Serum markers for early detection of patients with mesenteric ischemia after cardiac surgery
-
Daniel-Sebastian Dohle
, Carolin Bestendonk
Abstract
Objective
Mesenteric ischemia (MESI) is a rare but often fatal complication in patients after cardiac surgery. Non-specific clinical symptoms and lack of specific laboratory parameters complicate the diagnosis. We evaluated potential serum markers for MESI in cardiac surgery patients.
Methods
Between March and October 2012, serial serum samples of 567 elective cardiac surgery patients were collected 1, 24, and 48 h after the operation, and concentrations of potential markers for MESI [α-glutathione-S-transferase (αGST), intestinal fatty-acid-binding protein (iFABP), and D-lactate] were measured retrospectively. In patients requiring laparotomy, blood samples obtained 72, 48, 24, and 12 h before the laparotomy were additionally measured and compared to all other patients (control group).
Results
Laparotomy was performed in 18 patients at 11±7 days after cardiac surgery. MESI was found in 9/18 patients. Already 1 h after cardiac surgery, the serum concentrations of D-lactate (37±18 vs. 25±20 nmol/mL, p<0.01) and αGST (82±126 vs. 727±1382 μg/L, p<0.01) in patients undergoing laparotomy were increased compared to the control group. Between patients with and without MESI, differences were only found for iFABP 24 h after cardiac surgery (1.1±0.4 vs. 2.9±0.6 ng/mL, p=0.04) and up to 72 h before laparotomy (0.56±0.72 vs. 2.51±1.96 ng/mL, p=0.01).
Conclusions
D-lactate and αGST were early markers for gastrointestinal complications after cardiac surgery. Before laparotomy, lowered iFABP levels indicated MESI. Routinely used, these markers can help identify patients with gastrointestinal complications after cardiac surgery early, and might be useful for the evaluation of new therapeutic or preventive strategies.
Introduction
Mesenteric ischemia (MESI) is a rare complication in patients after cardiac surgery, with an incidence of 0.1–0.5% and high mortality rates between 60% and 100% [1], [2], [3].
The pathophysiological mechanisms for MESI after cardiac surgery are multimodal. It is well known that cardiopulmonary bypass (CPB) causes systemic inflammation [4], reduction of intestinal perfusion [5], microcirculatory alterations, loss of intestinal barrier function [6], and hemolysis [7]. However, postoperatively low cardiac output, intravascular volume deficiency, and the need for vasopressors restrict mesenteric perfusion. Unfortunately, mesenteric malperfusion itself is a trigger of all the above-mentioned factors, resulting in a circulus vitiosus process.
The diagnosis of MESI after cardiac surgery is difficult in the setting of mostly sedated, intubated patients, with low cardiac output, systemic inflammatory response syndrome, need for vasopressors, and usually many differential diagnoses for acute abdomen. Early diagnosis and surgical treatment have been suggested to be the only beneficial factors for the prognosis of these patients [2, 8, 9]. Recent publications indicated that different biomarkers have some impact on the detection of MESI [10], [11], [12]. Indeed, α-glutathione-S-transferase (αGST), intestinal fatty-acid-binding protein 2 (iFABP-2), and D-lactate are the most promising biomarkers for the early and accurate detection of MESI. These biomarkers are explained in detail below.
First, the GSTs are a family of enzymes involved in the detoxification of a range of toxic and foreign compounds within the cell by conjugation to glutathione, although different isoforms exist, such as α, π, θ, and μ, which are distributed in relatively specific organs [13]. For example, αGST is a unique biomarker for hepatic and intestinal injuries.
Second, FABPs display a high affinity for long-chain fatty acids and seem to function in metabolism and intracellular transport of lipids. Three distinct FABPs are known: liver FABP, iFABP, and ileal lipid binding protein distributed proximally to distally in the intestine. The iFABP is detectable along the entire length of the small intestine and is maximally represented near the medial segment [14].
Finally, D-lactate and L-lactate result from the reduction of pyruvate by D- and L-lactate dehydrogenases, respectively. These enzymes uniquely exist in bacteria or mammals, respectively. Therefore, the quantification of D-lactate has been revealed as a marker for bacterial translocation, which follows intestinal or colonic mucosal injury caused by ischemia or other reasons [15].
Therefore, the aims of this study were (i) to quantify and monitor in a time-dependent manner these markers in patients after cardiac surgery and (ii) to evaluate their potential in identifying patients at a risk for MESI.
Methods
Patient population and characteristics
Between March and October 2012, a total of 567 elective consecutive cardiac surgery patients were included in this study. Institutional Ethics Committee review and approval for this prospective study were obtained (12-5066-BO), and patients gave written informed consent. Patient-specific parameters were stored anonymized in a project-specific internal database. The mean age was 67±12 years, and 393/567 (69%) of the patients were male. Of the 567 patients, 495 (87.3%) were operated using CPB. The procedures performed with and without CPB, and the comorbidities are listed in Table 1.
Procedures and comorbidities of patients operated with and without cardiopulmonary bypass (CPB), coronary artery bypass grafting (CABG), aortic valve repair/replacement (AVR), mitral valve repair/replacement (MVR), ventricular assist device (VAD), off-pump coronary artery bypass grafting (OPCAB), transcatheter aortic valve replacement (TAVR), arterial hypertonus (aHTN), diabetes mellitus (DM), chronic obstructive pulmonary disease (COPD), and chronic atrial fibrillation (Chron. aFib).
| Operative procedures and comorbidities | Number (%), 567 (100) | |
|---|---|---|
| With CPB | ||
| 495 | (87.3) | |
| AVR | 35 | (6.2) |
| CABG | 242 | (42.7) |
| MVR | 25 | (4.4) |
| Aortic | 21 | (3.7) |
| Combination | 154 | (27.2) |
| VAD/transplant | 16 | (2.8) |
| Others | 12 | (2.1) |
| Without CBP | ||
| 72 | (12.7) | |
| OPCAB | 5 | (0.9) |
| TAVR | 40 | (7.1) |
| Others | 17 | (3.0) |
| Comorbidities | ||
| aHTN | 483 | (85.2) |
| DM | 162 | (28.6) |
| COPD | 152 | (26.8) |
| Active smoking | 107 | (18.9) |
| pAVD | 94 | (16.6) |
| BMI >30 | 150 | (26.5) |
| NYHA ≥3 | 458 | (80.8) |
| Chron. aFib | 87 | (15.3) |
| Previous stroke | 64 | (11.3) |
| Dialysis | 32 | (5.6) |
BMI, body mass index; NYHA, New York Heart Association; pAVD, peripheral arterial vascular disease.
Study protocol and analyzed serum samples
The remaining volume of all routinely taken blood samples in the intensive care unit (ICU) from all included cardiac surgery patients was aliquoted and stored at −80 °C directly after measurement of the routine parameters, which avoided the need for additional blood sampling in the interest of the patient. Patients were routinely treated according to the internal ICU protocols. Due to the high technical effort and considerable costs for the enzyme-linked immunosorbent assay (ELISA) and enzyme assays, αGST, iFABP-2, and D-lactate were measured retrospectively at the end of the study period in all available blood samples taken 1, 24, and 48 h after the cardiac surgery. In patients with laparotomy blood samples obtained 72, 48, 24, and 12 h before laparotomy, the markers were additionally measured retrospectively. Patients with laparotomy with and without MESI were compared to all other patients, defined as the control group.
In 57/567 (10%) patients, no serum samples were taken within the first postoperative hour; 65/567 patients were discharged from the ICU within the first 24 h, and 254/567 (45%) patients within the first 48 h. Serum samples taken 1, 24, and 48 h after cardiac surgery in 510 (90%), 502 (89%), and 313 (55%) patients, respectively, and up to 72 h before laparotomy in 18 patients were analyzed.
Biomarkers
The serum concentrations of iFABP-2 and αGST were analyzed using a commercially available ELISA kit (iFABP-2: Hölzel-Biotech, Köln, Germany; αGST: Otto Nordwald GmbH, Hamburg, Germany) according to the manufacturer’s recommendations. D-lactate was measured by a colorimetric enzyme assay (BioCat Biovision, Heidelberg, Germany) according to the manufacturer’s recommendations.
Data analysis and statistics
Data were collected prospectively and analyzed retrospectively. Statistical analysis and figures were done using GraphPad Prism version 7.0a for Mac (GraphPad Software, La Jolla, CA, USA) and Stata statistical software (version 11.2; StataCorp, College Station, TX, USA). Due to the small group size, continuous variables were compared by the Wilcoxon signed-rank test or t-test, with the α level set at 0.05 for statistical significance.
Results
MESI after cardiac surgery is associated with high mortality
Laparotomy due to expected MESI or clinical signs of an acute abdomen was performed in 18/567 (3.2%) patients after 11±7 days of cardiac surgery. The indication for laparotomy was primarily given based on clinical signs of an acute abdomen, continuous hyperlactatemia, or increasing need for vasopressors. Additional computed tomography imaging without evidence of any occlusive mesenteric malperfusion was performed in 7/18 patients. Angiography was performed in one patient. In 9/18 (50%) patients, MESI was found in the ischemic mesenteric segment. The sigma and colon were affected in 7/9 (78%) cases, and the small intestine in 2/9 (22%) cases. Histology revealed transmural necrosis in 5/9 (56%) patients and submucosal necrosis in 4/9 (44%) patients. In patients without resection, no abdominal pathology was found in 5/9 (56%) cases, paralytic ileus in 2/9 (22%) cases, as well as one obscure non-necrotic small intestine perforation and one necrotic gallbladder. Mortality occurred in 8/9 (89%) patients in the laparotomy group with MESI and in 6/9 (67%) patients in the laparotomy group without MESI.
Patients with laparotomy during the postoperative course were found to have significantly more cardiovascular risk factors with a higher rate of diabetes (61% vs. 27%, p=0.002), more smoking (50% vs. 18%, p<0.001), and more peripheral artery disease (61% vs. 15%, p<0.001). Furthermore, patients with laparotomy during the postoperative course had longer bypass times (151±74 vs. 120±55 min, p=0.023) with CPB time >150 min in 67% vs 21% of the patients (p<0.001). The rate of patients with need for inotropic support already before cardiac surgery was higher in the laparotomy group (38.9% vs. 3.5%, p<0.001). Also, postoperative atrial fibrillation (12% vs. 28%, p=0.043) and heart failure (8.6% vs. 39%, p<0.001) were more frequent in patients requiring laparotomy later. Laparotomy patients demonstrated significantly higher gastrointestinal complication scores (GICS) (12.97 vs. 3.13, p<0.001) and EuroSCORE II scores (30.75 vs. 6.6, p<0.001).
Markers after cardiac surgery and before laparotomy
Within the first 48 h after cardiac surgery, significantly higher αGST and D-lactate serum concentrations were found in patients with laparotomy compared to patients without laparotomy. The iFABP serum concentration was significantly lower in the laparotomy group 1 h after cardiac surgery.
Comparing laparotomy patients with and without MESI up to 3 days before laparotomy, no significant differences between these two groups were found for αGST and D-lactate, but the iFABP-2 levels were significant lower in the MESI group.
High αGST levels are associated with laparotomy
Already 1 h after cardiac operation, nearly 10-fold higher αGST concentrations were found in the serum of patients needing a laparotomy 11±7 days later compared to patients without laparotomy. After 24 h, the αGST concentrations in the laparotomy group were >25-fold higher compared to the control group and remained significantly higher after 48 h (Table 2, Figure 1A). However, the differences in αGST serum concentration between the laparotomy patients with and without MESI were not significant within 48 h after cardiac surgery (Table 2, Figure 2A). Up to 3 days before laparotomy, the αGST levels in MESI and non-MESI patients did not differ significantly but were even lower than the initial postoperative levels of the control group (Table 3, Figure 2A).
Mean values±standard deviation (SD) of αGST, D-lactate, and iFABP 1, 24, and 48 h after cardiac surgery for the control group and laparotomy group with subgroup analysis for MESI and non-MESI patients.
| Control, mean±SD (n) | Laparotomy, mean±SD (n) | p-Value | MESI, mean±SD (n) | Non-MESI, mean±SD (n) | p-Value | |
|---|---|---|---|---|---|---|
| αGST (μg/L) | ||||||
| 1 h | 82±126 (492) | 727±1382 (18) | <0.01 | 280±245 (9) | 1173±1884 (9) | 0.35 |
| 24 h | 73±167 (484) | 4549±9889 (18) | <0.01 | 3741±8542 (9) | 1459±3013 (9) | 0.63 |
| 48 h | 83±153 (295) | 2300±5895 (18) | <0.01 | 1010±1710 (9) | 3204 ±8282 (9) | 0.63 |
| D-lactate (nmol/mL) | ||||||
| 1 h | 25.1±20.1 (492) | 37.1±17.9 (18) | <0.01 | 36.3±20.8 (9) | 37.8±15.9 (9) | 0.51 |
| 24 h | 23.2± 21.1 (484) | 52.2±40.5 (18) | <0.01 | 58.7±40.9 (9) | 45.7±41.4 (9) | 0.23 |
| 48 h | 24.8±23.6 (295) | 58.7±54.3 (18) | <0.01 | 56.3±44.6 (9) | 61.1±65.2 (9) | 0.69 |
| iFABP (ng/mL) | ||||||
| 1 h | 4.05±4.04 (492) | 1.04±0.91 (18) | <0.01 | 0.78±0.71 (9) | 1.30±1.05 (9) | 0.29 |
| 24 h | 3.42±4.95 (484) | 2.03±1.86 (18) | 0.38 | 1.15±1.30 (9) | 2.90±1.99 (9) | 0.04 |
| 48 h | 4.28±6.12 (295) | 2.88±2.66 (18) | 0.95 | 1.89±1.78 (9) | 3.87±3.11 (9) | 0.12 |
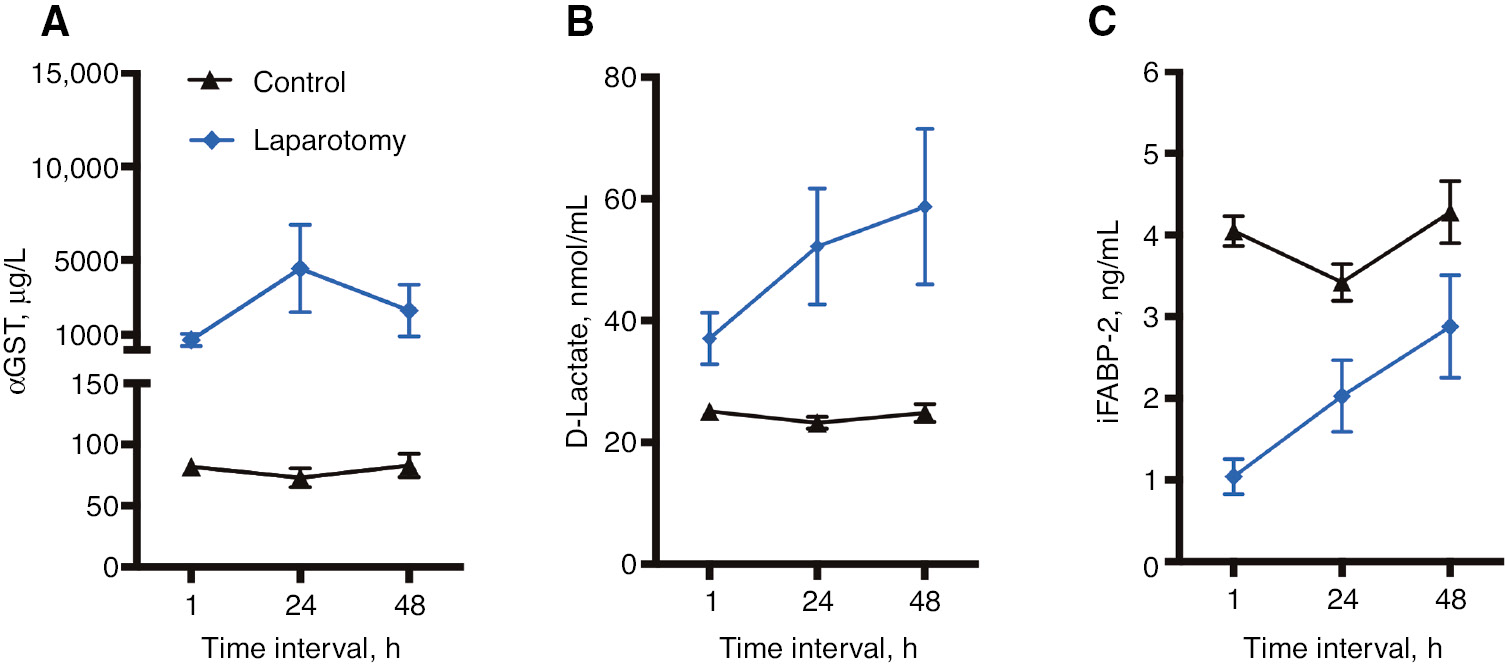
Already 1 h after cardiac surgery and 24 and 48 h thereafter, the serum concentrations of D-lactate and αGST in patients undergoing laparotomy were increased compared to the control group.
Timeline of αGST (A), D-lactate (B), and iFABP (C) mean serum concentrations and 95% confidence interval (CI) at 1, 24, and 48 h after cardiac surgery for the control group and the laparotomy group.
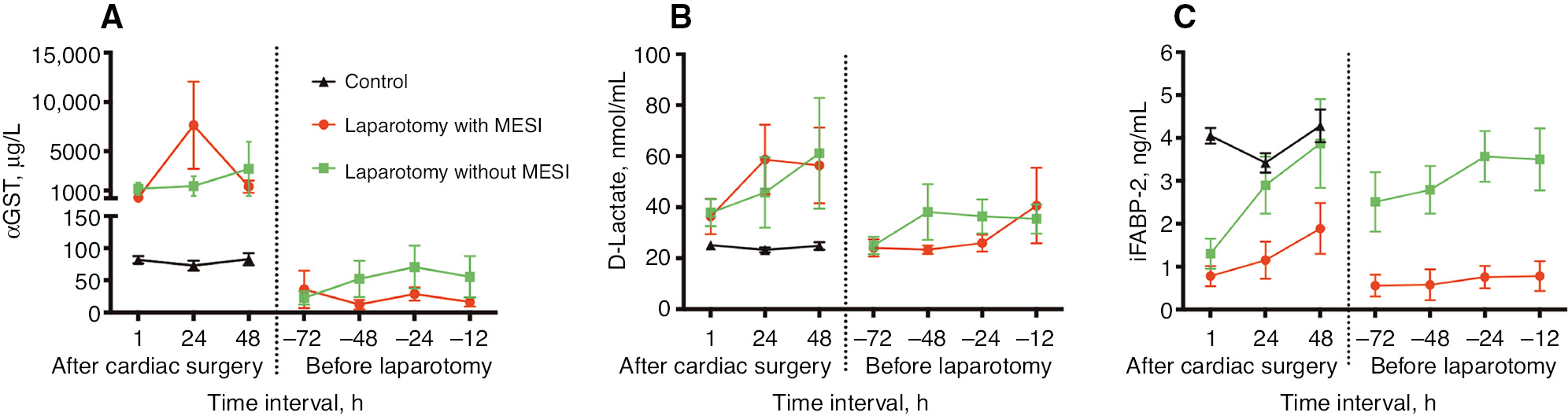
Comparing laparotomy patients with and without MESI up to 3 days before laparotomy, no significant differences between these two groups were found for αGST and D-lactate, but the iFABP-2 levels were significant lower in the MESI group.
Timeline of αGST (A), D-lactate (B), and iFABP (C) mean serum concentrations and 95% CI at 1, 24, and 48 h after cardiac surgery for the control, MESI, and non-MESI group at 1, 24, and 48 h after cardiac surgery and 72, 48, 24, and 12 h before laparotomy.
Mean serum concentration±SD of αGST, D-lactate, and iFABP 72, 48, 24, and 12 h before laparotomy for MESI and non-MESI patients. One patient underwent laparotomy 48 h after cardiac surgery.
| MESI, mean±SD (n) | Non-MESI, mean±SD (n) | p-Value | |
|---|---|---|---|
| αGST (μg/L) | |||
| −72 h | 35.8±82.4 (8) | 22.6±29.1 (8) | 0.40 |
| −48 h | 12.3±20.6 (8) | 52.1±79.8 (8) | 0.29 |
| −24 h | 28.6±30.9 (9) | 70.6±100.5 (9) | 0.55 |
| −12 h | 16.3±21.6 (9) | 55.4±96.7 (9) | 0.77 |
| D-lactate (nmol/mL) | |||
| −72 h | 24.1±9.5 (8) | 24.9±9.6 (8) | 0.83 |
| −48 h | 23.3±4.7 (8) | 38.1±30.9 (8) | 0.34 |
| −24 h | 25.9±9.8 (9) | 36.4±19.9 (9) | 0.09 |
| −12 h | 40.6±41.8 (9) | 35.4±17.1 (9) | 0.63 |
| iFABP (ng/mL) | |||
| −72 h | 0.56±0.72 (8) | 2.51±1.96 (8) | 0.01 |
| −48 h | 0.58±1.02 (8) | 2.79±1.57 (8) | 0.01 |
| −24 h | 0.76±0.78 (9) | 3.57±1.77 (9) | <0.01 |
| −12 h | 0.78±1.04 (9) | 3.50±2.17 (9) | 0.01 |
High D-lactate levels are associated with laparotomy
Significantly higher D-lactate concentrations were found in the laparotomy group compared to patients without laparotomy 1 h after cardiac surgery. Within the next 48 h, these differences amplified and D-lactate levels were more than twice as high in the laparotomy group (Table 2, Figure 1B). No significant differences between the laparotomy patients with and without MESI were found within the first 48 h after cardiac surgery (Table 2, Figure 2B). Before laparotomy, no significant differences between laparotomy patients with and without MESI were found. The measured D-lactate levels 72, 48, 24, and 12 h before laparotomy were comparable to the levels measured in the control group after cardiac surgery (Table 3, Figure 2B).
Low iFABP levels are associated with MESI
Only 1 h after cardiac surgery, serum iFABP concentrations between laparotomy and non-laparotomy patients differed significantly. The iFABP concentrations were lower by a factor of 4 in the laparotomy group. Within the next 48 h, the iFABP concentrations remained lower in the laparotomy group, but not significantly (Table 2, Figure 1C). Laparotomy patients with and without MESI both had similarly low iFABP-2 serum concentrations 1 h after cardiac surgery. After 24 h, MESI patients had significantly lower iFABP serum concentrations. However, the iFABP-2 levels of laparotomy patients without MESI were similar to those of patients without laparotomy after 24 and 48 h (Table 2, Figure 2C). Before laparotomy, significant differences between laparotomy patients with and without MESI were found. Already 72 h before laparotomy, MESI patients had significantly lower iFABP-2 levels compared to laparotomy patients without MESI. These differences remained significant 48, 24, and 12 h before laparotomy (Table 3, Figure 2C).
Discussion
The incidence of MESI after cardiac surgery in the literature varies. In a review with 35 included papers and a total of 151,652 patients, Rodriguez et al. calculated an incidence of 0.16% with 58% mortality [1]. Pang et al. described an even higher incidence (0.31%) and mortality (71%) [2], and retrospective autopsy studies found MESI in 0.49% of the patients, responsible for 11% of all postoperative deaths [3]. Interestingly, 96% of these had a non-occlusive MESI (NOMI), which is interesting regarding the definition and therapy of MESI, which will be addressed later. Our high laparotomy and MESI rate of 1.6% might be the result of a bias during the study period; however, it reflects the severely ill patient population with a mean EuroSCORE I of 11±14%. According to the GICS model introduced by Nilsson et al. [16], the expected MESI rate in our patient population is >1.9%, emphasizing the accuracy of the suggested score and the frailty of the observed patient cohort.
In the setting of cardiac and aortic surgery, some studies focused on αGST, D-lactate, and iFABP, and their elevation during operations with and without CPB [17], [18], [19]. On-pump coronary artery bypass grafting (CABG) patients showed significantly higher serum αGST levels at the end of surgery (10 μg/L) compared to off-pump CABG patients (4 μg/mL), normalizing 24 h after surgery [17]. Although the reported mean values are low compared to our total control group, they are similar to the mean values of an uncomplicated CABG subgroup (6 μg/mL), demonstrating the general impact of CPB on mesenteric perfusion and intestinal barrier function. In another study focusing on intestinal barrier function, Sun et al. found the highest D-lactate concentration (11 nmol/mL) 2 h postoperatively and the highest iFABP concentration (1.4 ng/mL) 6 h postoperatively in aortic valve repair/replacement patients. Both findings and values are conclusive with the results of our control group, again emphasizing damage and loss of intestinal barrier function during any operation using CPB. Also, in patients operated for abdominal aortic aneurysm (AAA), elevated D-lactate levels (33 vs. 11 nmol/mL [20]) were found in patients with histologically diagnosed ischemic colitis 24 and 48 h after open aortic reconstruction. Patients with a fatal intestinal necrosis after AAA repair had increased iFABP levels (0.6 vs. 4 ng/mL) early postoperatively and decreased concentrations on postoperative days 3 and 4 [21].
D-lactate and αGST showed no differences between patients with and without MESI, neither 72, 48, 24, or 12 h before laparotomy nor early after cardiac surgery. However, both markers were significantly increased in the laparotomy group within the first 2 days after cardiac surgery, suggesting a pathologic gastrointestinal process 11±7 days before clinical signs or imaging indicated laparotomy.
Interestingly, in our study, iFABP-2 was decreased in the laparotomy group compared to the control group early after cardiac surgery and increased again within the next 48 h, although the iFABP-2 concentration remained significantly lower in the MESI subgroup compared to the non-MESI subgroup. Before laparotomy, MESI patients had significantly lower iFABP-2 levels compared to non-MESI patients. These findings might be explained by our definition of MESI, which was a necrotic intestine diagnosed by laparotomy. In a human model, Schellekens et al. demonstrated the reduction of tissue and serum iFABP-2 within 120 min reperfusion after up to 60 min ischemia by subtotal destruction of the intestinal enterocytes and villus structures [22]. In histology of the resected intestinal segments in our study, transmural necrosis was found in 5/9 patients, and submucosal necrosis in 4/9 patients. In all specimens, no mucosa that could have released iFABP-2 was found.
The definition of MESI as intestinal ischemia treated by laparotomy and resection is unambiguous and used in most retrospective studies. However, it does not reflect the dynamics of this disease and its pathomechanism, which is non-occlusive (NOMI) in 95% [3] with an incidence after cardiac surgery of up to 10% [23]. We assume an even higher rate of mild and non-apparent MESI after most cardiac operations, demonstrated by elevated markers after CPB in all studies including ours. αGST and D-lactate seem to be suitable markers for early detection of gastrointestinal complications after cardiac surgery, and iFABP-2 could help differentiate patients with ischemia. Especially in high-risk patients with CPB times >150 min, pre- and postoperative need for inotropic support, atrial fibrillation, and high GICS and EuroSCORE, these markers might be used for differentiation. Nevertheless, resection of necrotic tissue is only damage control, and the benefit of a very early resection is questionable. Accepting the pathologic mechanism and the dynamic character, new therapeutic and protective approaches are required. Substances like glycine [24, 25] and pyruvate [26] demonstrated protective effects in MESI models in rats. The evaluated markers could not only be useful for early detection of patients with MESI, but also represent a new routine for evaluation of protective effects of different substances or conditioning maneuvers in further clinical studies.
Dedicated to: the memory of Prof. Dr. Dr. Herbert de Groot who died unexpectedly and much too early on May 10, 2016.
Author Statement
Research funding: We gratefully acknowledge the funding by the German Heart Foundation. Conflict of interest: Authors state no conflict of interest. Informed consent: Informed consent has been obtained from all individuals included in this study. Ethical approval: The research related to human use complies with all the relevant national regulations, institutional policies and was performed in accordance the tenets of the Helsinki Declaration, and has been approved by the authors’ institutional review board or equivalent committee.
Authors Contributions
Study conception and design: DSD, HJ, FP and HdG; Acquisition of data: DSD, CB and KHS; Analysis and interpretation of data: DSD, CB, HdG and AC; Drafting of manuscript: DSD, CB and MW Critical revision: AC, FP, HJ and KT.
Publication Funding
The German Society of Surgery funded the article processing charges of this article.
References
[1] Rodriguez R, Robich MP, Plate JF, Trooskin SZ, Sellke FW. Gastrointestinal complications following cardiac surgery: a comprehensive review. J Card Surg 2010;25:188–97.10.1111/j.1540-8191.2009.00985.xSuche in Google Scholar PubMed
[2] Pang PYK, Sin YK, Lim CH, Su JW, Chua YL. Outcome and survival analysis of intestinal ischaemia following cardiac surgery. Interact Cardiovasc Thorac Surg 2012;15:215–8.10.1093/icvts/ivs181Suche in Google Scholar PubMed PubMed Central
[3] Venkateswaran RV, Charman SC, Goddard M, Large SR. Lethal mesenteric ischaemia after cardiopulmonary bypass: a common complication? Eur J Cardiothorac Surg 2002;22:534–8.10.1016/S1010-7940(02)00373-1Suche in Google Scholar PubMed
[4] Asimakopoulos G. Systemic inflammation and cardiac surgery: an update. Perfusion 2001;16:353–60.10.1177/026765910101600505Suche in Google Scholar PubMed
[5] Ohri SK, Becket J, Brannan J, Keogh BE, Taylor KM. Effects of cardiopulmonary bypass on gut blood flow, oxygen utilization, and intramucosal pH. Ann Thorac Surg 1994;57:1193–9.10.1016/0003-4975(94)91355-2Suche in Google Scholar PubMed
[6] Rossi M, Sganga G, Mazzone M, Valenza V, Guarneri S, Portale G, et al. Cardiopulmonary bypass in man: role of the intestine in a self-limiting inflammatory response with demonstrable bacterial translocation. Ann Thorac Surg 2004;77:612–8.10.1016/S0003-4975(03)01520-0Suche in Google Scholar PubMed
[7] Vermeulen Windsant IC, de Wit NCJ, Sertorio JTC, van Bijnen AA, Ganushchak YM, Heijmans JH, et al. Hemolysis during cardiac surgery is associated with increased intravascular nitric oxide consumption and perioperative kidney and intestinal tissue damage. Front Physiol 2014;5:340.10.3389/fphys.2014.00340Suche in Google Scholar PubMed PubMed Central
[8] Abboud B, Daher R, Sleilaty G, Madi-Jebara S, Asmar El B, Achouch R, et al. Is prompt exploratory laparotomy the best attitude for mesenteric ischemia after cardiac surgery? Interact Cardiovasc Thorac Surg 2008;7:1079–83.10.1510/icvts.2008.176271Suche in Google Scholar PubMed
[9] Ghosh S, Roberts N, Firmin RK, Jameson J, Spyt TJ. Risk factors for intestinal ischaemia in cardiac surgical patients. Eur J Cardiothorac Surg 2002;21:411–6.10.1016/S1010-7940(02)00015-5Suche in Google Scholar PubMed
[10] Evennett NJ, Petrov MS, Mittal A, Windsor JA. Systematic review and pooled estimates for the diagnostic accuracy of serological markers for intestinal ischemia. World J Surg 2009;33:1374–83.10.1007/s00268-009-0074-7Suche in Google Scholar PubMed
[11] Acosta S, Nilsson T. Current status on plasma biomarkers for acute mesenteric ischemia. J Thromb Thrombolysis 2012;33:355–61.10.1007/s11239-011-0660-zSuche in Google Scholar PubMed
[12] Demir IE, Ceyhan GO, Friess H. Beyond lactate: is there a role for serum lactate measurement in diagnosing acute mesenteric ischemia? Dig Surg 2012;29:226–35.10.1159/000338086Suche in Google Scholar PubMed
[13] Mannervik B, Alin P, Guthenberg C, Jensson H, Tahir MK, Warholm M, et al. Identification of three classes of cytosolic glutathione transferase common to several mammalian species: correlation between structural data and enzymatic properties. Proc Natl Acad Sci USA 1985;82:7202–6.10.1073/pnas.82.21.7202Suche in Google Scholar PubMed PubMed Central
[14] Agellon LB, Toth MJ, Thomson ABR. Intracellular lipid binding proteins of the small intestine. Mol Cell Biochem 2002;239:79–82.10.1023/A:1020520521025Suche in Google Scholar PubMed
[15] Deitch EA, Morrison J, Berg R, Specian RD. Effect of hemorrhagic shock on bacterial translocation, intestinal morphology, and intestinal permeability in conventional and antibiotic-decontaminated rats. Crit Care Med 1990;18:529–36.10.1097/00003246-199005000-00014Suche in Google Scholar PubMed
[16] Nilsson J, Hansson E, Andersson B. Intestinal ischemia after cardiac surgery: analysis of a large registry. J Cardiothorac Surg 2013;8:156.10.1186/1749-8090-8-156Suche in Google Scholar PubMed PubMed Central
[17] Yamada T, Ochiai R, Takeda J, Kikuchi H, Ishibashi M, Watanabe K. Off-pump coronary artery bypass attenuates transient hepatocellular damage after myocardial revascularization. J Cardiothorac Vasc Anesth 2005;19:603–7.10.1053/j.jvca.2005.02.004Suche in Google Scholar PubMed
[18] van Boven W-JP, Gerritsen WB, Driessen AH, van Dongen EP, Klautz RJ, Aarts LP. Minimised closed circuit coronary artery bypass grafting in the elderly is associated with lower levels of organ-specific biomarkers: a prospective randomised study. Eur J Anaesthesiol 2013;30:685–94.10.1097/EJA.0b013e328364febfSuche in Google Scholar PubMed
[19] Sun Y-J, Song D-D, Diao Y-G, Zhou J, Zhang T-Z. Penehyclidine hydrochloride preserves the intestinal barrier function in patients undergoing cardiopulmonary bypass. J Thorac Cardiovasc Surg 2013;146:179–85.10.1016/j.jtcvs.2013.01.042Suche in Google Scholar PubMed
[20] Assadian A, Assadian O, Senekowitsch C, Rotter R, Bahrami S, Fürst W, et al. Plasma D-lactate as a potential early marker for colon ischaemia after open aortic reconstruction. Eur J Vasc Endovasc Surg 2006;31:470–4.10.1016/j.ejvs.2005.10.031Suche in Google Scholar PubMed
[21] Vermeulen Windsant IC, Hellenthal FA, Derikx JPM, Prins MH, Buurman WA, Jacobs MJ, et al. Circulating intestinal fatty acid-binding protein as an early marker of intestinal necrosis after aortic surgery: a prospective observational cohort study. Ann Surg 2012;255:796–803.10.1097/SLA.0b013e31824b1e16Suche in Google Scholar PubMed
[22] Schellekens DHSM, Grootjans J, Dello SAWG, van Bijnen AA, van Dam RM, Dejong CHC, et al. Plasma intestinal fatty acid-binding protein levels correlate with morphologic epithelial intestinal damage in a human translational ischemia-reperfusion model. J Clin Gastroenterol 2014;48:253–60.10.1097/MCG.0b013e3182a87e3eSuche in Google Scholar PubMed
[23] Groesdonk HV, Klingele M, Schlempp S, Bomberg H, Schmied W, Minko P, et al. Risk factors for nonocclusive mesenteric ischemia after elective cardiac surgery. J Thorac Cardiovasc Surg 2013;145:1603–10.10.1016/j.jtcvs.2012.11.022Suche in Google Scholar PubMed
[24] Petrat F, Drowatzky J, Boengler K, Finckh B, Schmitz KJ, Schulz R, et al. Protection from glycine at low doses in ischemia-reperfusion injury of the rat small intestine. Eur Surg Res 2011;46:180–7.10.1159/000324393Suche in Google Scholar PubMed
[25] Effenberger-Neidnicht K, Jägers J, Verhaegh R, de Groot H. Glycine selectively reduces intestinal injury during endotoxemia. J Surg Res 2014;192:592–8.10.1016/j.jss.2014.06.016Suche in Google Scholar PubMed
[26] Petrat F, Rönn T, de Groot H. Protection by pyruvate infusion in a rat model of severe intestinal ischemia-reperfusion injury. J Surg Res 2011;167:e93–101.10.1016/j.jss.2009.12.005Suche in Google Scholar PubMed
Supplementary Material
The article (https://doi.org/10.1515/iss-2018-0035) offers reviewer assessments as supplementary material.
©2018 Dohle D.-S., et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 License.
Artikel in diesem Heft
- Original Articles
- Young surgeons’ challenges at the start of their clinical residency: a semi-qualitative study
- Intermittent Pringle maneuver may be beneficial for radiofrequency ablations in situations with tumor-vessel proximity
- Computed tomography donor liver volumetry before liver transplantation in infants ≤10 kg: does the estimated graft diameter affect the outcome?
- Reversible biliary occlusion in a small animal model: first description of a new technique
- Short- and long-term outcomes for the surgical treatment of acute pulmonary embolism
- Serum markers for early detection of patients with mesenteric ischemia after cardiac surgery
- Case Report
- Acute heart failure due to giant left atrium: remote ECLS implantation for interhospital transfer and bridging to decision
Artikel in diesem Heft
- Original Articles
- Young surgeons’ challenges at the start of their clinical residency: a semi-qualitative study
- Intermittent Pringle maneuver may be beneficial for radiofrequency ablations in situations with tumor-vessel proximity
- Computed tomography donor liver volumetry before liver transplantation in infants ≤10 kg: does the estimated graft diameter affect the outcome?
- Reversible biliary occlusion in a small animal model: first description of a new technique
- Short- and long-term outcomes for the surgical treatment of acute pulmonary embolism
- Serum markers for early detection of patients with mesenteric ischemia after cardiac surgery
- Case Report
- Acute heart failure due to giant left atrium: remote ECLS implantation for interhospital transfer and bridging to decision

