Abstract
Based on the fundamental characteristics of high chromium vanadium-titanium magnetite (HCVTM), the effects of roasting temperature and roasting time on the phase transition and oxidation consolidation during the oxidation were investigated systematically. It was shown that the oxidation of HCVTM pellet was not a simple process but complex. With increasing roasting temperature and time, the compressive strength of oxidized pellet was improved. The phase transition during oxidation was hypothesized to proceed as follows: (1) Fe3O4 → Fe2O3; (2) Fe2.75Ti0.25O4 → Fe9TiO15 + FeTiO3 → Fe9TiO15 + Fe2Ti3O9; (3) Fe2VO4 → V2O3 → (Cr0.15V0.85)2O3; (4) FeCr2O4 → Cr2O3 → Cr1.3Fe0.7O3 + (Cr0.15V0.85)2O3. The oxidation consolidation process was divided into three stages: (1)oxidation below 1,173 K; (2) recrystallization consolidation at 1,173 – 1,373 K; (3) particle refining recrystallization-consolidation by the participation of liquid phase at 1,373 – 1,573 K. To obtain the HCVTM oxidized pellet with good quality, the rational roasting parameters included a roasting temperature of 1,573 K and a roasting time of 20 min.
Introduction
Vanadium-titanium magnetite (VTM) is an important mineral resource, and it has high comprehensive utilization value. According to the content of Cr2O3, VTM is divided into two major types of ordinary VTM and high chromium vanadium-titanium magnetite (HCVTM). The HCVTM used in this work is imported from Russian. With the big supply and demand gap of domestic iron ore in China, this type of iron ore is imported in large quantities because of its low price and high comprehensive utilization value of iron, vanadium, titanium, and chromium, but the fundamental characteristics and mineral structures of HCVTM are more complicated than those of the ordinary VTM [1, 2]. In nature, the iron ore is oxidized spontaneously and slowly. And the oxidation is also a common pretreatment before subsequent reduction to separate Fe from other oxides [3–6]. Due to the presence of different valence states of Fe, V, Ti and Cr, phase transitions and some complicated reactions occur during oxidation. It is important to understand the detail oxidation process.
In the past decades, the oxidation mechanism of VTM had been investigated by a number of researchers [7–11]. Takatoshi et al. [12] pointed that the oxidation process of VTM was correlatable to both mobility of cation and the oxidation condition such as a circulation of some active hydrothermal materials at low temperature. Eungyeul et al. [13] investigated the non-isothermal oxidation; they reported that titanomagnetite was oxidized to cubic maghemite and then transformed to rhombohedral titanohematite. In Gui et al.’s work [14], the oxidation temperature and time had obvious effects on the oxidation degree of VTM pellet. Chen et al. [15] applied the x-ray diffraction to analyze the oxidized titanomagnetite concentrates and found that the hematite and hematite–ilmenite solid solution were obtained at 773 – 973 K and 973 K, respectively. By Chen et al.’s work [16], their results indicated that titanomagnetite and FeTiO3 were oxidized to titanohematite and Fe2TiO5, respectively, when the temperature was above 773 K.
But till now, there was no considerable and sufficient information on the oxidation mechanism of HCVTM pellet, especially in the phase transitions and oxidation consolidation mechanism. Therefore, in this study, based on the fundamental characteristics of HCVTM, effects of roasting temperature and time on the oxidation were investigated. Meanwhile, the phase transitions and consolidation mechanism were analyzed during oxidation process. And then the HCVTM pellet oxidation consolidation process was discussed and summarized. Finally, the rational roasting parameters of HCVTM pellet were obtained.
Experimental
Materials
The chemical compositions of HCVTM and bentonite examined in the work were presented in Table 1. Except for Fe, there were other valuable components, such as 0.95 V2O5, 5.05 TiO2 and 0.61 Cr2O3 (mass %). The particle size was detected by using Mastersizer 2000 laser particle size analyzer. Comparing with the particle size distribution of HCVTM before and after grinding (Figure 1), it was evident that the volume percent of particle size less than 0.074 mm in raw HCVTM was only 29.98% but the percent was nearly 100% after wet grinding. The HCVTM disposed in a laboratory wet grinder would be used in the subsequent experiment.
Chemical compositions of HCVTM and bentonite (mass %).
| Item | TFe | V2O5 | Cr2O3 | TiO2 | Al2O3 | SiO2 | MgO | CaO | S | P | Na2O | K2O |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HCVTM | 62.12 | 0.95 | 0.61 | 5.05 | 3.18 | 2.12 | 0.92 | 0.22 | 0.04 | 0.01 | – | – |
| Bentonite | – | – | – | – | 14.47 | 67.45 | 4.61 | 2.47 | – | – | 1.68 | 1.19 |

The particle size distribution of HCVTM before (a) and after (b) wet grinding.
The key phase compositions of HCVTM were detected by X-ray diffraction are shown in Figure 2. It was indicated that HCVTM was mainly composed of magnetite (Fe3O4), titanomagnetite (Fe2.75Ti0.25O4), chromite (FeCr2O4) and coulsonite (Fe2VO4). And titanomagnetite was a solid solution of magnetite (Fe3O4) and ulvospinel (Fe2TiO4) with a chemical formula of Fe3–xTixO4 (x≈0.25) [17].

X-ray diffraction analysis of HCVTM.
The TG-DSC curve of HCVTM in air was presented in Figure 3. There were two obvious and strong exothermic peaks at about 1,073 K and 1,373 K, respectively, which suggested that violent reactions or phase transitions might bring out at the corresponding temperatures and the oxidation process of HCVTM could be divided into three stages.

TG-DSC curve of HCVTM.
Methods and procedure
The oxidation test contained three procedures: preparation of materials, pelletizing of green pellet and oxidation roasting (Figure 4). Green pellets were prepared using a laboratory balling disc with a diameter of 1,000 mm, an edge height of 200 mm, a tilting angle of 45° and a rotate speed of 18 rpm. Then the pellets with diameter of 10 – 12.5 mm were screened out and roasted in air using a muffle furnace. Pellet samples in the experiment were analyzed with XRD (XRD, Siemens D5000) and scanning electron microscope (SEM-EDS, American).

Three procedures of the oxidation test.
Results and discussion
Effect of roasting temperature on the oxidation of HCVTM pellet
Effect of roasting temperature on compressive strength of HCVTM oxidized pellet
The compressive strength (CS) of HCVTM oxidized pellet at different roasting temperatures was tested as Chinese GB/T 14201–1993, illustrated in Figure 5. Thus, CS increased with the temperature and the changes were described as three sections according to the TG-DSC curve of HCVTM (Figure 3). When the roasting temperature was less than 1,173 K, CS had a slight increase from 50 N to 516 N corresponding to the increase in temperature from 573 K to 1,173 K. With the temperature increased to 1,373 K, the rate of CS also increased. While the temperature was above 1,373 K, CS improved significantly.
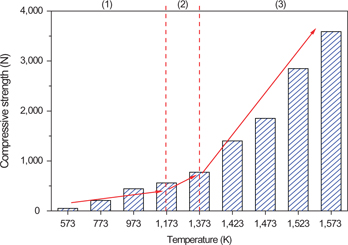
Effect of roasting temperature on the CS of HCVTM oxidized pellet.
The reasons that the CS was advanced by the increasing temperature could be summarized as follows: as the temperature rising, all the physical and chemical reactions inside pellet were accelerated and the recrystallization was promoted, which were both conducive to the sufficient oxidation. And then the structure of pellet was compact and the CS improved [18, 19]. When the roasting temperature exceeded 1,523 K, CS was 2,847 N which could meet the requirement of gas-based shaft furnace production.
Effect of roasting temperature on phase compositions of HCVTM oxidized pellet
HCVTM oxidized pellets roasted at different temperatures were analyzed by XRD and shown in Figure 6 and Table 2. At room temperature, the main phases inside HCVTM pellet were magnetite (Fe3O4), titanomagnetite (Fe2.75Ti0.25O4), chromite (FeCr2O4) and coulsonite (Fe2VO4). When the roasting temperature was below 773 K, the main phase compositions nearly kept the same. It was just because that the oxidation was slow leading to the low reaction rate as well as the lazy diffusion among particles, which made the phase has a slight change and difficult to be detected out. This phenomenon provided a proper evidence for the not so obvious improvement of CS at the low temperature (Figure 5).

XRD patterns of HCVTM pellet roasted at different temperatures.
Phase compositions of HCVTM oxidized pellet at different temperatures.
| T/K | Composition |
|---|---|
| Room temperature | Fe3O4, Fe2.75Ti0.25O4, FeCr2O4, Fe2VO4 |
| 573 K | Fe3O4, Fe2.75Ti0.25O4, FeCr2O4, Fe2VO4 |
| 773 K | Fe3O4, Fe2.75Ti0.25O4, FeCr2O4, Fe2VO4 |
| 973 K | Fe2O3, Fe3O4, Fe9TiO15, FeTiO3, Cr1.3Fe0.7O3, (Cr0.15V0.85)2O3 |
| 1,173 K | Fe2O3, Fe9TiO15, FeTiO3, Cr1.3Fe0.7O3, (Cr0.15V0.85)2O3 |
| 1,373 K | Fe2O3, Fe9TiO15, FeTiO3, Cr1.3Fe0.7O3, (Cr0.15V0.85)2O3 |
| 1,573 K | Fe2O3, Fe9TiO15, Fe2Ti3O9, Cr1.3Fe0.7O3, (Cr0.15V0.85)2O3 |
With the increase of the roasting temperature to 973 K, both the oxidations of magnetite (Fe3O4) and titanomagnetite (Fe2.75Ti0.25O4) were promoted. Pure magnetite was oxidized to hematite directly above 773 K [20]. Several new peaks appeared in the XRD pattern which could be attributed to the newly formed phase of titanohematite (Fe9TiO15). Besides, FeCr2O4 was “corroded” by magnetite and generated the solid solution of Cr1.3Fe0.7O3, but the vanadium in Fe2VO4 happened to transition and generated the solid solution of (Cr0.15V0.85)2O3.
At the roasting temperature of 1,173 K, Fe3O4 was not detected out and had been already totally oxidized to Fe2O3, so the peaks of Fe2O3 became stronger. As the temperature increased continuously, the oxidation was promoted and all the oxidation reactions were enhanced. At 1,373 K, except for the stronger diffraction peaks of each phase, the main phases in HCVTM oxidized pellet had no changes. However, because of the overlapping peaks, the decomposition from Fe9TiO15 to Fe2O3 and FeTiO3 was not verified at 973–1,373 K. When the temperature was up to 1,573 K, the titanium was present in the form of Fe9TiO15 and pseudorutile (Fe2Ti3O9) generated from the further oxidation of FeTiO3. But ulvospinel (Fe2TiO4), FeTiO3 and rutile (TiO2) were not detected out.
Effect of roasting temperature on the microstructure of HCVTM oxidized pellet
Figure 7 showed the SEM images with EDS analyses of HCVTM oxidized pellet at different temperatures. From the result, when the temperature was low, the particles were independent and angular, and the pores were coexisted as three shapes of big pores, small pores and intervals among particles; in general, the structure of pellet was loose. As the temperature increasing to 973 K, the number of pore decreased obviously. Magnetite was oxidized to hematite, and the corresponding magnetite of V, Ti and Cr was also oxidized to their own hematite or generated some special and complicated solid solutions (Points E and F in Figure 7(c)). The structure of HCVTM pellet became compact. Further increasing the temperature, both the recrystallization and crystal growth were enhanced, and the crystal bridges among particles were developed but still un-widespread, so the whole pellet structure was not contiguous. At 1,573 K, the crystal bridges further transited and grew, and there were plenty of liquid phases uniformly distributed in the pellet. Then the particles were interconnected and the inside structure was compact, which were both contribute to obtain a better CS. Combining the elemental proportions of point H obtained from the EDS spectrum and the results of XRD analysis, the phase composition of H was initially identified as a mixture of Fe9TiO15 and Fe2Ti3O9.

SEM images with EDS analyses (mass %) of HCVTM oxidized pellet at different temperatures.
The atomic iron to titanium ratio (Fe/Ti) of the white phase along analyzed lines in the HCVTM oxidized pellet at different temperatures was compared in Figure 8. The analyzed white phase contained Fe, V, Ti, Cr and O. Formation of a new titanohematite solid solution (Fe9TiO15) was seen from the XRD pattern (Figure 6), the SEM image (Figure 7) and the Fe/Ti ratio (Figure 8), and its percent was increased with the roasting temperature. At 973 K, the exsolved titanohematite had a higher content of Ti than that in the titanomagnetite matrix, which could be assigned to the higher mobility of Fe3+ in comparison with Ti4+. In the process of titanomagnetite oxidation, the concentration of Fe3+ increased, causing a compressive stress in the titanomagnetite lattice and triggering the structural transition of cubic spinel to rhombohedral titanohematite. During this transition, the Fe3+ migrated out of the fresh was formed rhombohedral lattice what accelerated the further oxidation of titanomagnetite. This process was characterized by the fluctuation of Fe/Ti ratio at a low roasting temperature (Figure 8(a)). With the increase of roasting temperature, the oxidation reactions were stable gradually resulting in the relatively uniform Fe/Ti ((Figure 8(b) and (c)). Generally, the Fe/Ti decreased and to be stable as the temperature increased.

The Fe/Ti ratio of the white phase in HCVTM oxidized pellet at 973 K (a), 1,173 K (b), and 1,373 K (c).
Phase transitions of valuable elements in HCVTM oxidized pellet
Combining the above analyses, reactions of the phases containing Ti in HCVTM pellet during oxidation process were summarized as follows:
The oxidation of titanomagnetite with the formation of a titanohematite solid solution (Fe9TiO15) and ilmenite (FeTiO3) at 773–973 K:
The further oxidation of ilmenite with the formation of pseudorutile (Fe2Ti3O9) at 1,373–1,573 K:
From the ternary phase diagram of FeO-Fe2O3-TiO2, the oxidation paths of Ti-bearing phases in HCVTM were also illustrated in Figure 9. As shown, ulvospinel and magnetite formed a solid solution of titanomagnetite (spinel, line ①); ilmenite and hematite formed a solid solution of titanohematite (rhombohedral, line②). There was a miscibility gap between titanomagnetite and titanohematite [21, 22]. The oxidation path followed the line marked [1], [2] and [3] corresponding to the reactions of (1), (2) and (3) respectively. The reaction (1) included the oxidation of Fe2+→ Fe3+, migration of iron ions (Fe2+ and Fe3+) and structural transition of spinel to rhombohedral. However, the reaction (2) included the structural transition of spinel to trigonal (ilmenite). At 1,373–1,573 K, the reaction (3) started; however, the degree of the transition from ilmenite to pseudorutile was quite low.

The ternary system of FeO-Fe2O3-TiO2.
The phase containing V transformed from coulsonite spinel (Fe2VO4) to the solid solution of V and Cr after 773 K with the reactions:
Accordingly, the Cr2O3, transformed from the chromite spinel (FeCr2O4), generated the solid solution with V2O3 and Fe2O3, respectively, together with the reaction (5) and the following reactions:
Consequently, the phase transition behaviors in HCVTM during the oxidation process were as follows: (1) Fe3O4 → Fe2O3; (2) Fe2.75Ti0.25O4 → Fe9TiO15 + FeTiO3 → Fe9TiO15 + Fe2Ti3O9; (3) Fe2VO4 → V2O3 → (Cr0.15V0.85)2O3; (4) FeCr2O4 → Cr2O3 → Cr1.3Fe0.7O3 + (Cr0.15V0.85)2O3.
Oxidation consolidation process of HCVTM oxidized pellet
As well as the ordinary magnetite pellet, the solid phase consolidation was the main method of oxidation consolidation for HCVTM pellet. Through the analyses on the change rules of CS, morphology evolutions and phase transitions, the detail oxidation consolidation process of HCVTM pellet was divided into three stages and presented as follows:
The first stage was oxidation. When the roasting temperature was less than 1,173 K, the main reactions were the oxidations of pure magnetite and titanomagnetite. In this stage, the phase composition was mainly composed of iron oxide. The mineral particles were angular and the number of pore in the pellet was more (Figure 7(a), (b) and (c)). The CS of pellet was relatively low, less than 600 N (Figure 5, section (1)).
The second stage was recrystallization consolidation. The temperature range was 1,173–1,373 K. Hematite was recrystallized and the crystal bridges among mineral particles appeared during this stage. Besides, the phases containing V, Ti and Cr continued to consolidate. The crystal bridges among particles developed but still un-widespread, so the whole pellet structure was not contiguous (Figure 7(d) and (e)). Therefore, the CS reached to 774 N (Figure 5, section (2)).
The third stage was the particle refining and recrystallization-consolidation by the attending of liquid phase. The temperature range was 1,373 ~ 1,573 K. In this stage, the silicate liquid phase generated gradually, by which the hematite and solid solution were separated to many small particles. And these small particles recrystallized and grew quickly. In addition, the small particles were conjoined by the crystal bridges and a small amount of slag. Then the pellet formed the compact whole and the oxidation consolidation was almost complete (Figure 7(f)). So, the HCVTM pellet had a relative high CS during this stage (Figure 5, section (3)).
It was noticed that the oxidation consolidation process almost corresponded to CS changes (Figure 5), microstructure transformations (Figure 7) and the TG-DSC curve in air (Figure 4). There were two similar key temperature points that just divided the oxidation process into three stages described as the above. Therefore, the analyses for oxidation consolidation process of HCVTM pellet were rational and credible.
Effect of roasting time on the oxidation of HCVTM pellet
Effect of roasting time on compressive strength of HCVTM oxidized pellet
Effect of roasting time on the CS of HCVTM oxidized pellet (1,573 K) was presented in Figure 10. By prolonging the roasting time, the CS showed an increasing tendency but not obvious. When the roasting time is short, the HCVTM pellet formed the double-layer structure due to the incomplete oxidation. The outer sphere was the recrystallization of hematite and titanohematite, while the core was magnetite recrystallization and slag consolidation. The concentric cracks resulted from the asynchronous shrink of the outer and core might weaken the CS. With the increasing time, the layer of hematite and titanohematite became thicker gradually, and then the complete oxidation of pellet had been achieved which was benefit to enhance the CS of pellet. When the roasting time was 10 min, the CS was 2,608 N which could meet the requirement for gas-based production.

Effect of roasting time on the CS of HCVTM oxidized pellet (1,573 K).
Effect of roasting time on the phase compositions of HCVTM oxidized pellet
The XRD patterns of HCVTM pellet roasted for different times (1,573 K) were shown in Figure 11. In any experimental case, the phases were almost the same just different on the intensity of diffraction peak for each phase. When the roasting time was short, the pores among particles were big which could provide the wide and enough contact areas for gas–solid reactions, and the interior diffusion resistance was small due to the thin oxidation product layer. Then the oxidation reaction was quick and violent, and the diffraction peaks of all oxides included Fe2O3, Fe9TiO15, Fe2Ti3O9, Cr1.3Fe0.7O3 and (Cr0.15V0.85)2O3 were strengthened evidently in the initial stage. Prolonging the roasting time, the link by the crystal bridge was close-knit leading to the decreasing reaction contact area. Moreover, the recrystallization of hematite and titanohematite was complete gradually and the oxidation reaction became slow. Therefore, the intensity of diffraction peaks increased inconspicuously.

XRD patterns of HCVTM pellet roasted at 1,573 K at different roasting times.
Effect of roasting time on the microstructure of HCVTM oxidized pellet
SEM images with EDS analyses of HCVTM pellet roasted for different times (1,573 K) are shown in Figure 12. Due to incomplete recrystallization of hematite and titanohematite at the beginning of roasting for 10 min, the mineral particles were single granular and the structure of the pellet was loose. By prolonging the time to 20 min, the recrystallization was accomplished gradually. Furthermore, the less pores in the pellet and the connection of single particles made the pellet become a compact whole, then the pellet had a good CS. If the roasting time was to prolong further, the excessively compact and thick oxidation product layer might cause some negative influences on the diffusion. Therefore, the microstructure of the pellet had no obvious changes after 20 min.
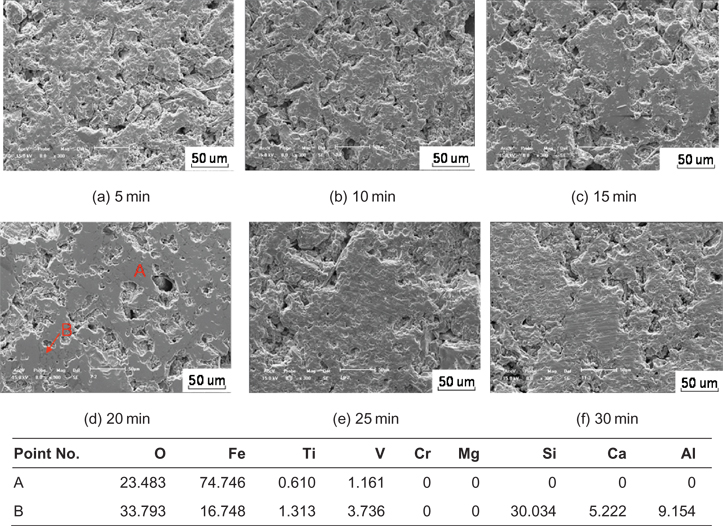
SEM images with EDS analyses (mass %) of HCVTM pellet roasted at 1,573 K at different times.
The Fe/Ti ratio along the analyzed line in the phase containing Fe, V, Ti, Cr and the Fe/Ti ratio at different roasting time was obtained in Figure 13. As shown, the Fe/Ti ratio decreased and became stable around 5 with the prolonging roasting time.

The Fe/Ti ratios of the white phase in HCVTM pellet roasted at 1,573 K for 5 min, 10 min, and 15 min.
Rational roasting parameters for HCVTM pellet
Based on the work, it was obvious that the CS improved with the increasing roasting temperature and time. When the temperature was more than 1,523 K or the time was above 10 min, the CS could meet the requirement of the gas-based shaft furnace production. However, at 1,573 K, the oxidation was sufficient and a large amount of liquid phases appeared and distributed uniformly in the pellet. Meanwhile, the particles were conjoined with each other. The pellet became a compact whole and the consolidation was almost achieved completely (Figure 7(f)). After roasting 20 min at 1,573 K, the recrystallization was accomplished gradually. The structure was more compact than that in the shorter roasting time and the pellet had a good CS (Figure 12(d)).
Taken together, the rational roasting parameters for HCVTM pellet included a roasting temperature of 1,573 K and a roasting time of 20 min. X-ray mapping study of the pellet roasted at the rational parameters and shown in Figure 14. The white was the phase containing Fe and the black or gray was slag. It was revealed that the most of Fe and Ti were connected with each other to form the phases (Fe9TiO15 and Fe2Ti3O9) according to its similar distribution. Both V and Cr dispersed in the whole pellet and generated the solid solutions (Cr1.3Fe0.7O3 and (Cr0.15V0.85)2O3) in both iron-bearing phase and slag phase. In general, Fe and O were more concentrated, while Cr, V and Ti, especially Cr and V which were difficult to be examined, were relatively disperse. Accordingly, it was necessary to further study the migration mechanism of the valuable elements, which can be seen in the following work.

Distribution of Fe, Ti, V, Cr and O in HCVTM pellet roasted at 1,573 K for 20 min.
Conclusions
Phase transitions and consolidation mechanism of HCVTM pellet by oxidation process were investigated by discussing the effects of roasting temperature and time. The following results could be summarized.
Both increasing roasting temperature and time could improve the CS of HCVTM pellet. The change rule of CS with the increasing temperature was described as three sections which were corresponding to the oxidation consolidation process of HCVTM pellet, respectively.
The phase transitions of valuable elements in HCVTM pellet during oxidation were obtained as follows: (1) Fe3O4 → Fe2O3; (2) Fe2.75Ti0.25O4 → Fe9TiO15 + FeTiO3 → Fe9TiO15 + Fe2Ti3O9; (3) Fe2VO4 → V2O3 → (Cr0.15V0.85)2O3; (4) FeCr2O4 → Cr2O3 → Cr1.3Fe0.7O3 + (Cr0.15V0.85)2O3.
The oxidation consolidation process of HCVTM pellet was divided into three stages: (1) oxidation (below 1,173 K), (2) recrystallization consolidation (1,173–1,373 K) and particle refining recrystallization-consolidation stage by the attending of liquid phase (1,373–1,573 K).
The rational roasting parameters for HCVTM pellet included a roasting temperature of 1,573 K and a roasting time of 20 min.
Funding statement: Funding: This work was financially supported by China National High Technology Research and Development Program of 863 Program (2012AA062302), China Fundamental Research Funds for the Central Universities (N130602003), National Natural Science Foundation of China (Grant No. 51574067).
References
[1] J. Tang, Y. Zhang, M.S. Chu and X.X. Xue, J. Northeast. Univ. Nat. Sci., 34 (2013) 956–960.10.4028/www.scientific.net/AMM.321-324.956Suche in Google Scholar
[2] J. Tang, Y. Zhang, M.S. Chu and X.X. Xue, J. Northeast. Univ. Nat. Sci., 34 (2013) 545–550.Suche in Google Scholar
[3] X. Fu, Y. Wang and F. Wei, Metall. Trans. A., 41A (2010) 1338–1348.10.1007/s11661-010-0173-ySuche in Google Scholar
[4] S.S. Liu, Y.F. Guo, G.Z. Qiu, T. Jiang and F. Chen, Trans. Nonferrous Met. Soc. China., 24 (2014) 3372–3377.10.1016/S1003-6326(14)63479-8Suche in Google Scholar
[5] X.J. Zuo, J.S. Wang, X.W. An, X.F. She and Q.G. Xue, Iron Steel Res. Int., 20 (2013) 12–18.10.1016/S1006-706X(13)60210-1Suche in Google Scholar
[6] Z.C. Huang, K. Wu, B. Hu, H. Peng and T. Jiang, Iron Steel Res. Int., 19 (2012) 1–4.10.1016/S1006-706X(12)60038-7Suche in Google Scholar
[7] S.Q. Zhang, B. Xie, Y. Wang, T. Guan, H.L. Cao and X.L. Zeng, Iron Steel Res. Int., 19 (2012) 33–38.10.1016/S1006-706X(13)60017-5Suche in Google Scholar
[8] W.O. Relly and S.K. Banerjee, Oxid. Titanomag., 29–37 (1967) 12.Suche in Google Scholar
[9] A. Randall and S. Albert, Metall. Trans. A, 24A (1991) 1993–1257.Suche in Google Scholar
[10] P. Nikolai and V. Hojatollash, Proc. Ocean Drill. Prog. Sci. Results, 106 (1990) 283–289.Suche in Google Scholar
[11] W.M. Zhou, V.V. Rob, P.R. Donald, D.M. Wang and Y.X. Zhang, J. Geophys. Res., 106 (2001) 6409–6421.10.1029/2000JB900447Suche in Google Scholar
[12] A. Takatoshi, K. Hajimu and F. Toshio, Earth Planet. Sci. Lett., 71 (1984) 263–278.10.1016/0012-821X(84)90091-8Suche in Google Scholar
[13] P. Eungyeul and O. Oleg, ISIJ. Int., 44 (2004) 74–81.10.2355/isijinternational.44.74Suche in Google Scholar
[14] G.H. Han, T. Jiang, Y.B. Zhang, Y.F. Huang and G.H. Li, Iron Steel Res. Int., 18 (2011) 14–19.10.1016/S1006-706X(11)60097-6Suche in Google Scholar
[15] D.S. Chen, B. Song, L.N. Wang, T. Qi, Y. Wang and W.J. Wang, Miner. Eng., 24 (2011) 864–869.10.1016/j.mineng.2011.03.018Suche in Google Scholar
[16] S.Y. Chen, M.S. Chu, J. Tang and X.L. Wu, J. Northeast. Univ. Nat. Sci., 34 (2013) 536–541.Suche in Google Scholar
[17] J. Dang, X.J. Hu, G.H. Zhang, X.M. Hou, X.B. Yang and K. Chou, High Temp. Mater. Proc., 32 (2013) 229–236.10.1515/htmp-2012-0128Suche in Google Scholar
[18] O. Sivrikaya and A. Arol, Int. J. Miner. Process., 123 (2013) 158–164.10.1016/j.minpro.2013.06.006Suche in Google Scholar
[19] S.P. Forsmo, P.Q. Samskoga and B.M. Björkman, Powder Technol., 181 (2008) 321–330.10.1016/j.powtec.2007.05.023Suche in Google Scholar
[20] W. Feitknecht, Appl. Chem., 423 (1964) 9.10.1351/pac196409030423Suche in Google Scholar
[21] A.F. Buddington and D.H. Lindsley, J. Petrol., 5 (1964) 310–357.10.1093/petrology/5.2.310Suche in Google Scholar
[22] S.E. Haggerty, Rev. Mineral, 25 (1991) 129.10.1111/j.1600-0536.1991.tb01806.xSuche in Google Scholar
©2017 by De Gruyter
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Artikel in diesem Heft
- Frontmatter
- Research Articles
- A New Method to Produce Ni–Cr Ferroalloy Used for Stainless Steel Production
- Mechanical and Electrochemical Characterization of Super-Solidus Sintered Austenitic Stainless Steel (316L)
- Effect of γ→α Phase Transformation on Refining Austenite Grains of Microalloyed Steel in Continuous Casting by Simulation
- Fatigue Life Improving of Drill Rod by Inclusion Control
- Influence of Basicity and MgO on Fluidity and Desulfurization Ability of High Aluminum Slag
- Effect of Sputtered AlY Coating on High-Temperature Oxidation Behavior of Stainless Steel
- Optimal Design of Nozzle for Supersonic Atmosphere Plasma Spraying
- Oxidation Behaviors of Inconel 740H in Air and Dynamic Steam
- Line-Profile Analysis Combined with Texture Analysis for Characterizing Dislocation Distribution in Texture Components of Cold-Rolled Copper Sheets
- Microstructure Analysis of HPb59-1 Brass Induced by High Current Pulsed Electron Beam
- Thermal Treatment Method for Synthesis and Characterization of the Octahedral Magnetic Nanostructures of Co3O4 from a New Precursor
- Phases Transition and Consolidation Mechanism of High Chromium Vanadium-Titanium Magnetite Pellet by Oxidation Process
- Effect of Fiber Laser Treating on Magnetic Domains in the Grain-Oriented Silicon Steel: Imaging Domains by Bitter, MFM and Kerr Microscopy
Artikel in diesem Heft
- Frontmatter
- Research Articles
- A New Method to Produce Ni–Cr Ferroalloy Used for Stainless Steel Production
- Mechanical and Electrochemical Characterization of Super-Solidus Sintered Austenitic Stainless Steel (316L)
- Effect of γ→α Phase Transformation on Refining Austenite Grains of Microalloyed Steel in Continuous Casting by Simulation
- Fatigue Life Improving of Drill Rod by Inclusion Control
- Influence of Basicity and MgO on Fluidity and Desulfurization Ability of High Aluminum Slag
- Effect of Sputtered AlY Coating on High-Temperature Oxidation Behavior of Stainless Steel
- Optimal Design of Nozzle for Supersonic Atmosphere Plasma Spraying
- Oxidation Behaviors of Inconel 740H in Air and Dynamic Steam
- Line-Profile Analysis Combined with Texture Analysis for Characterizing Dislocation Distribution in Texture Components of Cold-Rolled Copper Sheets
- Microstructure Analysis of HPb59-1 Brass Induced by High Current Pulsed Electron Beam
- Thermal Treatment Method for Synthesis and Characterization of the Octahedral Magnetic Nanostructures of Co3O4 from a New Precursor
- Phases Transition and Consolidation Mechanism of High Chromium Vanadium-Titanium Magnetite Pellet by Oxidation Process
- Effect of Fiber Laser Treating on Magnetic Domains in the Grain-Oriented Silicon Steel: Imaging Domains by Bitter, MFM and Kerr Microscopy

