Abstract
The clean utilization of the CuCl residue containing chloride produced from zinc hydrometallurgy residue is very important for the recycle of valuable metals. A new method of microwave roasting under oxygen enriched condition was put forward to strengthen the process of dechlorination. It was demonstrated that the dechlorination rate of CuCl residue by microwave roasting was higher than that of conventional roasting by tubular resistance furnace under the same conditions (temperature, holding time and atmosphere). After roasting at a temperature of 450°C for 120 min under an oxygen flow rate of 150 mL/min in microwave field, the dechlorination rate of CuCl residue was as high as 97%, increasing 17% compared to that of conventional roasting using tubular resistance furnace. The characterizations of phase variation of CuCl residue in microwave field by means of X-ray diffraction (XRD), scanning electron microscopy-energy dispersive spectrometer (SEM-EDS) and Raman spectroscopy indicated that the process of dechlorination by microwave roasting under the oxygen enriched condition was much more efficient than conventional roasting.
Introduction
Chlorine (Cl) is a harmful element in zinc hydrometallurgical process, because a concentration of chlorine ion in excess of 100 mg/L during the process of electrolysis will affect the stability of electro deposition process [1, 2]. Thus, Cl- contained in the solution should be removed before electrodeposits. One way of dechlorination methods from zinc sulfate solution is the addition of Cu+ to form CuCl. However, it is very difficult to find out an efficient and cleaner method to realize the comprehensive utilization the CuCl residue from zinc hydrometallurgy. In most smelters, the residue was washed with distilled water and alkaline solution such as Na2CO3 [3]. CuCl containing in the residue is turned to NaCl solution subsequently, causing a lot of water treatment and waste discharge problems.
Microwave irradiation has been widely investigated due to its unique heating advantage [4–9]. Microwave heats materials at a molecular level, which leads to homogeneous and quick thermal heating [10–14]. The microwave assisted roasting has been investigated a lot recently [15−17]. Wei et al. [18] put forward a new process of chlorine removal from zinc dross by microwave roasting to solve the problem of recycling the zinc dross. The results showed that the zinc dross is a good microwave absorbing mineral under the condition of roasting temperature of 700°C, duration time of 60 min and stirring speed of 15 r/min, the dechlorination rate is as high as 88%. Ma et al. [19] measured the complex permittivity and the temperature rising of zinc oxide dust from rotary kilns, showing that zinc oxide dust is a good microwave absorber and can be heated quickly in the microwave field, confirming the feasibility of removing F and Cl from zinc oxide dust by microwave roasting. Li et al. [20] carried out the investigation of removing Cl from zinc oxide dust by microwave roasting, showing that dechlorination rate of zinc oxide dust could reach over 95% under the conditions of roasting temperature of 650°C, duration time of 30 min and stirring speed of 60 r/min and air flow of 300 L/h. In general, dechlorination in microwave field could obtain a higher dechlorination rate under a shorter reaction time and a lower roasting temperature than roasting with kiln or multiple hearth furnace.
A lot of investigations on the copper-chlorine (Cu-Cl) cycle process being used to produce hydrogen have been carried out due to its lower process temperature and higher yield. The main chemical reactions of Cu-Cl-O system at different temperatures were listed in Table 1 [21, 22]:
The main chemical reactions of Cu–Cl–O system.
| Temperature | Reaction |
| About 375℃ | 2CuCl (s) + 2O2 (g) = Cu2OCl2 (s) |
| 431–457℃ | Cu2OCl2 (s) +O2 = 2CuO (s) +Cl2 (g) |
| 375–470℃ | Cu2OCl2 (s) = CuCl (s or l) +CuO (s) +Cl2 (g) |
| 375–530℃ | Cu2OCl2 (s) = 2CuCl (l) + 1/2O2 (g) |
| 400–470℃ | 3Cu2OCl2 (s) = CuCl2 (s) + 2CuCl (s or l) +3CuO (s) + 2Cl2 (g) |
| Over 550℃ | CuCl (l) = CuCl (g) |
| CuCl (l) + O2 (g) = CuO (s) + Cl2 (g) |
In order to obtain higher dechlorination, special attention should be paid to the optimization of experimental conditions, minimizing the impact of additional separation steps, and maximizing the dechlorination reaction of just producing CuO and Cl2.
The objective of present work is to strengthen the dechlorination process of CuCl residue by microwave roasting under oxygen enriched condition. The microwave and conventional roasting of CuCl residue was compared. And the influences of temperature, roasting time and gas flow rate on the dechlorination rate were investigated by a single experiment. Products obtained by microwave roasting at different reaction conditions and time were analyzed by techniques of X-ray diffraction (XRD), SEM/EDS (scanning electron microscope-energy dispersive spectrometer) and Raman spectroscopy.
Experimental
Materials
The raw materials, CuCl residue, were obtained from a zinc hydrometallurgical plant in Yunnan Province, China. The main chemical composition and phase of CuCl residue after complete drying is shown in Table 2 and Figure 1.
The chemical elements of CuCl residue from zinc hydrometallurgy.
| Composition | Cu | Cl | O | S | Zn |
| Content (%) | 54.68 | 17.27 | 3.34 | 4.74 | 6.55 |

XRD patterns of the raw materials.
It can be seen in Figure 1 that cuprous chloride (CuCl) and cuprous oxide (Cu2O) are the major phase composition in the CuCl residue, a minor amount of ZnS is also present.
Experimental device
The conventional experiments were carried out in a high-temperature tubular resistance furnace.
The microwave higher temperature reactor with the power of 3 kW and frequency of 2,450 MHz was illustrated in Figure 2, which were designed and developed by the Key Laboratory of Unconventional Metallurgy, Ministry of Education. The microwave system consists of two magnetrons, a multi-mode cavity and a quartz glass tube. It is equipped with a water-cooled condenser and a temperature controller (temperature controlled by the input microwave power during the dechlorination process, measured by the thermocouple type of nichrome-nickel silicon armor, placed such that it touched raw, with a measurement precision of ±0.5°C) to adjust the microwave power level for a preset temperature. The thermocouple pyrometer was inserted into the center of the sample for taking temperature measurements. The measurements were recorded by a designated computer.

Schematic diagram of the microwave reactor system.
Experiment procedures
Conventional experiments: A thin layer of 20 g dried and ground sample placed in a mullite boat was put inside the resistance furnace and heated to 300–500°C at the oxygen flow rate of 150 mL/min and held for a duration of 120 min. And the off-gas was treated by absorbing bottles.
Microwave experiments: A thin layer of 20 g dried and ground sample placed in a mullite boat was put inside the microwave reactor and heated to 300–500°C at the oxygen flow rate of 50–150 mL/min and held for duration of 0–120 min.
Analysis and characterization
The content of Cu and Zn in raw materials, intermediate materials and roasted product was determined by EDTA titration method. And Cl content was measured by using turbidimetric method (as AgCl).
The phase composition of the CuCl residue and the oxidizing reaction of microwave roasting were characterized by XRD (D/Max 2200, Rigaku, Japan) using CuKα radiation (λ = 1.5418 Å and a Ni filter, in the 2θ range 5–100° with the scanning rate of 0.25°/min.
The Raman spectra of samples were carried out at room temperature using a confocal microprobe Raman system (Renishaw Ramascope System 1000, UK). A 514-nm argon laser was used for excitation. Backscattered Raman signals were collected through a microscope and holographic notch filters in the spectrum scattering detection region ranges from 100 to 1,000 cm−1, with a spectral resolution of 2 cm−1. The samples were exposed for 10 s.
The morphological aspects of the CuCl residue and the oxidation reaction in microwave field were investigated by SEM (XL30ESEM-TMP, Philips, Holland). The SEM instrument was operated at 20 kV in a low vacuum, while the EDS (USA) attached to the SEM was used for chemical analysis.
Results and discussion
Conventional experiments
The dechlorination rate of CuCl residue after conventional roasting using resistance furnace at different temperature (300–500°C) with time of 120 min under different atmospheres were shown in Figure 3.
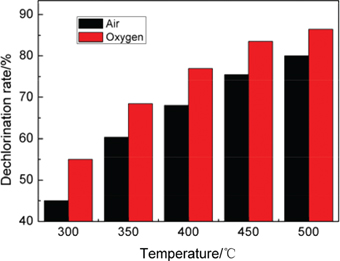
The dechlorination rate of CuCl residue after conventional roasting.
Figure 3 illustrated that both the reaction atmosphere and temperature of dechlorination process had great influence on the dechlorination rate. When the temperature reached to 450°C, the dechlorination rate achieved over 83% with 120 min roasting under the atmosphere of oxygen. And in the same conditions, the materials’ dechlorination rate after roasting under air atmosphere just reached around 80%. To obtain a higher dechlorination rate needs a longer reaction time and a higher roasting temperature in the dechlorination process by using tubular resistance furnace.
Microwave experiments
Temperature rise characteristic in microwave field
The rising temperature curve of sample (100 g) by microwave heating (1.5 kW) was shown in Figure 4.

The heating curve of CuCl residue under microwave irradiation.
It was demonstrated that after 15 min of microwave heating the temperature of the CuCl residue was heated up to 600°C. The CuCl residue could be heated quickly in the microwave field. And CuCl of the residue has the hyperactive response to the microwaves [13].
Effect of temperature, time and oxygen flow rate
The effect of temperature, time and oxygen flow rate which had great influences on the dechlorination efficiency were investigated under the optimal conditions as follow: microwave power: 1.5 kW, roasting time: 0–120 min, temperature: 300–500℃ and oxygen flow rate: 50–150 mL/min. The off-gas (Cl2) will be treated by ferrous chloride solution containing excessive iron filings in the absorbing bottles. The experimental results are shown in Figure 5.

The dechlorination rate of CuCl residue at different temperature, time and oxygen flow rate: (a) the effect of roasting time and oxygen flow rate at 450°C and (b) the effect of temperature.
It can be seen from Figure 5(a) that with the temperature got higher, the dechlorination rate increased strongly. When the microwave roasting temperature reached to 450°C, the dechlorination rate achieved over 96% with 90 min under oxygen enriched condition. While, the dechlorination rate after roasting just reached about 80% under air atmosphere compared to that of oxygen enriched condition. The dechlorination rate of CuCl residue roasted by microwave heating under oxygen enriched condition is much higher than the conventional roasting.
Figure 5(b) indicated that increased either roasting time or the oxygen flow rate, the dechlorination rate would increase. With the roasting time increased from 30 to 90 min at temperature of 450°C, the dechlorination rate increased to more than 40%. The oxygen flow rate at different roasting time also had effect on the dechlorination rate. With the oxygen flow rate increased, the dechlorination rate would increase. The dechlorination rate reached the highest at the oxygen flow rate of 150 mL/min.
Phase transformation process
The phase composition of the CuCl residue and the oxidizing reaction in microwave field were characterized by XRD, and the results are illustrated in Figure 6.

XRD patterns of the raw materials and roasted samples at 450°C: (a) raw materials, (b) for 30 min and oxygen, (c) for 60 min and oxygen, (d) for 90 min and oxygen, (e) for 90 min and air.
Figure 6(a) shows the crystalline compounds of the CuCl residue. It can be seen that cuprous chloride (CuCl) and cuprous oxide (Cu2O) are the major phase composition in the CuCl residue, a minor amount of ZnS also exist. Figure 6(b) shows the phase transformation of chlorine (Cl) containing in CuCl residue. By heating the samples up to 450℃ and holding time of 30 min (Figure 6(b)), the strong diffraction peaks of CuO are observed, CuCl peak got weak and Cu2O peak disappeared. The main phase of CuCl residue changed from CuCl and Cu2O to CuO, and a new phase of Cu2OCl2 was found during the roasting process. It can be inferred that the dechlorination reaction of CuCl in 30 min, transformation from Cu2O to CuO and new phase formation reaction happened. The oxidation reaction can be described as follows:
With the duration time increase from 30 and 60 min (Figure 6(c)), Cu2OCl2 peak turned weaker, and CuO phase’s peak turned stronger and sharper. It can be inferred that the decomposition reaction of Cu2OCl2 is the main chemical reaction, and the Cl in CuCl residue has been removed by transforming into Cl2. The chemical reaction can be described as follows:
With the duration time increase from 60 to 90 min (Figure 6(d)), Cu2OCl2 peak disappeared. The thermal decomposition of Cu2OCl2 is still the main reaction.
Figure 6(e) shows the major phase of CuCl residue after microwave roasting at 450°C for 90 min under air atmosphere. The phase of CuCl and Cu2OCl2 can be still founded in Figure 6(e). It can be found comparing to Figure 6(d) and (e) that roasting under oxygen enriched condition can promote the decomposition of the Cu2OCl2 and improve the dechlorination efficiency.
Figure 7 illustrated that the oxygen flow rate of dechlorination process had great influence on the dechlorination rate and the phase composition of roasted product. When the oxygen flow rate was low (50 and 100 mL/min), the phase of CuCl and Cu2OCl2 can be still founded in the roasted product with 90 min roasting. So in the process of dechlorination enough oxygen is necessary.

XRD patterns of the roasted samples at 450°C for 90 min under different oxygen flow rate.
Characterization by SEM-EDS
The microscopic structure of the mode of occurrence and the main elements are analyzed by SEM coupled with EDS, shown in Figure 8.

SEM and EDS analysis results of samples before and after microwave roasting: (a) raw materials and EDS result of spot “1”; and (b) microwave roasting at 450°C for 90 min and EDS result of spot “2.”
From Figure 8(a) and (b), it can be seen that the surface of the thermal decomposed residue is distinctly different from the raw sample. The results indicate that the surface structures of primary particles have a tighter and smoother surface morphology, with many small pits and a group of regular polyhedrons appearing on the CuCl residue surface. The surface of dechlorinated sample illustrated the formation of irregularly and coarse crystals, due to the elimination of chlorine from the original CuCl residue and the formation of CuO crystals. The EDS analysis results show a high dechlorination efficiency of microwave roasting under oxygen enriched condition.
Characterization by Raman
In order to analyze the oxidation mechanism of the CuCl residue, the oxidizing products in microwave field are investigated by micro-Raman spectroscopy. The Raman spectrum of the raw materials and oxidizing product of oxygen-enriched air roasting in microwave field, including cuprous chloride (CuCl), cuprous oxide (Cu2O), zinc sulfide (ZnS) and copper oxide (CuO), were presented in Figure 9, respectively.

Raman spectra of CuCl residue before and after microwave roasting at 450°C: (a) raw materials, (b) 30 min, (c) 60 min, (d) 90 min and (e) pure copper oxide.
Figure 9(a) shows six Raman active modes at 108, 152, 216, 425, 516 and 639 cm−1, respectively. The sharp first strong band at 108 and 152 cm−1 were due to the symmetric stretching vibrations and symmetric bending vibrations of CuCl [23], respectively. The Raman band at 425 and 516 cm−1 is directly assigned to ZnS, which is mainly due to characteristic vibrational Raman peak of nanometer ZnS [24]. The Raman spectrum of sample exhibited a sharp second strong band at 216 and 639 cm−1 is directly assigned to Cu2O [25, 26]. The cause of the Raman peaks at 639 cm−1 broad is due to the blending of the stretching vibration peak of Cu–O.
It can be seen from Figure 9(e) that the Raman peaks detected at 291, 340 and 623 cm−1, which are directly matched with CuO. The Raman spectra reveal three main phonon modes, at 291, 340 and 623 cm−1, corresponding to the Ag, B1g and B2g symmetries, respectively [27]. Figure 9(b), (c) and (d) show an unambiguous evidence of a continuous enhancement of the red-shift as well as the sharpened and strengthened of the Ag, B1g and B2g phonon modes when the holding time is sustained from 30 to 90 min of the investigated microwave treated samples. It has been documented that the phonon modes in single crystal CuO will shift slightly to a lower frequency and the peak will be broadened as a result of the nano-size effect [27]. As the diameter of the grain diameter increases, the Raman peak is expected to shift toward higher frequencies and the line shape will become symmetrical [28].
Conclusion
According to the heating curve of CuCl residue under microwave irradiation, the material was heated rapidly, showing that the CuCl residue has the hyperactive response to the microwaves.
Under the conditions of temperature 450°C, holding time 120 min and oxygen flow 150 mL/min in microwave field, the dechlorination rate of CuCl residue is as high as 97%, and the dechlorination rate increased more than 17% than that of roasting using tubular resistance furnace.
The comparison of XRD patterns shows that most of CuCl in residue has been removed by transforming into CuO. The EDS energy patterns confirm the high de-chlorination efficiency of microwave roasting. The Raman spectrum showed the existence of vibration bands of CuO in the roasted samples. The phase change of dechlorination process can be described as chemical reactions (1), (2), (3) and (4).
The dechlorination effect of CuCl residue from zinc hydrometallurgy by microwave roasting shows advantages such as high Cl removal rate, rapid heating and low roasting temperature, which extends a wide industrial prospect.
Funding statement: Funding: This work was funded by the National Natural Science Foundation of China (No. 51104073), Technology of People’s Republic of China, Yunnan Provincial Science and Technology Innovation Talents scheme, Technological Leading Talent (No. 2013HA002).
References
[1] N. Güresin and Y.A. Topkaya, Hydrometallurgy, 49 (1998) 179–187.10.1016/S0304-386X(98)00012-7Search in Google Scholar
[2] M.K. Jha, V. Kumar and R.J. Singh, Resour. Conserv. Recycl., 33 (2001) 1–22.10.1016/S0921-3449(00)00095-1Search in Google Scholar
[3] S.D. Lu, Y. Xia, C.Y. Huang, G.Q. Wu, J.H. Peng, S.H. Ju and L.B. Zhang, J. Cent. South Univ., 21 (2014) 1290–1295.10.1007/s11771-014-2065-6Search in Google Scholar
[4] Q.S. Liu, T. Zheng, N. Li, P. Wang and G. Abulikemu, Appl. Surf. Sci., 256 (2010) 3309–3315.10.1016/j.apsusc.2009.12.025Search in Google Scholar
[5] D.E. Clark and W.H. Sutton, Annu. Rev. Mater. Sci., 26 (1996) 299–331.10.1146/annurev.ms.26.080196.001503Search in Google Scholar
[6] S.A. Galema, Chem. Soc. Rev., 26 (1997) 233–238.10.1039/cs9972600233Search in Google Scholar
[7] M. Al-Harahsheh and S.W. Kingman, Hydrometallurgy, 73 (2004) 189–203.10.1016/j.hydromet.2003.10.006Search in Google Scholar
[8] Y. Li, R. Wang, F. Qi and C. Wang, Appl. Surf. Sci., 254 (2008) 4708–4715.10.1016/j.apsusc.2008.01.076Search in Google Scholar
[9] E.H. Grant and B.S.J. Halstead, Chem. Soc. Rev., 27 (1998) 213–224.10.1039/a827213zSearch in Google Scholar
[10] D.A. Jones, T.P. Lelyveld, S.D. Mavrofidis and S.W. Kingman, Resour. Conserv. Recycl., 34 (2002) 75–90.10.1016/S0921-3449(01)00088-XSearch in Google Scholar
[11] P. Lidström, J. Tierney, B. Wathey and J. Westman, Tetrahedron, 57 (2001) 9225–9283.10.1016/S0040-4020(01)00906-1Search in Google Scholar
[12] M. Oghbaei and O. Mirzaee, J. Alloy. Compd., 494 (2010) 175–189.10.1016/j.jallcom.2010.01.068Search in Google Scholar
[13] K.E. Haque, Int. J. Miner. Process, 57 (1999) 1–24.10.1016/S0301-7516(99)00009-5Search in Google Scholar
[14] R. Cecilia, U. Kunz and T. Turek, Chem. Eng. Process, 46 (2007) 870–881.10.1016/j.cep.2007.05.021Search in Google Scholar
[15] S. Guo, W. Li, J. Peng, H. Niu, M. Huang, L. Zhang, S. Zhang and M. Huang, Int. J. Miner. Process, 93 (2009) 289–293.10.1016/j.minpro.2009.09.001Search in Google Scholar
[16] A. Ghasemi, A. Hossienpour, A. Morisako, A. Saatchi and M. Salehi, J. Magn. Magn. Mater., 302 (2006) 429–435.10.1016/j.jmmm.2005.10.006Search in Google Scholar
[17] S.B. Cho, D.H. Kang and J.H. Oh, J. Mater. Sci., 31 (1996) 4719–4722.10.1007/BF00366375Search in Google Scholar
[18] Y.Q. Wei, J.H. Peng, L.B. Zhang, S.H. Ju, Y. Xia, Q. Zheng and Y.J. Wang, J. Cent. South Univ., 21 (2014) 2627–2632.10.1007/s11771-014-2222-ySearch in Google Scholar
[19] A.Y. Ma, L.B. Zhang, J.H. Peng, G. Ghen, C.H. Liu, H.Y. Xia and Y.G. Zuo, J. Microw. Power E.E., 48 (2014) 25–34.10.1080/08327823.2014.11689869Search in Google Scholar
[20] Z. Li, L. Zhang, A. Ma, J. Peng, J. Li and C. Liu, High Temp. Mat. PR-ISR., 33 (2014) 193–298.10.1515/htmp-2013-0037Search in Google Scholar
[21] A. Nixon, M. Ferrandon, M.H. Kaye and L. Trevani, J. Therm. Anal. Calorim., 110 (2012) 1095–1105.10.1007/s10973-011-1998-3Search in Google Scholar
[22] G.F. Naterer, K. Gabriel, Z.L. Wang, V.N. Daggupati and R. Gravelsins, Int. J. Hydrogen Energ., 33 (2008) 5439–5450.10.1016/j.ijhydene.2008.05.035Search in Google Scholar
[23] C. Ulrich, A. Göbel, K. Syassen and M. Cardons, Phys. Rev. Lett., 82 (1999) 351–354.10.1103/PhysRevLett.82.351Search in Google Scholar
[24] M. Scocioreanu, M. Baibarac, I. Baltog, I. Pasuk and T. Velula, J. Solid State Chem., 186 (2012) 217–223.10.1016/j.jssc.2011.12.012Search in Google Scholar
[25] R.C. Wang and H.Y. Lin, Mater. Chem. Phys., 13 (2012) 661–665.10.1016/j.matchemphys.2012.07.039Search in Google Scholar
[26] H. Gao, J. Zhang, M. Li, K. Liu, D. Guo and Y. Zhang, Curr. Appl. Phys., 13 (2013) 935–939.10.1016/j.cap.2013.01.049Search in Google Scholar
[27] M. He, M. Luo and P. Fang, J. Rare Earth, 24 (2006) 188–192.10.1016/S1002-0721(06)60091-4Search in Google Scholar
[28] M.H. Chou, S.B. Liu, C.Y. Huang, S.Y. Wu and C.L. Cheng, Appl. Surf. Sci., 254 (2008) 7539–7543.10.1016/j.apsusc.2007.12.065Search in Google Scholar
©2016 by De Gruyter
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Articles in the same Issue
- Frontmatter
- Research Articles
- Creep Behaviors Calculated by Varying Material Constants Obtained from Different Stress Regions for a Closed-End P91 Pipe
- Effect of Ultrasonic Treatment on Solidification Quality of ESR Ingots
- Synthesis of Posnjakite Nanoparticles in the Presence of a New Capping Agent
- Effects of Hot Isostatic Pressing (HIP) on Microstructure and Mechanical Properties of K403 Nickel-Based Superalloy
- Influence of Mechanical Alloying Time on Morphology and Properties of 15Cr-ODS Steel Powders
- Clean Utilization of CuCl Residue by Microwave Roasting under Oxygen-Enriched Condition
- Dry Sliding Wear Behavior of Cast A7075 and A7075/SAF 2205 Composite Material
- Synthesis and Characterization of Nanosized Manganese Oxyhydroxide Compounds by Sonochemical Method
- Synthesis and Characterization of Nanocrystalline Barium–Samarium Titanate
- Kinetics Calculation of the Non-isothermal Reduction of Pellet
- Deposition of Nano Tungsten Oxide on Glass Mat Using Hot Filament Chemical Vapor Deposition for High Catalytic Activity
- Preparation and Dielectric Properties of Si3N4/BN(CB) Composite Ceramic
- Structural and Magnetic Properties of Cr-Substituted NiCuZn Ferrite
Articles in the same Issue
- Frontmatter
- Research Articles
- Creep Behaviors Calculated by Varying Material Constants Obtained from Different Stress Regions for a Closed-End P91 Pipe
- Effect of Ultrasonic Treatment on Solidification Quality of ESR Ingots
- Synthesis of Posnjakite Nanoparticles in the Presence of a New Capping Agent
- Effects of Hot Isostatic Pressing (HIP) on Microstructure and Mechanical Properties of K403 Nickel-Based Superalloy
- Influence of Mechanical Alloying Time on Morphology and Properties of 15Cr-ODS Steel Powders
- Clean Utilization of CuCl Residue by Microwave Roasting under Oxygen-Enriched Condition
- Dry Sliding Wear Behavior of Cast A7075 and A7075/SAF 2205 Composite Material
- Synthesis and Characterization of Nanosized Manganese Oxyhydroxide Compounds by Sonochemical Method
- Synthesis and Characterization of Nanocrystalline Barium–Samarium Titanate
- Kinetics Calculation of the Non-isothermal Reduction of Pellet
- Deposition of Nano Tungsten Oxide on Glass Mat Using Hot Filament Chemical Vapor Deposition for High Catalytic Activity
- Preparation and Dielectric Properties of Si3N4/BN(CB) Composite Ceramic
- Structural and Magnetic Properties of Cr-Substituted NiCuZn Ferrite

