Abstract
Strontium carbonate (SrCO3) nanostructures were synthesized via simple hydrothermal method by Sr(NO3)2, ethylenediamine and hydrazine as reagents. The products were characterized with X-ray diffraction (XRD), scanning electron microscopy (SEM), energy-dispersive X-ray spectroscopy (EDX) and Fourier transform infrared spectroscopy (FT-IR). Different parameter’s effects on the product size and morphology were investigated. It was found that reagent concentration, reaction time and temperature play key roles in morphology of the obtained product.
Introduction
Nanomaterials and nanotechnologies attract tremendous attention in recent researches [1–4]. Strontium carbonate (SrCO3) is an important material in the production of glass for color television tubes and ferrite magnets for small DC motors [5], and also used in the production of iridescent and special glasses, pigments, driers, paints, pyrotechnics and catalysts [6,7]. Furthermore, SrCO3 has only one crystal phase, so it has been widely studied as a model system for biocrystallization [8,9]. It has been proven that SrCO3 materials have unique performance in low-temperature catalytic oxidation of VOC (volatile organic compound) and chemiluminescence sensors [10]. A variety of methods for the fabrication of SrCO3 such as self-assembled monolayers [11], ion entrapment method [12], hydrothermal method [13], microemulsion-mediated solvothermal method [14], homogeneous precipitation by enzyme-catalyzed reaction [15] have been reported. So far, SrCO3 particles with different morphologies have been produced, such as nanowires [16], flowerlike nanostructures [13], dendritic [16] and hexahedral ellipsoids [17]. In the present work, we synthesized SrCO3 nanostructures by the hydrothermal method. Different parameters such as Sr2+/ethylenediamine, reaction time and temperature were investigated on product size and morphology. Crystallinity and structure of the product were studied by X-ray diffraction (XRD) and Fourier transform infrared spectroscopy (FT-IR) spectroscopy. Chemical purity and stoichiometry of the product were characterized by Energy-dispersive X-ray spectroscopy (EDAX) analysis.
Experiment
Materials and physical measurements
All the chemical reagents used in this experiment such as Sr(NO3)2, ethylenediamine (C2H8N2) and hydrazine (N2H4.H2O) were of analytical grade and used as received without further purification. XRD patterns were recorded by a Rigaku D-max C III, X-ray diffractometer using Ni-filtered Cu Kα radiation. Scanning electron microscopy (SEM) images were obtained on Philips XL-30ESEM equipped with an energy-dispersive X-ray spectroscopy (EDX). FTIR spectra were recorded on a Shimadzu Varian 4300 spectrophotometer in KBr pellets.
Preparation of SrCO3 nanostructures
Different mole ratios of Sr(NO3)2 and ethylenediamine were dissolved at water and mixed together. Specified value of hydrazine was added to obtain solution and the resulting solution was transferred to autoclave. The reaction was done at 140–180°C for 6–24 h and then the autoclave was gradually cooled down to room temperature. The obtained powder was centrifuged and washed several times with water and absolute ethanol for removing probably byproducts. Finally, the product was dried at 80°C for 10 h. Experimental condition of SrCO3 formation was shown in Table 1.
Experimental condition of SrCO3 formation.
| Sample no. | Sr2+:en | Reaction time (h) | Reaction temperature (°C) |
| 1 | 1:1 | 24 | 180 |
| 2 | 1:2 | 24 | 180 |
| 3 | 1:3 | 24 | 180 |
| 4 | 1:4 | 24 | 180 |
| 5 | 1:5 | 24 | 180 |
| 6 | 1:6 | 24 | 180 |
| 7 | 1:4 | 6 | 180 |
| 8 | 1:4 | 12 | 180 |
| 9 | 1:4 | 18 | 180 |
| 10 | 1:4 | 30 | 180 |
| 11 | 1:4 | 24 | 140 |
| 12 | 1:4 | 24 | 150 |
| 13 | 1:4 | 24 | 160 |
| 14 | 1:4 | 24 | 170 |
Results and discussion
X-ray diffraction pattern
Figure 1 shows XRD spectra of the obtained product. As shown in Figure 1, all of the peaks are related to SrCO3 with orthorhombic (JCPDS = 05–0418). There are not any other peaks in this spectrum and it has been concluded that the as-synthesized product has high purity. The calculated crystal size by deby-sherrer formula was about 14.8 nm.
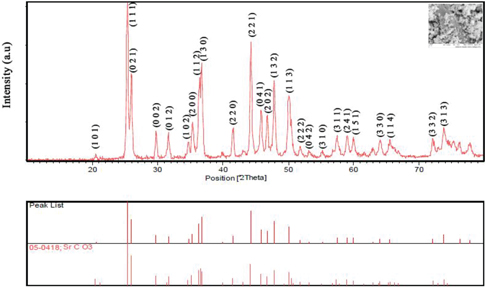
XRD of sample no. 11.
EDAX spectroscopy
Chemical purity of synthesized product was examined with EDAX spectroscopy. As shown in Figure 2, there are Sr, C and O peaks that verified the presence of SrCO3 in product. Si peak is related to the used substrate for EDAX analysis.

EDAX spectroscopy of sample no. 11.
FT-IR spectroscopy
Figure 3(a) shows FT-IR of sample no. 7. Related peaks to SrCO3 are seen in 1,771.34, 1,467.48, 1,070.34, 858.01 and 702.88 cm−1 [18,19]. Broad peak at 3,727.42 cm−1 is related to stretching vibration frequency of OH group at H2O molecule that has been adsorbed on the sample surface [2,3]. Strong absorption peak at 1,467.48 cm−1 is allocated to asymmetric stretching vibration of C–O band at CO32– and weak peak at 1,070.34 cm−1 is related to symmetric stretching vibration of C–O band at CO32–. Also two absorption peaks at 702.88 and 858.01 cm−1 can be assigned to the bending out-of-plane vibrations and in-plane vibrations [20]. Absorption peak at 1,771.34 cm−1 is related to bond stretching vibrations of C = O [21]. Figure 3(b) shows FT-IR of sample no. 11. The observed peaks in this spectrum are exactly similar to FT-IR of sample no. 7, and related peaks of SrCO3 are clearly seen at 1,771.59, 1,446.46, 170.35, 857.80 and 702.90 cm−1. Figure 3(c) shows the FT-IR spectra of purified sample no. 11. The purified sample was obtained as follows: as-synthesized sample no.11 was added to water and was heated on the heater. Then the reaction was done in autoclave at 140°C for 24 h. With the purification, the peak’s intensity of prototype was increased. Broad peak at 3,428.54 cm−1 is related to H2O molecule adsorption on the sample surface.

FT-IR of (a) sample no. 7, (b) sample no. 11 and (c) purified sample 11.
SEM analysis
Ethylenediamine concentration effect
Figure 4 shows Sr:en mole ratio effect on product size and morphology. When the mole ratio was selected to 1:1 (Figure 4(a)), microstructures that have been composed from microrods, irregular plates, small and large particles were obtained. By increasing the mole ratio to 1:2 (Figure 4(b)), regular rods and irregular particles were achieved. With further increase of concentration (1:3), some of the rods were broken and irregular particles, and plates have been formed (Figure 4(c)). In the mole ratio of 1:4, some of the rods were aggregated together and thick rods were obtained (Figure 4(d)). By increasing the mole ratio to 1:5 (Figure 4(e)) and 1:6 (Figure 4(f)), absence and presence of SrCO3 rods can be seen. So in specified concentration, one dimension growth of SrCO3 can be done.
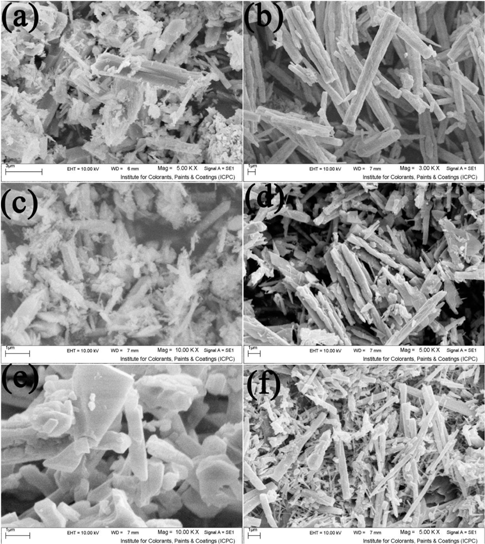
SEM image of (a–f) sample no. 1–6, respectively.
Time effect
For studying time effect on product size and morphology, the mole ratio and reaction temperature were fixed at 1:4 and 180°C, respectively. Then the reaction was done at different times. When the reaction time was selected to 6 h (Figure 5(a)), aggregated nanoparticles were obtained that by increasing the time to 12 h (Figure 5(b)), this nanoparticle became larger and lump-like masses were achieved. By increasing the time to 18 h (Figure 5(c)), the growth process has been continued and microrods were formed. When the reaction was done at 24 h (Figure 5(d)), the rods aggregated together and thicker rods were obtained. Finally with processing at 30 h (Figure 5(e)), aggregated nanoparticles were collected to micro-plates and microrod formation is still continued.
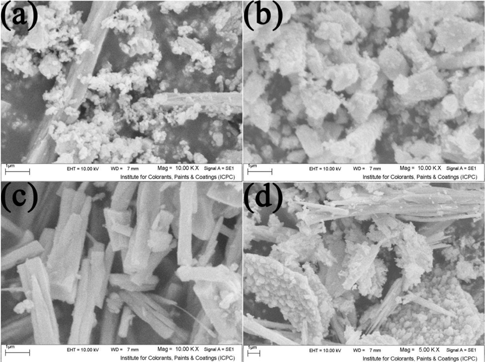
SEM image of (a–d) sample no. 7–10, respectively.
Temperature effect
For investigation of temperature effect on product size and morphology, the Sr2+:en mole ratio and reaction time were considered 1:4 and 24 h, respectively. When the reaction was proceeded at 140°C (Figure 6(a)), small and uniform particles were obtained. By increasing the temperature to 150°C (Figure 6(b)), the growth rate became dominant in comparison to nucleation rate and, therefore, the nanoparticles have been grown one dimensional and uniform microrods were formed. When the reaction was done at 160°C (Figure 6(c)), length and diameter of the rods have been increased and aggregated together. But the temperature was selected to 170°C (Figure 6(d)), the rods were broken and finally rods, particles and plates were achieved. The morphology change indicates that morphology and reaction temperature have close relationship.
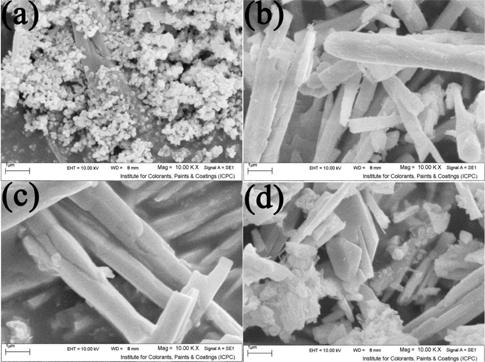
SEM image of sample no. 11–14.
Conclusion
SrCO3 nanostructures were synthesized via simple hydrothermal method. Many parameters were investigated on product size and morphology. It was found that Sr2+/ethylenediamine, reaction time and temperature play key role in morphology of the obtained product. The structure of the product was studied by X-ray diffraction pattern, and it was demonstrated that synthesized product is SrCO3 with orthorhombic phase. Other tool for characterization of the product was FT-IR spectroscopy. FT-IR spectra of SrCO3 showed that for achieving pure product, a purification step is necessary. Chemical purity of the obtained product was characterized with EDAX spectroscopy, and it was found that Sr, C and O are the elements present in the product.
Acknowledgements
Authors are grateful to the council of Iran National Science Foundation and University of Kashan for their unending effort to provide financial support to undertake this work.
References
[1] F.Mohandes, F.Davar and M.Salavati-Niasari, J. Magn. Magn Mater., 322 (2010) 872–877.Search in Google Scholar
[2] M.Salavati-Niasari, F.Davar and M.Farhadi, J. Sol-Gel Sci. Technol., 51 (2009) 48–52.Search in Google Scholar
[3] M.Salavati-Niasari, F.Davar and Z.Fereshteh, J. Alloys Compd., 494 (2010) 410–414.Search in Google Scholar
[4] M.Salavati-Niasari, N.Mir and F.Davar, J. Alloys Compd., 476 (2009) 908–912.Search in Google Scholar
[5] T.J.Bastow, Chem. Phys. Lett. 354 (2002) 156–159.10.1016/S0009-2614(02)00135-5Search in Google Scholar
[6] M.Erdemolu and M.Canbazolu, Hydrometallurgy, 49 (1998) 135–150.10.1016/S0304-386X(98)00018-8Search in Google Scholar
[7] G.Owusu and J.E.Litz, Hydrometallurgy, 57 (2000) 23–29.10.1016/S0304-386X(00)00091-8Search in Google Scholar
[8] J.Küther, G.Nelles, R.Seshadri, M.Schaub, H.J.Butt and W.Tremel, Chem. A Eur. J., 4 (1998) 1834–1842.10.1002/(SICI)1521-3765(19980904)4:9<1834::AID-CHEM1834>3.0.CO;2-6Search in Google Scholar
[9] J.Küther, R.Seshadri, G.Nelles, W.Assenmacher, H.J.Butt, W.Mader and W.Tremel, Chem. Mater., 11 (1999) 1317–1325.10.1021/cm980773aSearch in Google Scholar
[10] J.Shi, J.Li, Y.Zhu, F.Wei and X.Zhang, Anal. Chim. Acta, 466 (2002) 69–78.10.1016/S0003-2670(02)00549-4Search in Google Scholar
[11] J.Aizenberg, A.J.Black and G.M.Whitesides, J. Am. Chem. Soc., 121 (1999) 4500–4509.Search in Google Scholar
[12] D.Rautaray, S.R.Sainkar and M.Sastry, Langmuir, 19 (2003) 888–892.Search in Google Scholar
[13] S.Li, H.Zhang, J.Xu and D.Yang, Mater. Lett., 59 (2005) 420–422.Search in Google Scholar
[14] M.Cao, X.Wu, X.He and C.Hu, Langmuir, 21 (2005) 6093–6096.Search in Google Scholar
[15] I.Sondi and E.Matijević, Chem. Mater., 15 (2003) 1322–1326.Search in Google Scholar
[16] Q.Huang, L.Gao, Y.Cai and F.Aldinger, Chem. Lett., 33 (2004) 290–291.Search in Google Scholar
[17] L.Shi and F.Du, Mater. Lett., 61 (2007) 3262–3264.Search in Google Scholar
[18] S.M.Teleb, D.El-Sayed Nassr and E. M.Nour, Bull. Mater. Sci., 27 (2004) 483–485.Search in Google Scholar
[19] M.A.Alavi and A.Morsali, Ultrason. Sonochem., 17 (2010) 132–138.Search in Google Scholar
[20] L.Chen, Y.Shen, A.Xie, F.Huang, S.Li and Q.Zhang, Cryst. Res. Technol., 43 (2008) 797–800.Search in Google Scholar
[21] H.V.Tran, L.D.Tran, H.D.Vu and H.Thai, Coll. Surf. A Physicochem. Eng. Aspects, 366 (2010) 95–103.Search in Google Scholar
©2016 by De Gruyter
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Articles in the same Issue
- Frontmatter
- Numerical Simulation to Study the Effect of Arc Travelling Speed and Welding Sequences on Residual Stresses in Welded Sections of New Ferritic P92 Pipes
- Microstructural Evolution and Compressive Properties of Two-Phase Nb-Fe Alloys Containing the C14 Laves Phase NbFe2 Intermetallic Compound
- Optimization of Microwave Roasting for Dechlorination of CuCl Residue under Oxygen-Enriched Condition
- Evaluation of High Temperature Properties and Microstructural Characterization of Resistance Spot Welded Steel Lap Shear Joints
- Microstructural Changes of a Creep-Damaged Nickel-Based K002 Superalloy Containing Hf Element under Different HIP Temperatures
- Effect of Ultrasonic Treatment on the Solidification Microstructure of GCr15 Bearing Steel
- Effects of Ultrasonic Treatment on Microstructure and Properties of Al-Based Composites Reinforced by In Situ Al2O3 Nanoparticles
- High-Temperature Oxidation Behavior of Fe-Si-Ce Alloys
- Reaction Mechanism of Siderite Lump in Coal-Based Direct Reduction
- EAF Gas Waste Heat Utilization and Discussion of the Energy Conservation and CO2 Emissions Reduction
- Numerical Parametric Analysis of Bond Coat Thickness Effect on Residual Stresses in Zirconia-Based Thermal Barrier Coatings
- The Marker Conservation Law in Multiphase Systems
- Synthesis and Characterization of Strontium Carbonate Nanostructures via Simple Hydrothermal Method
Articles in the same Issue
- Frontmatter
- Numerical Simulation to Study the Effect of Arc Travelling Speed and Welding Sequences on Residual Stresses in Welded Sections of New Ferritic P92 Pipes
- Microstructural Evolution and Compressive Properties of Two-Phase Nb-Fe Alloys Containing the C14 Laves Phase NbFe2 Intermetallic Compound
- Optimization of Microwave Roasting for Dechlorination of CuCl Residue under Oxygen-Enriched Condition
- Evaluation of High Temperature Properties and Microstructural Characterization of Resistance Spot Welded Steel Lap Shear Joints
- Microstructural Changes of a Creep-Damaged Nickel-Based K002 Superalloy Containing Hf Element under Different HIP Temperatures
- Effect of Ultrasonic Treatment on the Solidification Microstructure of GCr15 Bearing Steel
- Effects of Ultrasonic Treatment on Microstructure and Properties of Al-Based Composites Reinforced by In Situ Al2O3 Nanoparticles
- High-Temperature Oxidation Behavior of Fe-Si-Ce Alloys
- Reaction Mechanism of Siderite Lump in Coal-Based Direct Reduction
- EAF Gas Waste Heat Utilization and Discussion of the Energy Conservation and CO2 Emissions Reduction
- Numerical Parametric Analysis of Bond Coat Thickness Effect on Residual Stresses in Zirconia-Based Thermal Barrier Coatings
- The Marker Conservation Law in Multiphase Systems
- Synthesis and Characterization of Strontium Carbonate Nanostructures via Simple Hydrothermal Method

