Abstract
The present study reports result from research into vapor–liquid–liquid phase equilibrium for n-octane highly diluted in water and water highly diluted in n-octane blends, using a dynamic method implemented in a constant volume CREC-VL-Cell. In the CREC-VL-Cell, a very high level of mixing is achieved, allowing for dispersions to be formed in the liquid phase and good mixing in the gas phase. This VL-Cell and its auxiliary equipment provide an increasing temperature ramp in the 30–110 °C range. It is found that the CREC-VL-Cell is of special value, for studying immiscible or partially miscible blends, such as is the case of n-octane in water. With the data obtained, which includes vapor pressures and temperatures, data analyses involving mass and molar balances, allow establishing overall liquid and vapor molar fractions. The recorded vapor pressures together with the calculated liquid and vapor molar fractions offer valuable data for VL thermodynamic model discrimination. For instance, it can be shown that vapor pressures, vapor and liquid molar fractions, as calculated with the Aspen-Hysys Peng Robinson Equation of State (Hysys-Aspen PR-EoS) provide only a first approximation of the experimental data, with significant discrepancies in the prediction of an n-octane disengagement temperatures. Thus, the determination of combined measured vapor pressures and calculated overall liquid molar fractions in the CREC-VL-Cell, offers a valuable and accurate procedure for thermodynamic model validation and discrimination.
Funding source: Natural Sciences and Engineering Research Council of Canada
Funding source: Syncrude Canada Ltd.
Acknowledgments
The authors would like to acknowledge Dr. Sujit Bhattacharya from Syncrude Canada Ltd. for his valuable comments and advice during the development of this research. The authors would also like to thank the Natural Science and Engineering Research Council of Canada (NSERC) and the industrial sponsor, Syncrude Canada Ltd., for the financial support provided to this work, under the NSERC-CRD program. Additionally, the authors would like to acknowledge Florencia de Lasa for her assistance with the editing of this manuscript.
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
Research funding: This study was funded by Natural Science and Engineering Research Council of Canada and Syncrude Canada Ltd.
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
Appendix A: Error analysis for liquid and vapor molar fractions in the CREC-VL-Cell
In the CREC-VL-Cell, the
Given that δ1, δ2 and δ3 can be bounded with standard relative errors of ±2.65, ±0.53 and ±1.39% respectively (Escobedo et al. 2021), the anticipated relative error in
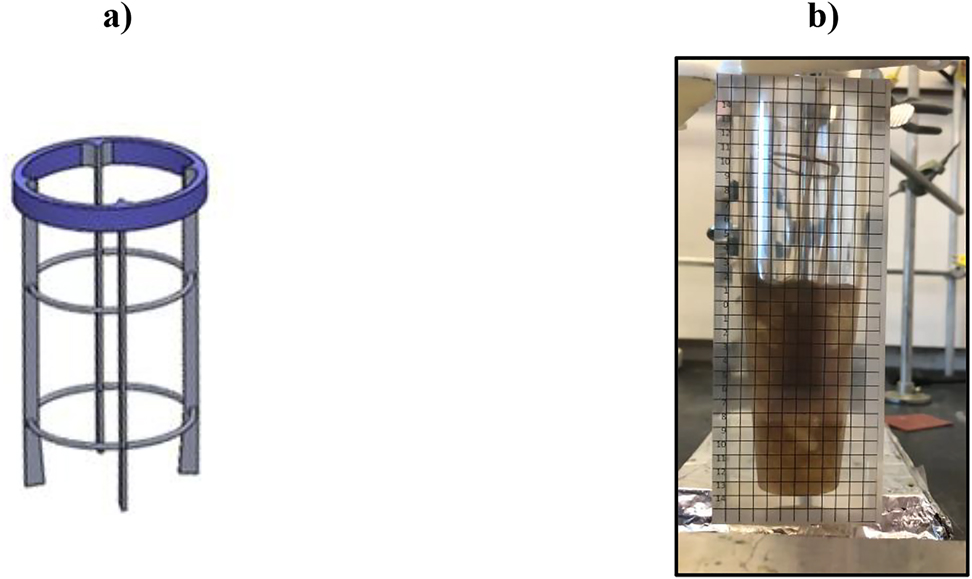
(a) Diagram of the baffles, used in the CREC-VL-Cell, and (b) Mixing of a 20 wt% n-octane + 80 wt% water blend, using a marine impeller and from the top suspended baffles covered 10% of their total height with the liquid phase.
Note: A 240 FPS high-speed camera and n-octane blend containing 0.0001 wt% bitumen dye is provided in a video, as Supplementary Material.
Appendix B: Multiphase fluid dynamics using video images
A plexiglass transparent unit was used to show the droplet size and droplet motion using a marine impeller. Short vertical baffles, as described in Figure B.1a were used to mitigate the issues with a marine impeller, such as (i) limited radial mixing and (ii) vortex formation. To achieve good visualization, a 0.0001 wt% of bitumen dye was employed to tag the hydrocarbon phase (n-octane) as shown in Figure B.1b. This was then reported in a separate video (see the Supplementary Material) using a high-speed camera at 240 frames/second (FPS). Video sequences were 10 times slower than regular motion.
Appendix C: Aspen-Hysys Model with Peng-Robinson Equation of State (PR-EoS)
Binary coefficients obtained from Aspen-Hysys with PR-EOS are defined in Table C.1.
Binary coefficients obtained from Aspen-Hysys® using the Peng-Robinson fluid package.
| N-octane | Water | |
|---|---|---|
| N-octane | 0.48 | |
| Water | 0.48 |
References
Avagyan, A. B. 2017. “Environmental Building Policy by the Use of Microalgae and Decreasing of Risks for Canadian Oil Sand Sector Development.” Environmental Science and Pollution Research 24 (25): 20241–53, https://doi.org/10.1007/s11356-017-9864-x.Suche in Google Scholar
Canada, G. of. 2015. Oil Resources. Also available at https://www.nrcan.gc.ca/energy/energy-sources-distribution/crude-oil/oil-resources/18085?wbdisable=true.Suche in Google Scholar
Canada National Energy Board. 2006. Canada’s Oil Sands Opportunities and Challenges 2015, https://doi.org/10.1002/14356007.a26.Suche in Google Scholar
Du, J., and W. R. Cluett. 2018. “Modelling of a Naphtha Recovery Unit (NRU) with Implications for Process Optimization.” Processes 6 (7): 74, https://doi.org/10.3390/pr6070074.Suche in Google Scholar
Escobedo, S., J. Kong, S. Lopez-Zamora, and H. de Lasa. 2021. “Synthetic Naphtha Recovery from Water Streams: Vapor-Liquid-Liquid Equilibrium (VLLE) Studies in a Dynamic VL-Cell Unit with High Intensity Mixing.” Canadian Journal of Chemical Engineering (in press).10.1002/cjce.24120Suche in Google Scholar
Ha, H. C. 1974. Vapor Pressures and Thermodynamics Properties of Benzene-Cyclohexane Solid Mixtures. McMaster University.Suche in Google Scholar
Henley, E. J., and J. D. Seader. 1981. “Equilibrium-Stage Separation Operations in Chemical Engineering.” The Chemical Engineering Journal 25(1): 122, https://doi.org/10.1016/0300-9467(82)85033-8.Suche in Google Scholar
Huber, M. L., A. Laesecke, and D. G. Friend. 2006. “Correlation for the Vapor Pressure of Mercury.” Industrial & Engineering Chemistry Research 45 (21): 7351–61, doi:https://doi.org/10.1021/ie060560s.Suche in Google Scholar
Kong, J. 2020. “Multiphase Equilibrium in A Novel Batch Dynamic VL-Cell Unit with High Mixing: Apparatus Design and Process Simulation.” Electronic Thesis and Dissertation Repsitory: 7283. https://ir.lib.uwo.ca/etd/7283.Suche in Google Scholar
Lopez-Zamora, S., J. Kong, S. Escobedo, and H. de Lasa. 2021. “Thermodynamics and Machine Learning Based Approaches for Vapor-Liquid-Liquid Phase Equilibria in N-Octane/Water Blends, as a Naphtha-Water Surrogate in Water Streams.” Processes 9 (3), https://doi.org/10.3390/pr9030413.Suche in Google Scholar
MAK Value Documentation. 2015. “Naphtha (Petroleum), Hydrotreated Heavy (MAK Value Documentation, 2010).” The MAK‐Collection for Occupational Health and Safety: Annual Thresholds and Classifications for the Workplace, https://doi.org/10.1002/3527600418.mb6474248yole4815.Suche in Google Scholar
Manasrah, A. D., and N. N. Nassar. 2020. “Oxy-Cracking Technique for Producing Non-combustion Products from Residual Feedstocks and Cleaning up Wastewater.” Applied Energy 280, https://doi.org/10.1016/j.apenergy.2020.115890.Suche in Google Scholar
Mohammadtabar, F., R. G. Pillai, B. Khorshidi, A. Hayatbakhsh, and M. Sadrzadeh. 2019. “Efficient Treatment of Oil Sands Produced Water: Process Integration Using Ion Exchange Regeneration Wastewater as a Chemical Coagulant.” Separation and Purification Technology 221: 166–74, doi:https://doi.org/10.1016/j.seppur.2019.03.070.Suche in Google Scholar
Navarro, P., M. Larriba, J. García, and F. Rodríguez. 2017. “Design of the Recovery Section of the Extracted Aromatics in the Separation of BTEX from Naphtha Feed to Ethylene Crackers Using [4empy][Tf2N] and [emim][DCA] Mixed Ionic Liquids as Solvent.” Separation and Purification Technology 180: 149–56, https://doi.org/10.1016/j.seppur.2017.02.052.Suche in Google Scholar
Rahimi, M., O. Jafari, and A. Mohammdifar. 2017. “Intensification of Liquid-Liquid Mass Transfer in Micromixer Assisted by Ultrasound Irradiation and Fe3O4 Nanoparticles.” Chemical Engineering and Processing: Process Intensification 111: 79–88, doi:https://doi.org/10.1016/j.cep.2016.11.003.Suche in Google Scholar
Romero Yanes, J. F., H. B. De Sant’Ana, F. X. Feitosa, M. Pujol, J. Collell, J. Pauly, F. P. Fleming, F. Montel, and J. L. Daridon. 2020. “Study of Liquid-Liquid and Liquid-Liquid-Vapor Equilibria for Crude Oil Mixtures with Carbon Dioxide and Methane Using Short-Wave Infrared Imaging: Experimental and Thermodynamic Modeling.” Energy & Fuels 34: 14109–23, doi:https://doi.org/10.1021/acs.energyfuels.0c03064.Suche in Google Scholar
Saniere, A., I. Hénaut, and J. F. Argillier. 2004. “Pipeline Transportation of Heavy Oils, a Strategic, Economic and Technological Challenge.” Oil and Gas Science and Technology 59 (5): 455–66, https://doi.org/10.2516/ogst:2004031.10.2516/ogst:2004031Suche in Google Scholar
Supplementary Material
The online version of this article offers supplementary material (https://doi.org/10.1515/ijcre-2021-0031).
© 2021 Walter de Gruyter GmbH, Berlin/Boston
Artikel in diesem Heft
- Frontmatter
- Editorial
- 10.1515/ijcre-2021-0153
- Special Issue Articles
- Gas-liquid downward flow through narrow vertical conduits: effect of angle of entry and tube-diameter on flow patterns
- Liquid antisolvent recrystallization and solid dispersion of flufenamic acid with polyvinylpyrrolidone K-30
- Forced convection heat transfer study of a blunt-headed cylinder in non-Newtonian power-law fluids
- Comparative studies on the separation of endocrine disrupting compounds from aquatic environment by emulsion liquid membrane and hollow fiber supported liquid membrane
- Instabilities of a freely moving spherical particle in a Newtonian fluid: Direct Numerical Simulation
- Numerical simulation of squeezing flow Jeffrey nanofluid confined by two parallel disks with the help of chemical reaction: effects of activation energy and microorganisms
- Development of inexpensive, simple and environment-friendly solar selective absorber using copper nanoparticle
- Effect of contacting pattern and various surfactants on phenol extraction efficiency using emulsion liquid membrane
- Foam drainage enhancement in foam fractionation for dye removal: process optimization by Taguchi methodology and grey relational analysis
- Phase equilibrium in n-octane/water separation units: vapor pressures, vapor and liquid molar fractions
Artikel in diesem Heft
- Frontmatter
- Editorial
- 10.1515/ijcre-2021-0153
- Special Issue Articles
- Gas-liquid downward flow through narrow vertical conduits: effect of angle of entry and tube-diameter on flow patterns
- Liquid antisolvent recrystallization and solid dispersion of flufenamic acid with polyvinylpyrrolidone K-30
- Forced convection heat transfer study of a blunt-headed cylinder in non-Newtonian power-law fluids
- Comparative studies on the separation of endocrine disrupting compounds from aquatic environment by emulsion liquid membrane and hollow fiber supported liquid membrane
- Instabilities of a freely moving spherical particle in a Newtonian fluid: Direct Numerical Simulation
- Numerical simulation of squeezing flow Jeffrey nanofluid confined by two parallel disks with the help of chemical reaction: effects of activation energy and microorganisms
- Development of inexpensive, simple and environment-friendly solar selective absorber using copper nanoparticle
- Effect of contacting pattern and various surfactants on phenol extraction efficiency using emulsion liquid membrane
- Foam drainage enhancement in foam fractionation for dye removal: process optimization by Taguchi methodology and grey relational analysis
- Phase equilibrium in n-octane/water separation units: vapor pressures, vapor and liquid molar fractions

