Abstract
The wear behavior of cast A7075 and A7075/SAF 2205 composite material fabricated by vacuum-assisted investment flask casting was investigated under dry sliding condition. The wear tests were carried out using a “ball-on-disc” type tribometer. In the wear tests, 100Cr6 and ZrO2 balls were used as counterparts and the load, total distance and rotating speed were selected as 10 N, 100 m and 100 rpm, respectively. The results were evaluated using the friction coefficient–distance diagram, weight loss and wear rate. All worn surfaces were examined by scanning electron microscope and wear characteristics of the materials were discussed as a function of the microstructural features. It was concluded that composite material had lower friction coefficient, less weight loss and slower wear rate than that of cast material.
Introduction
Aluminum alloys are favorable materials for industrial, structural and transport applications due to their low density, good mechanical properties and excellent corrosion resistance [1]. In order to enhance their mechanical properties (i.e. specific modulus, strength and wear resistance), several hard particles (SiC, Al2O3, TiC and B4C) are added as reinforcing materials to form an aluminum matrix composite [2–7]. Such composites are also produced by the addition of steel-based particles in aluminum matrix and superior mechanical properties are obtained compared to aluminum alloys [8–12].
As it is well known, metal matrix composites (MMC) can be fabricated by solid/liquid/vapor phase methods (i.e. powder metallurgy, spray atomization, co-deposition, plasma spraying, stir casting and squeeze casting, etc.) [13–19]. In engineering applications, casting is an inexpensive technique for the production of MMCs, which proposes many other options for materials and processing conditions. At the present time, there is still a quest to reduce manufacturing costs of MMCs. Cost reductions could be achieved either by developing cheaper fabrication methods or using cheaper materials that provide desirable mechanical properties [20, 21]. In our previous studies, we adapted a vacuum-assisted block mold investment casting technique to fabricate near-net shape MMC [3, 22]. This technique is also suitable for the production of A7075/SAF 2025 composite material. The aim of this study is to investigate the tribological characteristics of the fabricated composite material and discuss its enhanced surface properties compared to A7075 alloy under dry sliding conditions.
Experimental
Materials
A7075 aluminum alloy and SAF 2205 duplex stainless steel chips were selected as matrix and reinforcing materials, respectively. Tables 1 and 2 represent the chemical compositions of A7075 and SAF 2205 alloys.
The chemical composition of A7075 aluminum alloy (wt.%).
| Mg | Mn | Si | Cu | Fe | Cr | Ti | Zn | Al |
| 2.40 | 0.30 | 0.40 | 1.60 | 0.50 | 0.26 | 0.2 | 5.30 | Balance |
The chemical composition of SAF 2205 duplex stainless steel (wt.%).
| C | Cr | Ni | Mo | Mn | Si | P | S | N | Fe |
| 0.02 | 22.56 | 5.42 | 2.95 | 1.29 | 0.457 | 0.031 | 0.014 | 0.170 | Balance |
Casting and heat treatment applications
The solid investment casting mold was prepared with a cylindrical wax pattern having 21 mm diameter and 50 mm height. The wax pattern was fastened into a rubber flask base and a stainless steel perforated flask was placed on the base. Perforated flask holes were covered with an adhesive band. Plaster bonded (plaster/silica) commercial investment powder was mixed into water using 0.40 powder/water ratio. The slurry was filled into the flask under vibration and the flask was placed into an electrical furnace for dewaxing and burnout processes. To obtain successful burnout regime, the mold was heated up to 700°C gradually. The steel-based preform was placed into mold just 10 min before casting for preheating of the preform without excessive oxidation. Casting process illustrated in Figure 1 was carried out at 830°C using 10−5 Pa pressure. Age hardening was carried out on the composite specimens to eliminate hardness difference between the matrix and reinforcing materials. Solution heat treatment was performed at 460°C for 2 h and age hardening was performed at 190°C for 5 h.

Casting process used in this study.
Hardness test
The effect of the SAF 2205 duplex stainless steel chips on the mechanical properties of A7075 alloy was investigated with Vickers hardness tests using hardness tester Bulut HVS1000. Hardness tests were carried out under 300 g test load and average of five measurement was reported. The hardness values were determined as 162.01 ± 10 HV and 269.67 ± 9 HV for cast and composite materials, respectively.
Wear test under dry sliding condition
For tribological characterization wear tests were performed under dry sliding condition and wear characteristics were then determined based on the appearances of worn surfaces and wear-induced loss amounts. Wear tests were realized using a “ball-on-disc” type tribometer (Nanovea). Related test parameters are listed in Table 3. Volume loss and specific wear rate values were calculated according to ASTM G99-05 standard using optically measured wear track dimensions for both of the tested materials.
Parameters used in wear tests.
| Counterpart material | 100Cr6 | ZrO2 |
| Counterpart hardness | 65 HRC | 6.5–7 (mohs) |
| Rotational speed | 0.10 m/s | |
| Normal load | 10 N | |
| Sliding distance | 100 m | |
Metallographic sample preparation and microscopic examinations
All samples for the microstructural characterization were prepared by grinding with 320, 600 and 1,000 mesh size SiC abrasives, respectively, and then ground surfaces were polished with 3 μm and then 1 μm diamond solution. Etching was carried out with Keller reagent in order to determine the phases within the matrix. Zeiss Axiotech 100 light microscope (LM) and Jeol JSM 6060 scanning electron microscope (SEM) were used for both metallographic and worn surface examinations. IXRF model energy dispersive x-ray spectrometer (EDS) was used for characterization of the phases.
Results and discussion
Microstructure
Figure 2 shows the microstructures of A7075 material where the matrix consists of typical dendritic structure with several intermetallics formed in interdendritic spaces (Figure 2(a)). The Al-Zn-Mg precipitates provide the basis for the 7xxx wrought and cast alloys. Two phases can form by eutectic decomposition in commercial Al-Zn-Mg alloys: MgZn2 (ɳ) and Al2Mg3Zn3. Depending on the zinc/magnesium ratio, copper-free alloys are strengthened by metastable precursors to either MgZn2 or Al2Mg3Zn3 (ɵ). In Al-Zn-Mg-Cu alloys, copper and aluminum substitute for zinc in MgZn2 to form Mg(Zn,Cu,Al)2. Particles of Al2CuMg can also form in these alloys by eutectic decomposition and solid-state precipitation [23–25]. In addition to these intermetallics, Al7Cu2Fe, Al23CuFe4, Al6Fe, and Mg2Si particles have been observed in A7075 alloy in other studies [26, 27]. EDS analyses of the sample show that the dark particles marked by black arrows in the image contain Mg, Zn and Si, suggesting that they are Mg2 (Zn, Si). The matrix has two types of bright particles marked by white arrows: (i) the particles in the form of Chinese script contain Al, Fe, Mg, Zn and Cu, (ii) the particles having globular morphology contain Al, Mg, Zn and Cu (Figure 2(b)). The variation of microstructural features during aging has not been studied; however, it was well known that the precipitation sequence in A7075 alloy is described by GP zones→ ɳ’ → ɳ and the high mechanical strength developed by the alloy in the T6 temper is associated with high density metastable precipitates, distributed homogeneously in the aluminum matrix [24, 28–31].

(a) LM and (b) SEM micrographs showing the cast structure of A7075 alloy. The matrix consists of dendritic α phase including several intermetallics.
The composite material was also characterized by LM and SEM examinations (Figure 3). Two principal precipitates are formed during the solidification of the cast samples due to the interdiffusion of aluminum and steel. Figure 3(a) and 3(b) shows one of these precipitates formed in plate/needle-like shape. The presence of chromium as a major alloying element in the steel matrix leads to the formation of ternary phases and EDS analyses indicate that the phase is Fe2Al13Cr2. Another precipitate is determined as an intermetallic layer around steel matrix and EDS analyses point to Fe5Al4Cr2 intermetallic (Figure 3(c)). As it is well known, these intermetallic phases are harder and more brittle than the matrix and the nature of the interface between the steel and Al-matrix in the cast composite metal was found to have a prominent effect on their deformation behavior [32].

(a) LM and (b and c) SEM micrographs showing the composite structure.
Evaluation of wear test data
Figure 4 shows the variation of friction coefficient values as a function of distance for two different counterpart materials for cast A7075 and A7075/SAF 2025 composite materials. With both 100Cr6 and ZrO2 interaction, A7075 alloy has a higher friction coefficient than that of A7075/SAF 2025 composite material. The diagram given in Figure 4(a) indicates that the friction coefficient value for A7075 alloy is in the range of 0.10–0.20; however, this value is in a narrower range like 0.12–0.15 for the composite material. As mentioned in Section “Microstructure”, both cast and its composite structures consist of several intermetallics giving strength to the matrix. Composite material exhibits lower friction coefficient than the cast alloy due to its higher hardness. However, the effect of hardness on the wear is complicated by the fact that different wear mechanisms can prevail depending on the microstructure and also operating conditions. The wear intensity may decrease by increasing the hardness of the materials in contact [33]. On the other hand, when a harder counterpart material is selected like ZrO2, the friction of coefficients reaches to higher values and this concept suits for both materials studied, as illustrated in Figure 4(b). As it is well known, in sliding of a hard ball on a plate, frictional forces become a function of increased shear strength and decreased contact area making the friction coefficient higher [34]. Figure 5 shows the change of volume loss in wear and specific wear rate of A7075 and composite materials as a function of counterpart material. The diagram clearly indicates that values of wear loss and wear rate measured for cast alloy are higher than those of composite material in interaction against both counterparts; however, the harder counterpart (ZrO2 ball) causes more wear. Some characteristics such as roughness, plastic deformation, grooving, tendency to adhesion during sliding, surface fatigue, tribochemical reactions affect the wear [34]. The examination of worn surfaces reveals the wearing features which will be evaluated in Section “Examinations on worn surfaces”.

The diagram showing the variation of friction coefficients as a function of distance with the interaction of 100Cr6 (a) and ZrO2 counterpart (b).

The diagram showing the variation of wear loss and specific wear rate of A7075 and A7075/SAF 2025 composite materials with the interaction of 100Cr6 and ZrO2 counterpart in dry sliding conditions.
Examinations on worn surfaces
In order to understand the wear characteristics under dry sliding condition, the worn surfaces of the materials were investigated using SEM. In sliding contact, surface damage is caused by abrasion, adhesion, surface fatigue and also tribochemical reactions [29]. Figure 6 shows the surface degradation on the materials. The materials exhibit typical grooves and adhesion layers on the worn surfaces contacted with 100Cr6/ZrO2 ball. Grooves resulting from micro-cutting are evident in both non-reinforced and reinforced structure indicating abrasive wear (Figure 6(a) and 6(c)). In abrasion, micro-cuttings, fatigue due to repeated ploughing, fracture due to microcracking (Figure 6(b)) of the base body (A7075 or A7075/SAF 2025) caused by the counterpart’s hard asperities or by hard particles such as intermetallics removed from the matrices in the interfacial medium lead to wear. During the test, oxidation occurs and micro-crack coalescence causes fracture of the oxidized surfaces. Figure 6(a) and 6(c) gives good evidences to understand the wear resistance of two different materials. In Al-matrix, the width of wear track is wider than that of steel part. As given in Figure 5, wear rates were evaluated as a function of both matrix and counterpart type. A7075 has higher wear rate than that of its composite contacted with harder metal/ceramic counterpart and ceramic-based counterpart increases the rate by a factor of 2. The worn surfaces also indicate that ceramic counterpart causes severe wear by forming adhesion layers (Figure 6(b) and 6(d)). In adhesion, after possibly extant protective surface layers have been broken through, microwelds form above all on the plastically deformed microcontacts between the base body and counterpart. If the strength of the adhesive bonds is greater than that of the softer friction partner, material eventually detaches from the deformed surface of the softer friction partner and is transferred to the harder one (metal/ceramic ball). The transferred material can either remain on the harder friction partner or detach or even return as given in Figure 6.
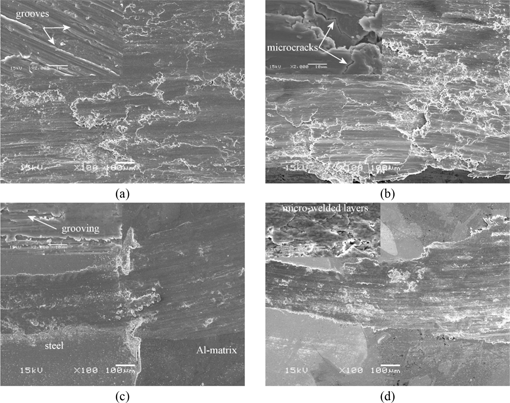
SEM micrographs showing the worn surfaces of cast A7075 (a and b) and A7075/SAF 2025 composite (c and d) materials contacted with 100Cr6 (a and c), ZrO2 counterpart (b and d).
Conclusion
In this study, dry sliding wear behavior of cast A7075 and A7075/SAF 2025 chip composite material was investigated. SAF 2205 chips were selected as reinforcing material due to higher mechanical properties of duplex stainless steel. Also, recycling was ensured and cost of the composite specimen was reduced by using SAF 2205 chips. Metallurgical bonding occurred between the matrix and reinforcing materials by liquid/solid process with infiltration of liquid metal. Also, intermetallic phases determined with microstructure analyses were the evidence of metallurgical bonding between the matrix and reinforcing materials.
A7075 alloy has a higher friction coefficient than that of A7075/SAF 2025 composite material. Composite material has a lower friction coefficient owing to higher hardness value than A7075 alloy. Also, the values of wear loss and wear rate measured for cast alloy are higher than those of composite material in the interaction with all counterparts.
References
[1] J.R. Davis, Aluminum and Aluminum Alloys, ASM International, Ohio (1993).Search in Google Scholar
[2] B. Venkataraman and G. Sundararajan, Wear, 245 (2000) 22–38.10.1016/S0043-1648(00)00463-4Search in Google Scholar
[3] A. Kısasöz, K.A. Güler and A. Karaaslan, Trans. Nonferrous Met. Soc. China, 22 (2012) 1563–1567.10.1016/S1003-6326(11)61356-3Search in Google Scholar
[4] R. İpek, J. Mater. Process. Tech., 162–163 (2005) 71–75.10.1016/j.jmatprotec.2005.02.207Search in Google Scholar
[5] B. Terry and G. Jones, Metal Matrix Composites: Current Developments and Future Trends in Industrial Research and Applications, Elsevier, Amsterdam (1990).Search in Google Scholar
[6] V.K. Lindroos and M.J. Talvitie, J. Mater. Process. Tech., 53 (1995) 273–284.10.1016/0924-0136(95)01985-NSearch in Google Scholar
[7] K.M. Shorowordi, T. Laoui, A.S.M.A. Haseeb, J.P. Celis and L. Froyen, J. Mater. Process. Tech., 142 (2003) 738–743.10.1016/S0924-0136(03)00815-XSearch in Google Scholar
[8] R.B. Bhagat, J. Mater. Sci., 24 (1989) 1496–1502.10.1007/BF02397092Search in Google Scholar
[9] R.P. Baron, J.A. Wert, D.A. Gerard and F.E. Wawner, J. Mater. Sci., 32 (1997) 6435–6445.10.1023/A:1018686505563Search in Google Scholar
[10] C. Colin, Y. Marchal, E. Boland and F. Delannay, J. Phys. IV, 3 (1993) 1749–1752.Search in Google Scholar
[11] D. Mandal, B.K. Dutta and S.C. Panigrahi, Mater. Sci. Eng. A, 492 (2008) 346–352.10.1016/j.msea.2008.03.031Search in Google Scholar
[12] D. Mandal, B.K. Dutta and S.C. Panigrahi, Wear, 257 (2004) 654–664.10.1016/j.wear.2004.02.006Search in Google Scholar
[13] A. Baradeswaran and A.E. Perumal, Compos Part B, 54 (2013) 146–152.10.1016/j.compositesb.2013.05.012Search in Google Scholar
[14] V.V.B. Prasad, K.S. Prasad, A.K. Kuruvilla, A.B. Pandey, B.V.R. Bhat and Y.R. Mahajan, J. Mater. Sci., 26 (1991) 460–466.10.1007/BF00576543Search in Google Scholar
[15] T.S. Srivatsan and E.J. Lavernia, J. Mater. Sci., 27 (1992) 5965–5981.10.1007/BF01133739Search in Google Scholar
[16] R. Tiwari, H. Herman, S. Sampath and B. Gudmundsson, Mater. Sci. Eng. A, 144 (1991) 127–131.10.1016/0921-5093(91)90217-BSearch in Google Scholar
[17] A. Mortensen and I. Jin, Int. Mater. Rev., 37 (1992) 101–128.10.1179/imr.1992.37.1.101Search in Google Scholar
[18] A. Çanakçı, F. Arslan and I. Yaşar, J. Mater. Sci., 42 (2007) 9536–9542.10.1007/s10853-007-1896-zSearch in Google Scholar
[19] K.A. Güler, A. Kisasöz, and A. Karaaslan, Russ. J. Non Ferr. Met., 54 (2013) 320–324.10.3103/S1067821213040068Search in Google Scholar
[20] K.A. Güler, A. Kisasöz and A. Karaaslan, Int. J. Mater. Res., 102 (2011) 304–308.10.3139/146.110476Search in Google Scholar
[21] P.A. Karnezis, G. Durrant and B. Cantor, Mater. Charact., 40 (1998) 97–109.10.1016/S1044-5803(97)00106-XSearch in Google Scholar
[22] A. Kısasöz, K.A. Güler and A. Karaaslan, Mater. Test., 53 (2011) 54–57.10.3139/120.110202Search in Google Scholar
[23] ASM Handbook. Vol. 09, ASM International, Ohio-USA (1990).Search in Google Scholar
[24] N. Mahathaninwong, T. Plookphol, J. Wannasin and S. Wisutmethangoon, Mater. Sci. Eng. A, 532 (2012) 91–99.10.1016/j.msea.2011.10.068Search in Google Scholar
[25] X. Li and M.J. Starink, Mater. Sci. Tech., 17 (2001) 1324–1328.10.1179/026708301101509449Search in Google Scholar
[26] N. Mahathaninwong, Y. Zhou, S.E. Babcock, T. Plookphol, J. Wannasin and S. Wisutmethangoon, Mater. Sci. Eng. A, 556 (2012) 107–113.10.1016/j.msea.2012.06.064Search in Google Scholar
[27] G. Wang, Z. Zhao, J. Cui and Q. Guo, Acta Metall. Sin., 25 (2012) 160–168.Search in Google Scholar
[28] F. Viana, A.M.P. Pinto, H.M.C. Santos and A.B. Lopes, J. Mater. Process. Tech., 92–93 (1999) 54–59.10.1016/S0924-0136(99)00219-8Search in Google Scholar
[29] Ş.H. Atapek and Ş. Polat, Met. Sci. Heat Treat., 55 (2013) 181–183.10.1007/s11041-013-9602-zSearch in Google Scholar
[30] A. Karaaslan, I. Kaya and H. Atapek, Met. Sci. Heat Treat., 9–10 (2007) 443–447.10.1007/s11041-007-0083-9Search in Google Scholar
[31] A. Karaaslan, I. Kaya and H. Atapek, Mater. Test., 50 (2008) 256–258.10.3139/120.100882Search in Google Scholar
[32] L.J. Vendra, J.A. Brown and A. Rabiei, J. Mater. Sci., 46 (2011) 4574–4581.10.1007/s10853-011-5356-4Search in Google Scholar
[33] K. Holmberg and A. Matthews, Coatings Tribology Techniques and Applications in Surface Engineering, Elsevier, Oxford (2009).Search in Google Scholar
[34] K.H.Z. Gahr, Microstructure and Wear of Materials, Elsevier,New York (1987).Search in Google Scholar
©2016 by De Gruyter
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Articles in the same Issue
- Frontmatter
- Research Articles
- Creep Behaviors Calculated by Varying Material Constants Obtained from Different Stress Regions for a Closed-End P91 Pipe
- Effect of Ultrasonic Treatment on Solidification Quality of ESR Ingots
- Synthesis of Posnjakite Nanoparticles in the Presence of a New Capping Agent
- Effects of Hot Isostatic Pressing (HIP) on Microstructure and Mechanical Properties of K403 Nickel-Based Superalloy
- Influence of Mechanical Alloying Time on Morphology and Properties of 15Cr-ODS Steel Powders
- Clean Utilization of CuCl Residue by Microwave Roasting under Oxygen-Enriched Condition
- Dry Sliding Wear Behavior of Cast A7075 and A7075/SAF 2205 Composite Material
- Synthesis and Characterization of Nanosized Manganese Oxyhydroxide Compounds by Sonochemical Method
- Synthesis and Characterization of Nanocrystalline Barium–Samarium Titanate
- Kinetics Calculation of the Non-isothermal Reduction of Pellet
- Deposition of Nano Tungsten Oxide on Glass Mat Using Hot Filament Chemical Vapor Deposition for High Catalytic Activity
- Preparation and Dielectric Properties of Si3N4/BN(CB) Composite Ceramic
- Structural and Magnetic Properties of Cr-Substituted NiCuZn Ferrite
Articles in the same Issue
- Frontmatter
- Research Articles
- Creep Behaviors Calculated by Varying Material Constants Obtained from Different Stress Regions for a Closed-End P91 Pipe
- Effect of Ultrasonic Treatment on Solidification Quality of ESR Ingots
- Synthesis of Posnjakite Nanoparticles in the Presence of a New Capping Agent
- Effects of Hot Isostatic Pressing (HIP) on Microstructure and Mechanical Properties of K403 Nickel-Based Superalloy
- Influence of Mechanical Alloying Time on Morphology and Properties of 15Cr-ODS Steel Powders
- Clean Utilization of CuCl Residue by Microwave Roasting under Oxygen-Enriched Condition
- Dry Sliding Wear Behavior of Cast A7075 and A7075/SAF 2205 Composite Material
- Synthesis and Characterization of Nanosized Manganese Oxyhydroxide Compounds by Sonochemical Method
- Synthesis and Characterization of Nanocrystalline Barium–Samarium Titanate
- Kinetics Calculation of the Non-isothermal Reduction of Pellet
- Deposition of Nano Tungsten Oxide on Glass Mat Using Hot Filament Chemical Vapor Deposition for High Catalytic Activity
- Preparation and Dielectric Properties of Si3N4/BN(CB) Composite Ceramic
- Structural and Magnetic Properties of Cr-Substituted NiCuZn Ferrite

