research: a cross-sectional examination of stigmatizing language within medical literature
- Clinical Image
- Superficial spreading melanoma within a nevus spilus
Articles in the same Issue
- Frontmatter
- General
- Review Article
- Glucocorticoid use in rheumatoid arthritis patients and the onset of pneumonia: a systematic review and meta-analysis
- Medical Education
- Original Article
- Barriers to research opportunities among osteopathic medical students
- Musculoskeletal Medicine and Pain
- Case Report
- Structural abnormalities and osteopathic considerations in primary immunodeficiencies
- Neuromusculoskeletal Medicine (OMT)
- Original Article
- Does the osteopathic pedal pump reduce lower limb volume in healthy subjects?
- Neuromusculoskeletal Medicine (OMT)
- Clinical Practice
- Enabling health potential: exploring nonlinear and complex results of osteopathic manual medicine through complex systems theory
- Pediatrics
- Review Article
- Person-centered language and pediatric ADHD research: a cross-sectional examination of stigmatizing language within medical literature
- Clinical Image
- Superficial spreading melanoma within a nevus spilus

