Abstract
Objectives
Medicinal leeches have long been recognized for the bioactive compounds present in their saliva. These compounds have been of interest due to their potential therapeutic properties. This research aimed to explore the impact of both medicinal leech application and the application of medicinal leech saliva extract on wound healing in a rat model with a dorsal random flap in vivo.
Methods
In this in vivo study, a dorsal random skin flap model was created in female Wistar albino rats. Rats were randomly assigned to three groups: control (flap only), medicinal leech therapy (MLT), and leech saliva extract (LSE) injection. Histological, immunohistochemical (VEGF), and ELISA-based biochemical analyses were performed to assess wound healing parameters on postoperative day 7.
Results
The flap necrosis area (%) in Group II and Group III was significantly lower than the control group (p<0.05). Vascular Endothelial Growth Factor (VEGF) (+) cell (%), neovascularisation, epithelial regeneration, and granulation tissue thickness in Group II and Group III were significantly higher than the control group (p<0.05). Also, inflammatory cells in group III were substantially lower than in the control group (p<0.05).
Conclusions
To our knowledge, this study is the first in the literature to examine the effect of medicinal leech extract injection in the flap model. These findings emphasize the potential therapeutic benefits of medicinal leeches and their saliva extract in promoting efficient wound healing, with implications for future clinical applications.
Introduction
Wounds, characterized by skin or tissue integrity disruption, often result from accidents, trauma, or surgery, leading to structural and functional deterioration [1]. Wound healing unfolds through successive, overlapping phases – homeostasis, inflammation, proliferation, and remodelling. Initiated after tissue injury, physiological wound healing establishes a sophisticated signalling network among different cell types and skin compartments [2]. In addressing such wounds caused by tumour removal, burns, or cuts, dermatological surgery frequently employs skin flaps, which consist of skin and subcutaneous tissue with a robust vascular supply [3]. Sometimes, applying these flaps becomes necessary to treat and promote the healing of such complex wounds effectively. Despite their widespread use, random skin flaps, especially in distal regions, are prone to necrosis, posing a risk of flap loss. Consequently, research globally focuses on methods to reduce necrosis rates and enhance flap survival, with complementary and supportive treatments at the forefront of investigation [4], 5].
Medicinal Leech Therapy (MLT), also known as hirudotherapy, has been employed for centuries to treat various diseases [6]. Leech saliva contains over a hundred bioactive peptides and proteins, exhibiting anti-coagulant, anti-microbial, anti-inflammatory, and analgesic effects [7]. The therapeutic efficacy of medicinal leeches is primarily attributed to the rich biochemical composition of their secretions [8]. Hirudin, a potent thrombin inhibitor, plays a key role in anticoagulation, while other molecules such as calins inhibit platelet aggregation; bdellins and eglins act as protease inhibitors with anti-inflammatory potential. Additionally, hyaluronidase facilitates tissue permeability, destabilase contributes to fibrin degradation, and acetylcholine and histamine-like substances cause vasodilation [9]. MLT is recognized for its effectiveness in increasing perfusion and promoting rapid wound healing. Numerous experimental animal studies in the literature have explored the impact of medicinal leech application on wound healing [10], 11].
Although previous studies have explored the use of medicinal leeches in various wound models, there is a notable lack of research directly evaluating the effects of isolated leech saliva extract (LSE) in an in vivo flap model. Therefore, the main aim of this study is to investigate and compare the therapeutic effects of both medicinal leech therapy (MLT) and direct subcutaneous injection of LSE on skin flap survival and wound healing. By integrating histopathological, immunohistochemical (VEGF), and biochemical analyses, this study seeks to provide novel insight into the in vivo efficacy of leech saliva constituents. To the best of our knowledge, this is the first study to assess LSE in a random skin flap model in rats.
Materials and methods
Animal experiments
This study was approved by the Gazi University Animal Experiments Local Ethics Committee (Approval no: G.Ü. ET – 22.081). Experimental procedures involving animals were conducted at Gazi University – Laboratory Animal Breeding and Experimental Research Centre (GUDAM, Ankara), adhering to ethical standards outlined in the “Guidelines for the Care and Use of Laboratory Animals”. Female rats (Rattus norvegicus albinus) with an average body weight of 250 ± 30 g were utilized for this investigation. Throughout the experiment, the rats were provided with a standard pellet diet and had unrestricted access to water, maintaining ad libitum conditions.
Experimental design
In this experimental study, 18 animals were randomly assigned to three groups, each comprising six animals (Figure 1). It is important to note that an unfortunate incident occurred during the experiments, resulting in two deaths in the Medicinal Leech Therapy (MLT) and Medicinal Leech Saliva Extract (LSE) experimental groups.
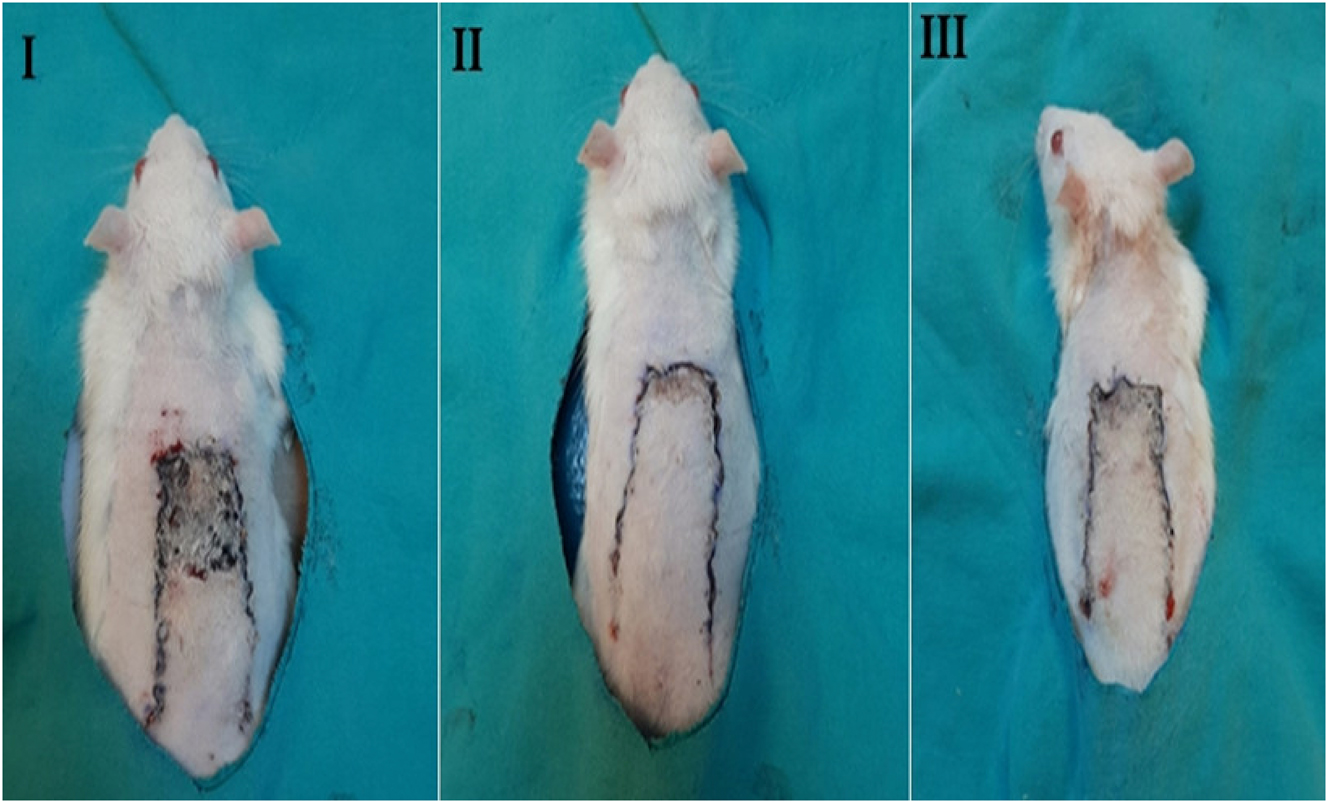
Quantitative comparison of flap necrosis area (%) among the three experimental groups on postoperative day 7. Group I (control): flap surgery without treatment. Group II (MLT): treated with medicinal leeches applied to the flap center. Group III (LSE): treated with subcutaneous injection of leech saliva extract.
On the seventh post-operative day, rats were euthanized through intra-cardiac blood collection under anaesthesia. Dorsal skin flaps were excised proximally and distally around the suture line for histopathological evaluation. Some tissues were preserved in containers with 10 % buffered formaldehyde, while the remaining samples were frozen in liquid nitrogen for biochemical analysis and stored at −80 °C. A homogeniser with a metal blade was used for the tissue homogenization. It was performed to obtain homogenates from the proximal and distal tissues of the flap areas in the experimental groups (Heidolph, SilentCrusher).
Random flap model
All rats were anesthetized under appropriate conditions by intraperitoneal injection of ketamine (80 mg/kg) and xylazine (10 mg/kg). After anaesthesia, the hair of the rats was shaved with a razor. The surgical operation was performed under aseptic conditions under general anaesthesia. The dorsal random flap model was applied to all rats in all groups. After the anatomical borders of the flaps (6 × 2 cm) were determined, the random dorsal flap was elevated over the deep muscle fascia. All flaps were then repositioned in their original positions with simple sutures.
Obtaining leech saliva extract and the content of leech saliva
The Mediterranean medicinal leech, Hirudo verbana Carena, 1820 (Clitellata, Hirudinea, Hirudo), was used in this study [12]. The leeches were sourced from an approved sterile leech farm in Isparta, Turkiye. The species and origin of the leeches were confirmed using a stereo zoom microscope (Euromex NZ.1903-S, Germany) on established morphological criteria of the medicinal leeches [13], 14]. Leech saliva collection involved feeding the leeches with a phagostimulant solution containing 0.15 M NaCl and 1 mM arginine. The saliva was obtained by squeezing the leeches from the posterior end forward immediately after feeding. This method, modified by Baskova et al., is commonly used in the literature [15]. The collected saliva underwent filtration through a 0.22 μm filter and was then divided into Eppendorf tubes. The extract was stored at −20 °C in a light-protected manner. The total protein content of the Medicinal Leech Saliva Extract (LSE) was determined using the Bradford method (Bradford Protein Assay Kit, ABP Biosciences, USA), revealing a 50 μg/mL concentration in our study [16].
Application of the medicinal leeches and LSE
In group I (control), only a flap operation was performed without leech saliva extract or medicinal leech treatment.
In group II (medicinal leech treatment), sterile leeches were applied to the flap centres. The weights of the leeches were recorded before and after the application (they sucked 0.5 mL of blood on average). The leeches were euthanized with 90 % ethanol after feeding.
In group III (LSE treatment), 0.5 mL of LSE was subcutaneously injected at the flap centres after the flap operation.
Skin flap survival
All rats in all groups were photographed on the seventh post-operative day. Then, all the images obtained with the camera in all groups were examined. Necrotic and surviving flap areas in all images were analysed using the ImageJ v1.0 (Oracle Corporation, USA) computer program. The calculated necrosis area was divided by the total flap area, and this ratio was multiplied by 100 and expressed as “Necrosis Rate (%)”: [Necrosis Area Ratio (%) = (Necrosis Area/Total Area) * 100].
Histopathological procedures
Histopathological studies were conducted at Gazi University, Faculty of Medicine, Department of Histology and Embryology Laboratories. Samples underwent fixation in a 10 % neutral formaldehyde solution for 72 h, followed by dehydration and embedding in molten paraffin. Paraffin blocks were sectioned into 4–5 µm thickness, and histomorphological changes were analysed using Haematoxylin-Eosin (H & E) and Masson’s trichrome staining methods under a computer-aided imaging system light microscopy (Leica DM4000, Germany). Micromorphologically examined areas were evaluated by taking photographs in the Leica QWin version 3.0 (Leica Microsystems, Switzerland). Scoring was made after histopathological procedures [17].
Immunohistochemical procedures
Immunohistochemistry procedures for VEGF were performed using a rabbit polyclonal anti-VEGF antibody (Cat: 114409 Lot: 05310, Fine Test, Wuhan, China). Sections (5 μm) were incubated at 60 °C overnight, dewaxed in xylene for 30 min, and passed through a decreasing alcohol series. Tissue sections were arrayed on an immunohistochemistry bar, drawn with PAP-Pen (Thermo Scientific), and washed with PBS (Phosphate Buffer Saline, pH: 7.4). Samples were treated with a serum-blocking solution for 10 min, followed by overnight incubation with the VEGF primary antibody at +4 °C. After incubation, a 3 % hydrogen peroxide solution was applied to inhibit endogenous peroxidase activity, followed by washing with PBS for 15 min to prevent non-specific binding. A secondary antibody with biotin was then applied, and the samples were washed with PBS for 9 min. Chromogen-containing diaminobenzidine (DAB) substrate was applied until a visible immune reaction occurred. Mayer’s haematoxylin was used as a background dye, and the samples were dehydrated, cleared in xylol, and covered with a coverslip using entellan. The samples were photographed with Leica QWin version 3.0 (Leica Microsystems, Switzerland). The uptake of the antibodies indicated in the cell counts provided in 10 independent fields selected for each slide was evaluated over one hundred cells. Scoring was made for immunohistochemical evaluation [18].
ELISA
The biochemical studies were conducted at Gazi University, Faculty of Medicine Department of Medical Biochemistry Laboratory. Serum or tissue IGF-1, TGF-α, and HIF-1-α were measured using original commercial enzyme-linked immunosorbent assay (ELISA) kits (ELK Biotechnology Co., Ltd., Wuhan, China). The working principle of the mentioned kit is based on sandwich enzyme immunoassay.
Statistical analysis
The data obtained from the study were statistically evaluated with the IBM SPSS 21 (IBM SPSS Inc., Chicago, IL) program. Data were expressed as mean and SEM (standard error of the mean). Variance among groups was evaluated using two-factor ANOVA, single-factor ANOVA, followed by post–hoc Tukey’s range test for multiple comparisons. The groups were also compared to find the source of the statistically significant difference. All analyses were accepted at a 95 % confidence interval and 0.05 significance level, and values below this value were considered statistically significant.
Results
MLT and LSE application increased the flap survival rate by decreasing the rate of necrosis in the flap area
There was a statistically significant difference between the groups regarding necrosis area (%) (p=0.028). The flap necrosis area (%) in the MLT-treated rats (12.04 ± 0.90) and LSE–treated rats (16.25 ± 0.92) were found to be significantly lower than the control group (Figure 1). Although the flap necrosis area (%) in the LSE–treated rats was lower than in the MLT–treated rats, no statistically significant difference was detected between these two groups (Figure 2). It was determined that the rate of necrosis area (%) was the highest in the control group (respectively *p=0.026, 0.046).
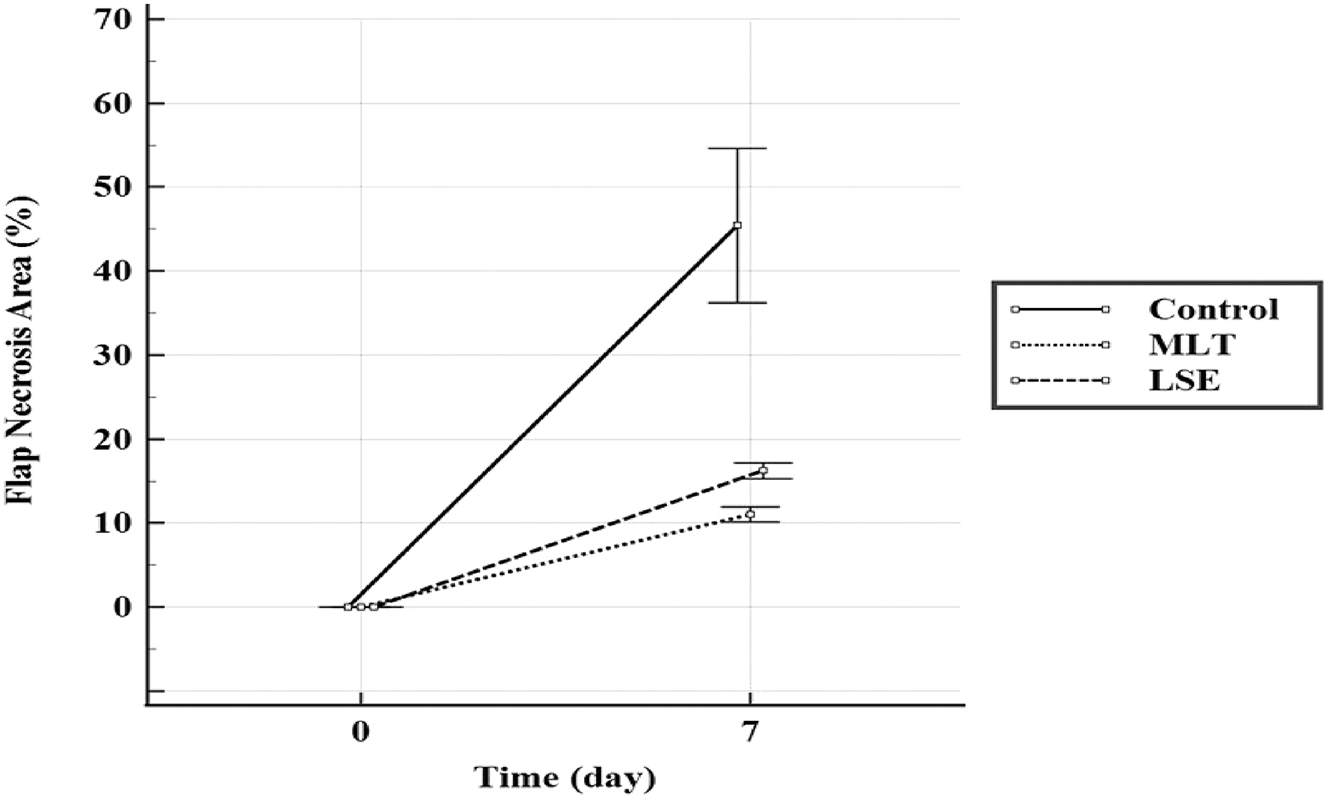
Comparison of flap necrosis area in the experimental groups.
LSE accelerated wound healing in rats with a dorsal random flap model
Histological assessments of the flaps involved an evaluation of both proximal and distal regions, considering parameters such as epithelial regeneration, granulation tissue thickness, fibroblast proliferation, neovascularization, and the presence of inflammatory cells. Immunohistochemical analysis was also conducted, explicitly assessing the percentage of positive Vascular Endothelial Growth Factor (VEGF) cells (Tables 1 and 2). Haematoxylin & Eosin and Masson’s trichrome staining methods were employed for comprehensive histopathological examinations, providing insights into tissue morphology, cellular composition, and the progression of regenerative processes within the flaps’ proximal and distal segments.
Comparison of histopathological and immunohistochemical parameters in proximal flap tissue among experimental groups. The parameters include epithelial regeneration, granulation tissue thickness, fibroblast proliferation, neovascularization, presence of inflammatory cells, and percentage of VEGF-positive cells.
| Parameter | Group I | Group II | Group III | p-Value |
|---|---|---|---|---|
| Epithelial regeneration | 2.25 ± 0.25 | 3.25 ± 0.25 | 3.75 ± 0.25 | 0.013 |
| Granulation tissue thickness | 1.75 ± 0.25 | 2.50 ± 0.29 | 3.25 ± 0.48 | 0.023 |
| Fibroblast proliferation | 2.00 | 2.75 ± 0.48 | 3.25 ± 0.25 | 0.138 |
| Neovascularisation | 1.50 ± 0.29 | 3.00 | 3.50 ± 0.29 | 0.0001 |
| Inflammatory cell presence | 3.00 ± 0.41 | 2.25 ± 0.25 | 1.50 ± 0.29 | 0.025 |
| VEGF (+) cells (%) | 42.25 ± 1.25 | 67.00 ± 1.96 | 74.25 ± 2.17 | 0.0001 |
-
Values are expressed as mean ± standard deviation. Group I: control; group II: medicinal leech therapy (MLT); group III: leech saliva extract (LSE). p-Values in bold indicate statistical significance at the α=0.05 level.
Comparison of histopathological and immunohistochemical parameters in distal flap tissue among experimental groups. The parameters include epithelial regeneration, granulation tissue thickness, fibroblast proliferation, neovascularization, presence of inflammatory cells, and percentage of VEGF-positive cells.
| Parameter | Group I | Group II | Group III | p-Value |
|---|---|---|---|---|
| Epithelial regeneration | 1.75 ± 0.25 | 3.00 | 3.50 ± 0.29 | 0.001 |
| Granulation tissue thickness | 1.50 ± 0.29 | 2.25 ± 0.25 | 2.75 ± 0.25 | 0.007 |
| Fibroblast proliferation | 1.75 ± 0.25 | 2.50 ± 0.29 | 3.00 ± 0.41 | 0.070 |
| Neovascularisation | 1.25 ± 0.25 | 2.50 ± 0.29 | 3.25 ± 0.25 | 0.0001 |
| Inflammatory cell presence | 3.25 ± 0.48 | 2.50 ± 0.29 | 2.00 ± 0.41 | 0.146 |
| VEGF (+) cells (%) | 38.50 ± 1.55 | 63.25 ± 1.38 | 69.00 ± 1.68 | 0.0001 |
-
Values are expressed as mean ± standard deviation. Group I: control; group II: medicinal leech therapy (MLT); group III: leech saliva extract (LSE). p-Values in bold indicate statistical significance at the α=0.05 level.
Histological evaluation of flap tissues for epithelial regeneration and granulation formation
In this study, comprehensive histological evaluations were undertaken to investigate the impact of MLT and LSE applications on cellular wound repair mechanisms in rats with a random flap model (Figure 3). Notably, in the proximal flap tissue, rats treated with LSE exhibited a significant enhancement in epithelial regeneration and the development of well-formed granulation tissue compared to the control group (p<0.05). Furthermore, in the distal flap tissue, rats subjected to both MLT and LSE treatments demonstrated a noteworthy increase in epithelial regeneration and the presence of robust granulation tissue when compared to the control group (p<0.05). These findings highlight the positive effects of both MLT and LSE in promoting epithelial regeneration and facilitating the formation of well-developed granulation tissue.
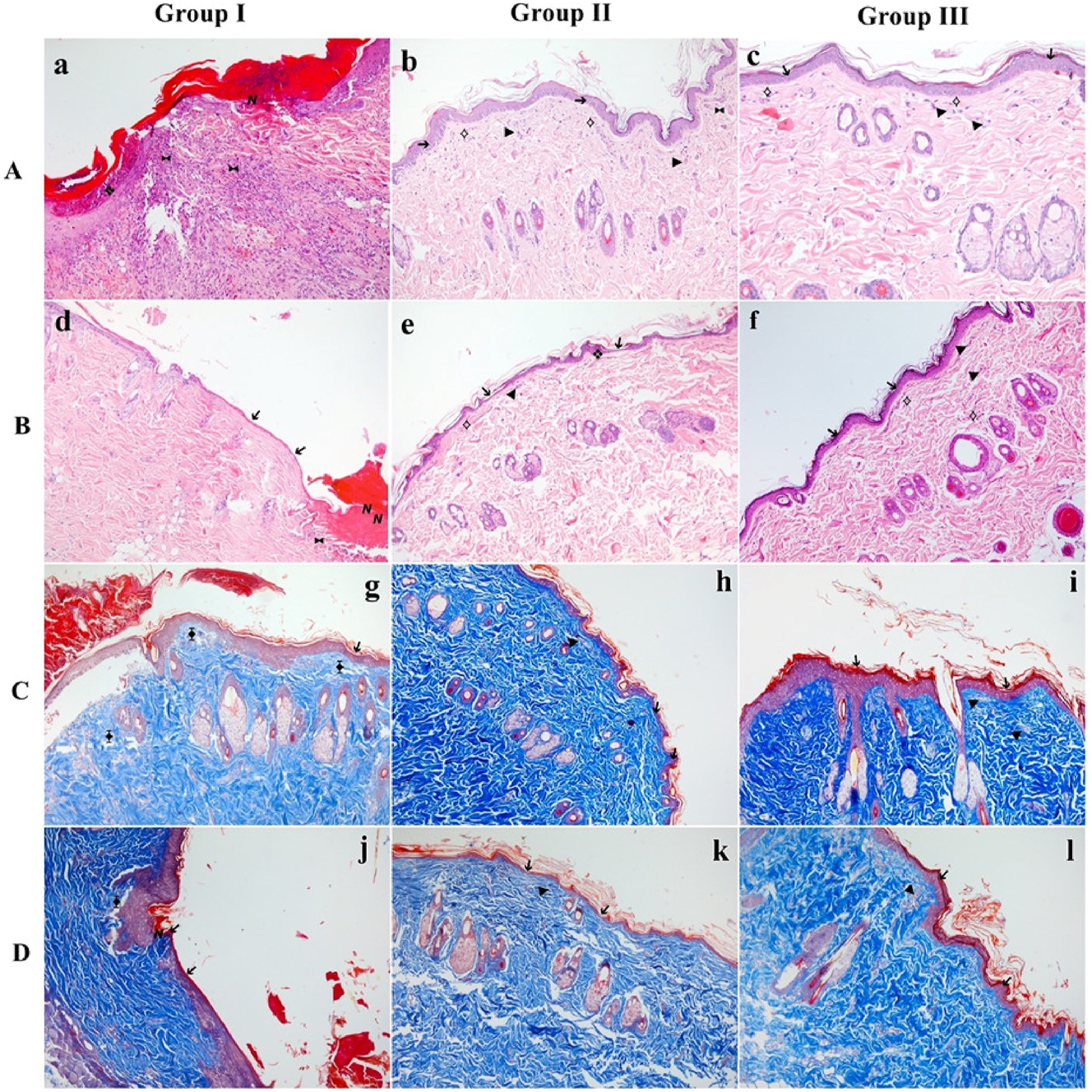
Representative histological images of flap tissues from each group. (A, B) Sections stained with Hematoxylin & Eosin (H&E) to assess epithelial structure, granulation, inflammatory infiltration, and neovascularization. (C, D) Sections stained with Masson’s trichrome to visualize collagen deposition and connective tissue organization. (A, C) Proximal regions of the flap. (B, D) Distal regions of the flap. Arrows and symbols indicate key histological findings: ➔ epithelial regeneration, ❖ granulation tissue, ⟡ fibroblast proliferation, ► neovascularization, ⧓ presence of inflammatory cells, ⧱ decreased collagen density, Ν necrosis. Group I: control; group II: MLT; group III: LSE.
Histological analysis of flap tissues for neovascularization and presence of inflammatory cells
MLT and LSE-treated rats showed an increasing neovascularisation for both proximal and distal flap tissue compared with the control group (p<0.05) (Figure 3A and B). Also, LSE caused a decrease in the presence of inflammatory cells in proximal flap tissue compared with the control group (p<0.05). In the distal flap tissue, there was no statistically significant difference between the experimental groups regarding the presence of inflammatory cells.
Histological analysis of flap tissues for fibroblast proliferation and collagen density
In the proximal and distal flap tissue, there was no statistically significant difference between the experimental groups regarding fibroblast proliferation (p>0.05). It was observed that the collagen density in the control group was lower than in the other groups. However, no statistically significant difference was found between the experimental groups (p>0.05) (Figure 3C and D).
MLT and LSE increases VEGF (+) cell (%) in the flap area
MLT and LSE-treated rats showed an increase in VEGF (+) cells (%) for both proximal and distal flap tissue when compared with the control group (p<0.05) (Figure 4A and B). Although the VEGF (+) cell (%) was observed at the highest level in group III, no significant difference was detected between groups II and III (p<0.05).
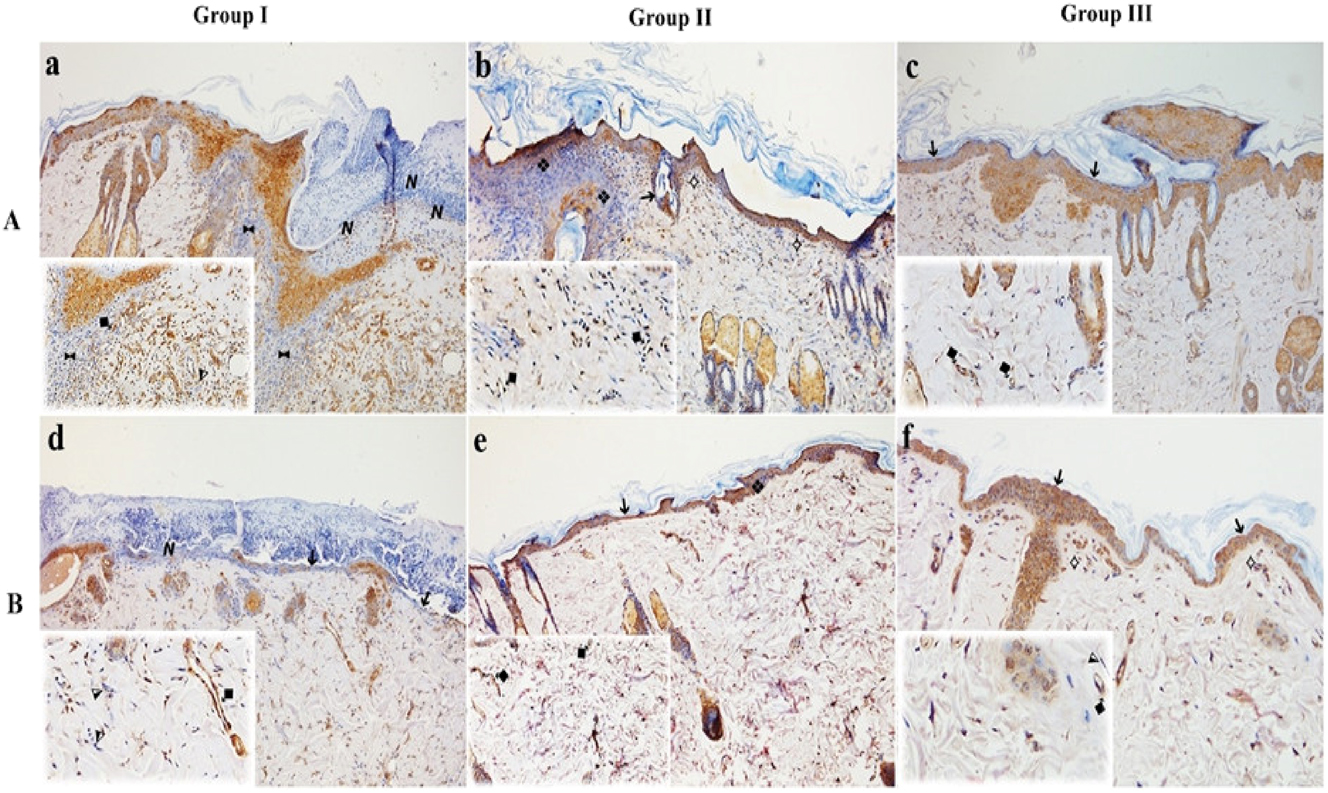
Immunohistochemical analysis for VEGF expression in proximal (A) and distal (B) flap tissue. VEGF-positive endothelial cells are indicated by ⧪, and VEGF-negative cells by ⧨. Increased VEGF staining is evident in groups II and III, reflecting enhanced angiogenic activity. Group I: control; group II: MLT; group III: LSE.
No difference was observed in terms of biochemical markers
There was no statistically significant difference in serum samples, proximal and distal tissue samples between the experimental groups in terms of HIF-1-α (ng/mL), TGF-α (pg/mL), and IGF-1 (ng/mL) concentrations (p<0.05).
Discussion
Today, MLT and products obtained from medicinal leeches are used worldwide. The mechanism of action of medicinal leech saliva, which contains more than 100 bioactive substances, is still a matter of curiosity, and various scientific studies are carried out on medicinal leeches and medicinal leech saliva. This study aimed to investigate the effects of MLT and LSE on wound healing in the flap area via biochemical and histological methods. In our research, sterile medicinal leeches of H. verbana were used.
In the initial stage, the flap necrosis area was compared to the total flap area, revealing that the control group had the highest necrosis area (%). However, Groups II and III, treated with medicinal leech and medicinal leech extract, exhibited significantly lower necrosis areas compared to the control group. These results imply that the application of medicinal leech and its extract increased flap survival rates by reducing necrosis area (%). Anti-coagulant substances in medicinal leech saliva, such as hirudin and destabilase, and vasodilator substances, like acetylcholine, enhance blood flow. Therefore, our study suggests that the decreased flap necrosis area (%) and the improved flap survival rates are attributed to the enhanced blood flow in the treated areas [19].
In the second stage of our study, histopathological evaluation of tissue samples revealed that Groups II and III, treated with medicinal leech and its extract, exhibited significantly higher epithelial regeneration, granulation tissue thickness, and neovascularization compared to the control group, with Group III showing the most pronounced effects. These results indicate a positive contribution of LSE to the wound-healing process. Notably, the low inflammatory cell count in the LSE-treated group suggests a potential anti-inflammatory effect.
Similar positive effects of MLT on wound healing were observed in studies by Darestani et al. [20] and Zakian et al. [21], where MLT promotes faster wound closure and reduces inflammation. In another study by Mousavi et al., MLT demonstrated efficacy in reducing inflammatory cells, necrosis, and tissue damage in a rat model of acute venous congestion attributed to anti-inflammatory components in medicinal leech saliva [22]. Consistent with these findings, Bilden et al. also reported improved healing outcomes in rats with incisional wounds following MLT application, highlighting the broad therapeutic potential of leech therapy in different wound models [23].
Contrasting our study design, Schlaudraff et al. investigated MLT’s effects on flap survival in a different model, exposing random flaps to ischemia and applying H. medicinalis [24]. Their findings suggested that multi-session leech application might not be suitable in cases of insufficient arterial support. In our research, the improved flap survival and wound healing in both MLT and LSE-treated groups could be linked to adequate arterial support.
In summary, our study and previous research highlight the positive impact of medicinal leech and its extract on various aspects of wound healing, including epithelial regeneration, tissue thickness, neovascularization, and anti-inflammatory effects.
In the third phase of our study, we assessed the percentage of VEGF-positive cells in proximal and distal tissue samples using immunohistochemistry. VEGF plays a crucial role in wound healing, promoting angiogenesis by stimulating endothelial cell activities. Unlike most tissues, areas with active angiogenesis, such as the skin, ovaries, and uterus, exhibit increased VEGF levels during wound healing [25], 26].
In a study by Yingxin et al., the impact of natural hirudin, obtained from leech secretion, on VEGF gene expression in flap-modelled rats was investigated. The study revealed a significant increase in VEGF gene expression with natural hirudin compared to control and recombinant hirudin groups, improving flap survival by reducing necrosis [27]. In contrast to their PCR-based gene expression evaluation, our study focused on VEGF-positive cell percentages. Compared to the control group, it increased proximal and distal flap tissue in rats treated with Medicinal Leech Therapy (MLT) and Medicinal Leech Extract (LSE).
Hirudin, a potent natural thrombin inhibitor found in medicinal leech secretion, is believed to enhance VEGF levels, which are crucial for angiogenesis. Additionally, the literature suggests in vitro studies showing that LSE increases VEGF expression. In a study by Ünal et al., LSE’s effects on a healthy cell line, HUVEC, demonstrated increased VEGF gene expression and cell migration without inducing apoptosis or necrosis [28]. Consistent with these findings, a recent study by Ünal et al. further reported that lyophilized leech saliva extract exerted anti-proliferative effects on breast cancer cells while promoting migration in healthy endothelial cells, supporting its dual potential in cancer and wound healing contexts [29].
Our study consistently found elevated VEGF-positive cell counts, enhanced epithelial regeneration, and increased neovascularization. These findings indicate that the applied methods in our flap model positively influence the wound-healing process, promoting the formation of new blood vessels.
In the last stage of our study, serum samples were taken from all animals in all groups. In addition, tissue homogenates were obtained by taking tissue samples from the proximal and distal parts of the flap regions. HIF-1-α, TGF-α, and IGF-1 parameters were analyzed by ELISA test from all these samples. However, no statistically significant differences in these growth factor concentrations were observed among the experimental groups.
HIF-1-α, TGF-α, and IGF-1 play crucial roles in different stages of wound healing, including proliferation and angiogenesis, with mitogenic effects. HIF-1-α, particularly important for cell survival under hypoxic conditions, regulates various aspects of wound healing, such as cell division, growth factor release, and matrix synthesis. TGF-α, a key growth factor in routine healing, is highly expressed in skin tissue. At the same time, IGF-1 acts as a chemotactic agent for endothelial cells and promotes fibroblast and keratinocyte proliferation and migration [30], [31], [32], [33]. In the intricate orchestration of wound healing, HIF-1-α and TGF-α emerge as pivotal players, steering the early stages of the reparative journey. Yet, within this biological tapestry, the roles of TGF-β and the orchestrated motility of macrophages weave additional threads into the narrative of recovery [34], 35]. It remains crucial to underscore that the landscape of wound healing is not a static tableau but rather a dynamic, intricate process. Despite significant strides in understanding, the precise mechanisms governing this complex biological phenomenon are still under scrutiny in ongoing scientific exploration.
Our study is distinctive in the literature, representing the first instance of using H. verbana leeches and direct leech saliva injection under in vivo conditions. The findings suggest that medicinal leech extract application can be an effective agent for wound healing. Our research significantly contributes to the existing literature by exploring the in vivo application of leech saliva extract and examining a variety of parameters in experimental animals.
While our findings offer promising evidence supporting the use of medicinal leech therapy and its extract in wound healing, there are several limitations to consider. First, the study involved a relatively small sample size, and two animal losses during the experiment reduced statistical power. Second, although histological and immunohistochemical analyses were performed, molecular investigations (e.g., VEGF mRNA expression, angiogenic gene panels) were not included. Third, the dose of 0.5 mL LSE at 50 μg/mL protein concentration was chosen to assess the effects of the crude extract. The optimal concentration has not yet been established, as protein content can vary considerably depending on the individual leech and collection season. Furthermore, the exact concentration and individual effects of the active compounds in LSE were not isolated or quantified. Future studies should incorporate proteomic profiling of LSE, explore dose-dependent responses, and examine longer-term outcomes. Additionally, translational research into human tissue models and clinical settings may pave the way for therapeutic applications of LSE in reconstructive surgery.
Funding source: Gazi University
Acknowledgments
We thank the Department of Medical Biochemistry and the Department of Histology-Embryology of Gazi University.
-
Research ethics: This study was approved by the Gazi University Animal Experiments Local Ethics Committee (Approval no: G.Ü. ET – 22.081).
-
Informed consent: Not applicable.
-
Author contributions: K.Ü. and M.E.E. conceptualized the study; K.Ü. curated the data. Formal analysis was performed by K.Ü., M.E.E., and D.D.; H.A. identified and supplied the leeches. Investigation was conducted by K.Ü., M.E.E., and D.D.; Methodology was developed by K.Ü., M.E.E., D.D., and E.D.; Plastic-reconstructive procedures were carried out by E.D. Project administration was led by K.Ü.; Resources were provided by K.Ü., D.D., H.A., E.D., and K.F.; Supervision was performed by K.Ü. Visualization was handled by M.E.E. and D.D.; K.Ü. and M.E.E. wrote the original draft. K.Ü., M.E.E., and D.D. reviewed and edited the manuscript.
-
Use of LLM, AI and MLT: We acknowledge the assistance of artificial intelligence tool Grammarly (Grammarly, Inc., San Francisco, USA) for improving the language of this manuscript.
-
Conflict of interest: The authors state no conflcit of interest.
-
Research funding: This study received financial support from Gazi University – Scientific Research Projects (BAP) Coordination Unit with Project ID: TYL-2022-8029.
-
Data availability: Data and material are available upon reasonable request from the corresponding author.
References
1. George, BI, Janis, JE, Attinger, CE. The basic science of wound healing. Plast Reconstr Surg 2006;117:12S–34S. https://doi.org/10.1097/01.prs.0000225430.42531.c2.Search in Google Scholar PubMed
2. Biermann, JS, Siegel, G. Orthopaedic knowledge Update®: musculoskeletal tumors 4. Philadelphia: Lippincott Williams & Wilkins; 2020.Search in Google Scholar
3. Chilukuri, S, Leffell, DJ. Basic principles in flap reconstruction. In: Flaps and grafts in dermatologic surgery. Philadelphia, PA: Elsevier; 2007:15–29 pp.10.1016/B978-1-4160-0316-8.50007-7Search in Google Scholar
4. Yun, IS, Kim, YS, Roh, TS, Lee, WJ, Park, TH, Roh, H, et al.. The effect of red ginseng extract intake on ischemic flaps. J Invest Surg 2017;30:19–25. https://doi.org/10.1080/08941939.2016.1215577.Search in Google Scholar PubMed
5. Kirkil, C, Yigit, MV, Özercan, IH, Aygen, E, Gültürk, B, Artas, G. The effect of ozonated olive oil on neovascularizatıon in an experimental skin flap model. Adv Skin Wound Care 2016;29:322–7. https://doi.org/10.1097/01.asw.0000484172.04260.46.Search in Google Scholar PubMed
6. Koeppen, D, Aurich, M, Pasalar, M, Rampp, T. Medicinal leech therapy in venous congestion and various ulcer forms: perspectives of Western, Persian and Indian medicine. J Tradit Complementary Med 2020;10:104–9. https://doi.org/10.1016/j.jtcme.2019.08.003.Search in Google Scholar PubMed PubMed Central
7. Nair, HK, Ahmad, NW, Lee, HL, Ahmad, N, Othamn, S, Mokhtar, NSHM, et al.. Hirudotherapy in wound healing. Int J Low Extrem Wounds 2022;21:425–31. https://doi.org/10.1177/1534734620948299.Search in Google Scholar PubMed
8. Cooper, EL, Mologne, N. Exploiting leech saliva to treat osteoarthritis: a provocative perspective. J Tradit Complementary Med 2017;7:367–9. https://doi.org/10.1016/j.jtcme.2016.11.005.Search in Google Scholar PubMed PubMed Central
9. Sig, AK, Guney, M, Uskudar Guclu, A, Ozmen, E. Medicinal leech therapy–an overall perspective. Integr Med Res 2017;6:337–43. https://doi.org/10.1016/j.imr.2017.08.001.Search in Google Scholar PubMed PubMed Central
10. Lee, C, Mehran, RJ, Lessard, M-L, Kerrigan, CL. Leeches: controlled trial in venous compromised rat epigastric flaps. Br J Plast Surg 1992;45:235–8.10.1016/0007-1226(92)90085-CSearch in Google Scholar PubMed
11. Lozano, DD, Stephenson, LL, Zamboni, WA. Effect of hyperbaric oxygen and medicinal leeching on survival of axial skin flaps subjected to total venous occlusion. Plast Reconstr Surg 1999;104:1029–32. https://doi.org/10.1097/00006534-199909040-00019.Search in Google Scholar PubMed
12. Tessler, M, de Carle, D, Voiklis, ML, Gresham, OA, Neumann, JS, Cios, S, et al.. Worms that suck: phylogenetic analysis of Hirudinea solidifies the position of acanthobdellida and necessitates the dissolution of rhynchobdellida. Mol Phylogenet Evol 2018;127:129–34. https://doi.org/10.1016/j.ympev.2018.05.001.Search in Google Scholar PubMed
13. Davies, RW, Govedich, FR. Annelida: euhirudinea and acanthobdellidae. In: Ecology and classification of North American freshwater invertebrates. San Diego, CA: Academic Press; 2001, 2:465–504 pp.10.1016/B978-012690647-9/50014-4Search in Google Scholar
14. Nesemann, H, Neubert, E. Bd. 6/2: Annelida, Clitellata: Branchiobdellida, Acanthobdellea, Hirudinea. Heidelberg, Germany: Spektrum; 1999:187 p.Search in Google Scholar
15. Baskova, I, Zavalova, L, Basanova, A, Sass, A. Separation of monomerizing and lysozyme activities of destabilase from medicinal leech salivary gland secretion. Biochemistry 2001;66:1368–73.10.1023/A:1013333829196Search in Google Scholar PubMed
16. Bradford, MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;72:248–54. https://doi.org/10.1006/abio.1976.9999.Search in Google Scholar
17. Tan, WS, Arulselvan, P, Ng, S-F, Mat Taib, CN, Sarian, MN, Fakurazi, S. Improvement of diabetic wound healing by topical application of Vicenin-2 hydrocolloid film on Sprague Dawley rats. BMC Compl Alternative Med 2019;19:1–16. https://doi.org/10.1186/s12906-018-2427-y.Search in Google Scholar PubMed PubMed Central
18. Mao, Y, Zhang, D-W, Wen, J, Cao, Q, Chen, R-J, Zhu, J, et al.. A novel LMP1 antibody synergizes with mitomycin C to inhibit nasopharyngeal carcinoma growth in vivo through inducing apoptosis and downregulating vascular endothelial growth factor. Int J Mol Sci 2012;13:2208–18. https://doi.org/10.3390/ijms13022208.Search in Google Scholar PubMed PubMed Central
19. Ünal, K, Erol, ME, Ayhan, H. Literature review on the effectiveness of medicinal leech therapy in the wound healing. Ankara Med J 2023;23:151–64. https://doi.org/10.5505/amj.2023.20280.Search in Google Scholar
20. Darestani, KD, Mirghazanfari, SM, Moghaddam, KG, Hejazi, S. Leech therapy for linear incisional skin-wound healing in rats. J Acupunct Meridian Stud 2014;7:194–201. https://doi.org/10.1016/j.jams.2014.01.001.Search in Google Scholar PubMed
21. Zakian, A, Ahmadi, HA, Keleshteri, MH, Madani, A, Tehrani-Sharif, M, Rezaie, A, et al.. Study on the effect of medicinal leech therapy (Hirudo medicinalis) on full-thickness excisional wound healing in the animal model. Res Vet Sci 2022;153:153–68. https://doi.org/10.1016/j.rvsc.2022.10.015.Search in Google Scholar PubMed
22. Mousavi, SA, Ghasemi, M, Mousavi, SJ, Mousavi Darka, SS, Bagheri, V. Comparison of leeching and heparin therapy in management of acute venous congestion of limbs in rat. Pharm Biomed Res 2016;2:25–30. https://doi.org/10.18869/acadpub.pbr.2.3.25.Search in Google Scholar
23. Bilden, A, Kara, Ö, Kahraman, M, Çağlayan, N, Çiçek, M. Efficiency of medical leech on experimentally induced incisional wound healing in rats. J Complementary Integr Med 2025;22:288. https://doi.org/10.1515/jcim-2024-0351.Search in Google Scholar PubMed
24. Schlaudraff, KU, Bezzola, T, Montandon, D, Pepper, MS, Pittet, B. Mixed arterio-venous insufficiency in random skin flaps in the rat: is the application of medicinal leeches beneficial? J Surg Res 2008;150:85–91. https://doi.org/10.1016/j.jss.2008.01.012.Search in Google Scholar PubMed
25. Yaniz-Galende, E, Hajjar, R. Stem cell and gene therapy for cardiac regeneration. In: Cardiac regeneration and repair. Cambridge, UK: Woodhead Publishing; 2014:347–79 pp.10.1533/9780857096708.4.347Search in Google Scholar
26. Laurent, GJ, Shapiro, SD. Encyclopedia of respiratory medicine. San Diego, CA: Academic Press Elsevier; 2006.Search in Google Scholar
27. Yingxin, G, Guoqian, Y, Jiaquan, L, Han, X. Effects of natural and recombinant hirudin on VEGF expression and random skin flap survival in a venous congested rat model. Int Surg 2013;98:82–7. https://doi.org/10.9738/cc171.1.Search in Google Scholar PubMed PubMed Central
28. Ünal, K, Tirik, N, Erol, ME, İbrahİmkhanli, L, Elçİ, MP, Ayhan, H. The investigation of effects of medicinal leech saliva extract on the breast fibroblast cell line in vitro: an experimental study. J Tradit Med Complementary Ther 2023;6:142. https://doi.org/10.5336/jtracom.2022-92875.Search in Google Scholar
29. Ünal, K, Tirik, N, Erol, ME, Güngörmüş, M, Ayhan, H. Effects of lyophilized leech saliva extract on cell migration and apoptosis in MDA-MB-231 breast cancer and HUVEC cell lines. Jordan J Biol Sci 2025;18:9.10.54319/jjbs/180102Search in Google Scholar
30. Hong, WX, Hu, MS, Esquivel, M, Liang, GY, Rennert, RC, McArdle, A, et al.. The role of hypoxia-inducible factor in wound healing. Adv Wound Care 2014;3:390–9. https://doi.org/10.1089/wound.2013.0520.Search in Google Scholar PubMed PubMed Central
31. Sinno, H, Prakash, S. Complements and the wound healing cascade: an updated review. Plast Surg Int 2013;2013:146764. https://doi.org/10.1155/2013/146764.Search in Google Scholar PubMed PubMed Central
32. Schultz, G, Clark, W, Rotatori, DS. EGF and TGF‐α in wound healing and repair. J Cell Biochem 1991;45:346–52. https://doi.org/10.1002/jcb.240450407.Search in Google Scholar PubMed
33. Garoufalia, Z, Papadopetraki, A, Karatza, E, Vardakostas, D, Philippou, A, Kouraklis, G, et al.. Insulin-like growth factor-I and wound healing, a potential answer to non-healing wounds: a systematic review of the literature and future perspectives. Biomed Rep 2021;15:1–5. https://doi.org/10.3892/br.2021.1442.Search in Google Scholar PubMed PubMed Central
34. Yamakawa, S, Hayashida, K. Advances in surgical applications of growth factors for wound healing. Burns Trauma 2019;7:10. https://doi.org/10.1186/s41038-019-0148-1.Search in Google Scholar PubMed PubMed Central
35. Hutami, IR, Izawa, T, Khurel-Ochir, T, Sakamaki, T, Iwasa, A, Tanaka, E. Macrophage motility in wound healing is regulated by HIF-1α via S1P signaling. Int J Mol Sci 2021;22:8992. https://doi.org/10.3390/ijms22168992.Search in Google Scholar PubMed PubMed Central
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Reviews
- Efficacy of mangosteen as local drug delivery for improving periodontal health in adults with periodontitis: a systematic review and meta-analysis of randomized controlled trials
- Catechins in cancer therapy: integrating traditional and complementary approaches
- Phytochemical innovations in oral cancer therapy: targeting oncogenic pathways with natural compounds
- Opinion Paper
- Naturopathy and the Ottawa Charter: a synergistic model for community health promotion in rural India
- Research Articles
- Investigating the therapeutic potential of medical leech and leech saliva extract in flap survival: an in vivo study using rats
- Alstonia boonei stem bark aqueous extract ameliorates elevated parasitemia levels, normalises blood glucose, modulates inflammatory biomarkers and enhances antioxidant status in Plasmodium berghei-infected/diabetic mice
- Nutritional, antioxidant, enzyme inhibitory and toxicity assessments of an herbal formulation using in vitro, ex vivo, and in vivo approaches
- Molecular docking, molecular dynamic simulation, and ADME analysis of Moringa oleifera phytochemicals targeting NS5 protein: towards the development of novel anti-dengue therapeutics
- Impact of licorice supplementation on cardiac biomarkers and histomorphological changes in rats
- Effects of minas frescal cheese enriched with Lactobacillus acidophilus La-05 on bone health in a preclinical model of chronic kidney disease
- Teratogenic effect of unregistered traditional Chinese medicine containing Atractylodis lancea radix, Glycyrrhiza glabra radix, Rheum officinale rhizome, and Angelica dahurica radix on fetal morphology of BALB/c mice
- Network pharmacology based investigation of the multi target mechanisms of Murraya koenigii (curry leaves) in non-alcoholic steatohepatitis (NASH)
- Protective effects of citronellol on Escherichia coli septicemia-derived liver lesions in Wistar rats
- The effects of spilanthol (SA3X) supplementation on muscle size and strength in healthy men – a randomized parallel-group placebo-controlled trial
- Navigating complementary and alternative medicine use, medication adherence, and herb–drug interaction risks among gout patients: a multicenter cross-sectional study in indonesia
- Evaluation of photobiomodulation for modulating peripheral inflammation via the lumbosacral medullary region
- Traditional alternative and complementary medicine: a review of undergraduate courses and curricula in Peru
- Congress Abstracts
- Natural Health Products Research Society of Canada Natural Health Products and Cancer Mini-Symposium 2025
Articles in the same Issue
- Frontmatter
- Reviews
- Efficacy of mangosteen as local drug delivery for improving periodontal health in adults with periodontitis: a systematic review and meta-analysis of randomized controlled trials
- Catechins in cancer therapy: integrating traditional and complementary approaches
- Phytochemical innovations in oral cancer therapy: targeting oncogenic pathways with natural compounds
- Opinion Paper
- Naturopathy and the Ottawa Charter: a synergistic model for community health promotion in rural India
- Research Articles
- Investigating the therapeutic potential of medical leech and leech saliva extract in flap survival: an in vivo study using rats
- Alstonia boonei stem bark aqueous extract ameliorates elevated parasitemia levels, normalises blood glucose, modulates inflammatory biomarkers and enhances antioxidant status in Plasmodium berghei-infected/diabetic mice
- Nutritional, antioxidant, enzyme inhibitory and toxicity assessments of an herbal formulation using in vitro, ex vivo, and in vivo approaches
- Molecular docking, molecular dynamic simulation, and ADME analysis of Moringa oleifera phytochemicals targeting NS5 protein: towards the development of novel anti-dengue therapeutics
- Impact of licorice supplementation on cardiac biomarkers and histomorphological changes in rats
- Effects of minas frescal cheese enriched with Lactobacillus acidophilus La-05 on bone health in a preclinical model of chronic kidney disease
- Teratogenic effect of unregistered traditional Chinese medicine containing Atractylodis lancea radix, Glycyrrhiza glabra radix, Rheum officinale rhizome, and Angelica dahurica radix on fetal morphology of BALB/c mice
- Network pharmacology based investigation of the multi target mechanisms of Murraya koenigii (curry leaves) in non-alcoholic steatohepatitis (NASH)
- Protective effects of citronellol on Escherichia coli septicemia-derived liver lesions in Wistar rats
- The effects of spilanthol (SA3X) supplementation on muscle size and strength in healthy men – a randomized parallel-group placebo-controlled trial
- Navigating complementary and alternative medicine use, medication adherence, and herb–drug interaction risks among gout patients: a multicenter cross-sectional study in indonesia
- Evaluation of photobiomodulation for modulating peripheral inflammation via the lumbosacral medullary region
- Traditional alternative and complementary medicine: a review of undergraduate courses and curricula in Peru
- Congress Abstracts
- Natural Health Products Research Society of Canada Natural Health Products and Cancer Mini-Symposium 2025

