Abstract
Human disc large (DLG1) is a scaffolding protein that through the interaction with diverse cell partners participates in the control of key cellular processes such as polarity, proliferation and migration. Experimental data have mainly identified DLG1 as a tumor suppressor. An outstanding point for DLG1 protein is that altered DLG1 expression and DLG1 gene mutations were observed in different pathologies, including cancer and neurological and immunological disorders. Evident changes in DLG1 abundance and/or cell localization were identified in a number of studies suggesting its participation in molecular mechanisms responsible for the development of such illnesses. In this review, we focus on some of the latest findings regarding DLG1 alterations in different diseases as well as its potential use as a biomarker for pathological progression. We further address the current knowledge on the molecular mechanisms regulating DLG1 expression and the posttranslational modifications that may affect DLG1 cell localization and functions. Despite the advances in this field, there are still open questions about the precise molecular link between alterations in DLG1 expression and the development of each specific pathology. The complete understanding of this concern will give us new scenarios for the design of promising diagnosis and therapeutic tools.
Introduction
The polarity protein discs large 1 (DLG1) belongs to the family of molecular scaffolding proteins known as membrane associated guanylate kinases (MAGUKs). As for the other family members, DLG1 contains multi-PDZ (PSD95/DLG/ZO-1) domains, a Src homology 3 interaction module and an enzymatically inert guanylate kinase domain. An accumulating body of evidence indicates that the modular organization and scaffolding properties of MAGUKs play crucial roles in important cellular processes and features of different cell types, like the regulation of signaling pathways which are linked to the control of tissue growth, differentiation, cell migration and cell architecture plasticity (Won et al., 2017).
DLG1 is a component of the conserved Scribble polarity complex. Genetic and functional analyses highlighted the relevance of DLG1 in cell processes which require intracellular asymmetry, including the establishment and maintenance of cell polarity, as well as asymmetric cell division and cell migration (Knoblich, 2008; O’Neill et al., 2011; Golub et al., 2017; Stephens et al., 2018). In epithelial cells, DLG1 is localized to the adherens junctions in association with components of the cytoskeleton, where it coordinates junction formation and stability while contributing to the maintenance of apical-basal polarity (Laprise et al., 2004). Several mammalian DLG1 binding partners have been identified and many of them are key regulators of cellular signaling networks, highlighting the significant role of this scaffolding protein in mammalian cells. In addition, DLG1 was shown to be targeted by multiple human viral oncoproteins including the human papillomavirus (HPV) E6, the adenovirus E4-ORF1 and the human T cell leukemia virus type 1 (HTLV-1) Tax (Gardiol et al., 1999; Gardiol et al., 2002; Hirata et al., 2004; Kong et al., 2014; Marziali et al., 2017). These interactions have been implicated in the transforming and tumorigenic properties of the viral oncoproteins, giving DLG1 a key participation in viral pathogenesis. However, other works stand for the idea of DLG1 contributing to human epithelial cancers unrelated to viral infections as well.
Loss of apicobasal polarity has been recognized as a fundamental step in tumorigenesis. In this regard, initial experiments in Drosophila demonstrated that the lack of DLG1 expression leads to uncontrolled epithelial cell proliferation and neoplastic transformation, thereby defining DLG1 as a potential tumor suppressor. This hypothesis was extrapolated to mammals considering that epithelial cells from invasive carcinomas generally exhibit DLG1 null expression. Although DLG1 has historically been characterized as a tumor suppressor (Elsum et al., 2012; Sandoval et al., 2013) other studies challenged this notion. In fact, DLG1 varies in expression among malignancies and is known to play different and sometimes opposing roles in processes involved in tumor progression, including differentiation, cytokinesis, proliferation, cell migration and control of the microenvironment of the tumor (Roberts et al., 2012; James and Roberts, 2016; Stephens et al., 2018). These data suggest that DLG1 can acquire oncogenic attributes in some specific contexts; emphasizing the importance of DLG1 deregulation in human carcinogenesis, as described.
Even though most of the knowledge about DLG1 derives from experiments in the epithelial context, DLG1 is also expressed in cells from the nervous and immunological systems. In regard to the former, a growing number of reports have suggested DLG1 participation in several exocytotic and endocytotic pathways by directing components of the vesicle trafficking machinery either to the plasma membrane or to transport vesicles, regulating neuron receptor localization and clustering (Walch, 2013). Furthermore, DLG1 contributes to nerve cell functions by regulating various signaling pathways that organize the architecture and functionality of the synapses (Fourie et al., 2014). Meanwhile, the activity of DLG1 in immune cell biology has been ascertained for different T-cell types. In general, DLG1 mediates signal transduction downstream to T-cell receptors by facilitating microtubule polarization at the immunological synapses and by bridging significant kinases with their phosphorylation targets in such cell region (Round et al., 2005; Lasserre et al., 2010).
During the last years, different excellent revisions have addressed the plethora of interactions between DLG1 and cellular or viral partners that have contributed to explaining how DLG1 participates in the processes described above (Saito et al., 2018; Stephens et al., 2018). In this work, we will present the current knowledge about the expression of DLG1 in diverse human pathologies through alterations in its abundance and localization as well as specific genetic changes in DLG1 gene. We will also discuss emerging evidence that supports a key dual role for DLG1 in cancer, with both oncogenic or tumor suppressor activities according to the biological scenario. Moreover, we will extend the analysis to pathologies related to neurological and inflammatory processes. Finally, we will summarize the available data on the regulation of DLG1 expression and its impact on the development of cancer and other human pathologies.
DLG1 Expression in different pathologies
Epithelial cancer progression
A particular interest in DLG1 is due to its altered expression during the malignant progression of epithelial tumors (Figure 1A). Initial evidence was gathered from the analysis of HPV-induced cervical carcinogenesis. Cell culture experiments had shown that DLG1 was targeted for proteasomal degradation by the transforming oncoprotein E6 from high-risk HPV (Gardiol et al., 1999, 2002). Such findings prompted an investigation of DLG1 expression in the evolution of cervical lesions. In this sense, immunohistochemistry assays revealed a loss of DLG1 expression in invasive stages of the disease (Watson et al., 2002; Cavatorta et al., 2004). The fact that HPV E6 oncogene were highly upregulated in such stages suggested DLG1 proteasomal degradation, which was then supported by experiments showing that cervical cancer cell lines restored DLG1 levels and localization at the cell borders upon E6 ablation or proteasome inhibition (Massimi et al., 2004). However, striking changes in DLG1 expression were also observed in the early onset of disease, namely the preceding low-grade intraepithelial lesions (LSIL). In certain LSIL cases, reduced localization at the cell borders was evidently accompanied by increased expression in the cytoplasmic region (Cavatorta et al., 2004, 2017). Altogether, these initial analyses served to associate loss of DLG1 expression with cervical invasive stages, supporting the hypothesis of tumor suppressor functions of DLG1. Moreover, they also raised the possibility of a role of DLG1 mislocalization during the onset of cervical pathology. In addition, these analyses led to an interest to study the scenario in epithelial tumors unrelated to HPV infection. In this sense, biopsies from different colon adenocarcinomas were analyzed for DLG1 expression and, interestingly, some changes were detected. Semi-differentiated colon adenocarcinomas exhibited a reduction of DLG1 expression at the cell borders with a diffuse and increased cytoplasmic staining (Cavatorta et al., 2004; Gardiol et al., 2006). Furthermore, DLG1 protein was nearly undetectable in poorly differentiated stages. DLG1 expression was also analyzed in lung, larynx, breast and, more recently, in hepatocellular cancers. In all cases, a clear downregulation of its levels was found in late stages of malignancy (Fuja et al., 2004; Byeon et al., 2011; Szymanowska-Narloch et al., 2013; Wu et al., 2016). Moreover, in some of these reports, DLG1 upregulation and loss from cell borders were also observed, as well as mislocalization in intermediate lesions.
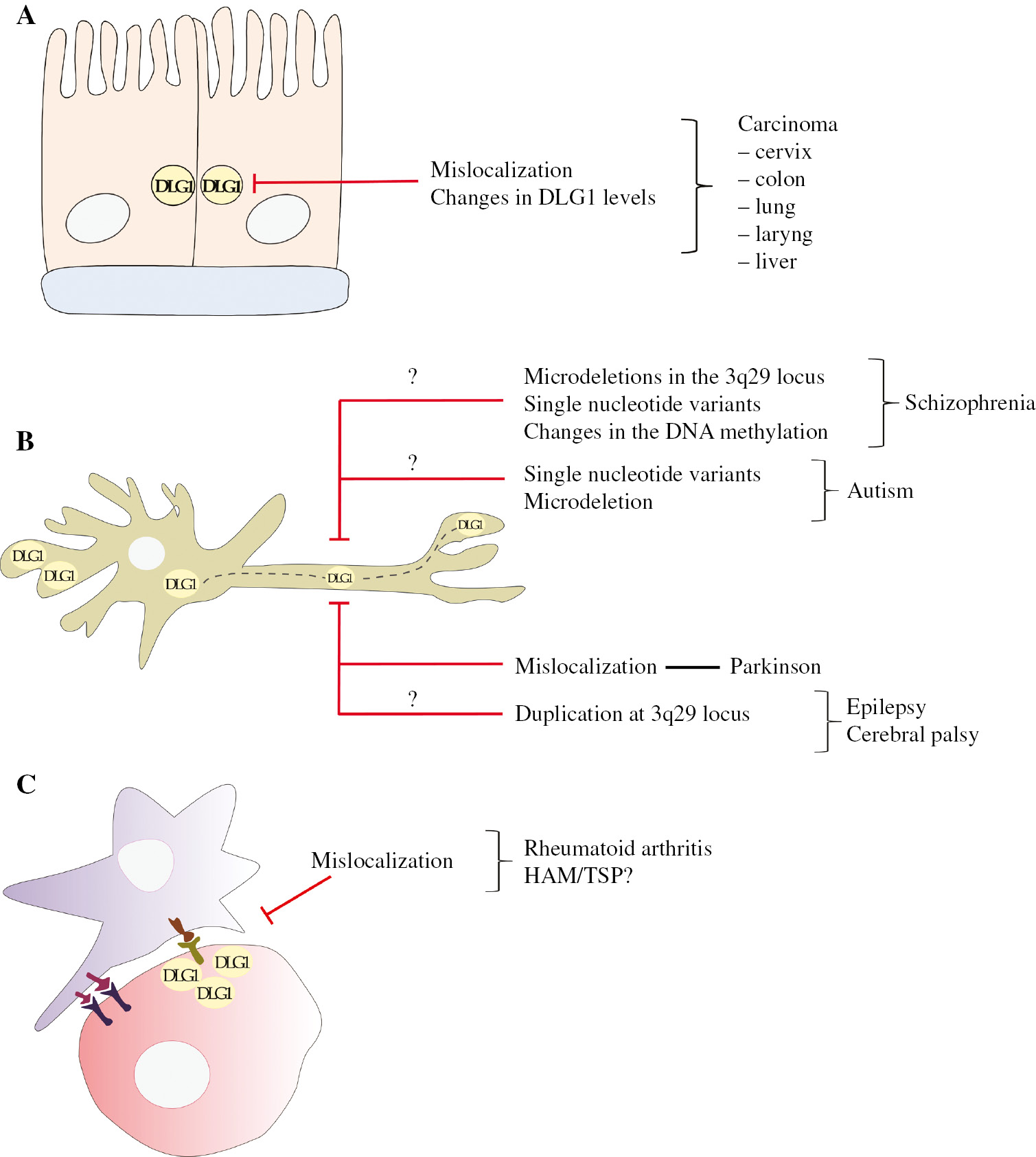
Alterations in DLG1 expression are associated to different human pathologies.
(A) Loss of DLG1 normal cell localization at the cell borders and changes in its abundance are observed during epithelial tumor development at diverse anatomical sites. (B) Different neurological diseases are linked to diverse genetic mutations in the DLG1 gene and probably to changes in DNA methylation status. (C) Redistribution of DLG1 in T-lymphocytes with loss of its expression at the immunological synapses can deregulate signaling pathways, driving to inflammatory illnesses.
Taken together, the results described suggest a common pattern for DLG1 expression during epithelial tumor evolution. Loss of DLG1 expression could indeed be considered a late stage of epithelial malignancy. On the other hand, the loss of DLG1 from its regular site of cell-to-cell interactions, accompanied by eventual overexpression, may take place in less severe lesions. The intriguing question of possible effects of such misexpression on the pathology outcome (better or worse prognosis) have been addressed in some exciting recent works. In the case of cervical cancer, our group performed a follow-up analysis associating the DLG1 staining pattern at LSIL stage with progression of malignancy or regression to a normalization of the epithelium (as frequently occurs at this stage). Interestingly, all LSIL cases with a membrane-associated DLG1 staining, resembling that of a normal tissue, regressed. However, LSILs with DLG1 misdistribution and overexpression progressed to high-grade intraepithelial lesions. In another study which analyzed colorectal tumor samples, increased and cytoplasmic expression of DLG1 was closely associated with malignancy (Zhu et al., 2017). Interestingly, such patients suffered a more aggressive form of the disease being reflected on enhanced tumor evasion and low overall survival. Finally, research carried out in endometrial cancer tissues showed that patients with DLG1 cytoplasmic staining were linked to nodal metastasis and poorer overall survival than those with a strong or weak membrane-associated expression (Sugihara et al., 2016). Therefore, these data clearly suggest that DLG1 misexpression is highly associated with tumor progression or more malignant forms of the disease. Considering DLG1 scaffolding properties, it is possible that its contribution or implication in cancer relates to its inability to interact with the correct partners in a spatial/temporal manner. As DLG1 redistribution to the cytoplasm is normally achieved during the S phase of the cell cycle, this pattern of expression could be associated with a proliferative state of epithelial cells. In the context of a tumor cell, such misexpressions are likely to synergize with abnormal expression of wild-type or mutated oncogenes in order to fully contribute to cell transformation. In line with this, more studies are necessary to understand the precise mechanism by which DLG1 deregulation contributes to malignancy. Nevertheless, it is important to note that the DLG1 staining pattern could serve to predict evolution of disease.
Neurological disorders
DLG1 contributes to the maintenance of neuronal homeostasis. DLG1 participates in the trafficking of glutamate and aspartate receptors and α-subunits of the potassium channels Kv1.4 and Kv4.2 to the cell membrane, all of which are crucial for basal synaptic transmission and synaptic plasticity (Gardoni et al., 2007; Fourie et al., 2014). Accordingly, there is mounting evidence that associates DLG1 deficiency or DLG1 mutant variants with the development of a number of neurological disorders (Figure 1B) (Soler et al., 2018). Perhaps the best defined is schizophrenia (SZ), which relies on altered glutamate neurotransmission (Sato et al., 2008; Uezato et al., 2012). Studies analyzing schizophrenic post-mortem brains reported a marked loss of DLG1 expression in the prefrontal cortex region. Microdeletions in the 3q29 locus, which contains the DLG1 gene, were found in such patients (Toyooka et al., 2002; Mulle et al., 2010; Quintero-Rivera et al., 2010; Kirov et al., 2012; Glassford et al., 2016). More recently, additional causes that may contribute to SZ susceptibility in regard to DLG1 expression have been reported. A study employing DNA methylation chip arrays demonstrated changes in the methylation pattern of the DLG1 gene and the upstream region, whereas other reports showed SZ-associated non-synonymous single nucleotide variants (SNVs) within the DLG1 coding sequence (Fromer et al., 2014; Purcell et al., 2014; Xing et al., 2016; McKinney et al., 2017). Remarkably, similar genetic variations in the DLG1 gene were demonstrated in further neurological pathologies. SNV and microdeletion were observed in autism-spectrum disorders while single nucleotide polymorphisms (SNP, which represent SNV that are at least as 1% frequent in population) were detected in cases of frontotemporal dementia. This last disorder affects the frontal-temporal lobes of the brain and is linked to progressive deterioration of behavior and cognition (Rabinovici and Miller, 2010). Interestingly, an abnormal hypomethylation state of DLG1 promoter region was also found in this pathology (Taskesen et al., 2017).
Parkinson’s disease (PD) is a degenerative disorder of the central nervous system, and the etiological causes are poorly understood. In animal models, a reduced expression and altered cellular distribution of the rat DLG1 homologue, SAP97, was observed in Parkinson’s drug induced experiments (Nash et al., 2005). Furthermore, microarray studies analyzing gene expression in whole blood identified DLG1 as a candidate gene for PD (Sun et al., 2014). These reports suggest a potential involvement of DLG1 misexpression in the pathobiology of this neurological disorder.
Finally, interstitial microduplication of 3q29 has recently been described in patients with widely variable phenotypes. A case report study described a 1.607Mb duplication at 3q29 locus, involving several genes including DLG1, in an individual with intellectual disability, epilepsy and cerebral palsy. The association of this genetic change with DLG1 expression level and with these severe clinical manifestations requires further investigation to understand whether or not DLG1 dysfunctions could play a role in these syndromes (Fernández-Jaén et al., 2014).
Inflammatory and autoimmune diseases
Regulatory T cells (Tregs) are central players in maintaining immune tolerance to self-antigens, preventing autoimmune diseases (Kitagawa and Sakaguchi, 2017). Treg immunosuppressive functions rely on TCR engagement and activation of downstream signaling pathways (Li and Rudensky, 2016). Evidence suggests that DLG1 accumulation at the immunological synapse (IS) must be particularly important for Tregs activation as these cells were shown to recruit a four-fold higher level of DLG1 than other T-cell types (Zanin-Zhorov et al., 2012, 2017). Importantly, DLG1 was demonstrated to modulate both nuclear factor of activated T cells (NFAT) and phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt) signaling pathways whose precise regulation is necessary for proper Treg functions. In this regard, DLG1 interacts to p38 MAP kinase and stimulates its activity which is required for NFAT activation and immunosuppressive cytokine production. In addition, DLG1 confers stability to PTEN phosphatase, which in turn downregulates the PI3K activity that normally impairs Treg functions (Zanin-Zhorov et al., 2012, 2017).
Current evidence indicates an association of autoimmune and inflammatory diseases with a reduced capacity of Tregs to recruit a sufficient amount of DLG1 at IS. One example is rheumatoid arthritis (RA), an autoimmune disease where diminished activity of Tregs has been observed. In RA patients Tregs are defective in suppressing CD8+ T-cell proliferation and, surprisingly, they were shown to accumulate lower levels of DLG1 at IS than Tregs from healthy individuals (Figure 1C) (Zanin-Zhorov et al., 2012). A similar scenario might occur in patients with the neuro-inflammatory disease known as HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP). This pathology is linked to HTLV-1 infection and is characterized by a high infiltration of HTLV-1 infected Tregs into the central nervous system. Interestingly, such cells not only present low suppressive activity but also change to a pro-inflammatory phenotype (Araya et al., 2014). This phenomenon was explained in part by the activity of the HTLV-1 Tax protein, a key player for HTLV-1 replication, which is expressed at high levels in HAM/TSP (Araya et al., 2011; Enose-Akahata et al., 2017). HTLV-1 Tax has been reported to downregulate foxp3 expression (a transcription factor crucial for Tregs suppressive activities); however, it may use further mechanisms to interfere with Tregs functions. In this regard, we and others have demonstrated that Tax can directly interact with DLG1 and promote its hyperphosphorylation leading to DLG1 mislocalization from the cell borders to the MTOC/Golgi region (Figure 1C) (Hirata et al., 2004; Marziali et al., 2017) . Thus, this interaction might interfere with DLG1 accumulation at IS upon TCR engagement, negatively affecting suppressive responses of infected Tregs. Interestingly, HAM/TSP individuals have a tendency to develop RA and studies using transgenic mouse models have confirmed a role of HTLV-1 Tax in the onset of such disease (Habu et al., 1999; Yakova et al., 2005).
Mechanisms of regulation of DLG1 expression
The above-mentioned facts remark the importance of DLG1 functions, and most likely of its interactions in specific areas of the cell in different cell types and tissues. Although the emergent interest in DLG1 deregulation as a central matter in the development of pathologies and in the identification of prognostic biomarkers, the molecular mechanisms that control DLG1 levels and localization, in physiological or pathological conditions, are still poorly understood. In the following sections we will discuss the current state of knowledge on the mechanisms that may contribute to regulating DLG1 protein abundance and cell distribution (Figure 2).
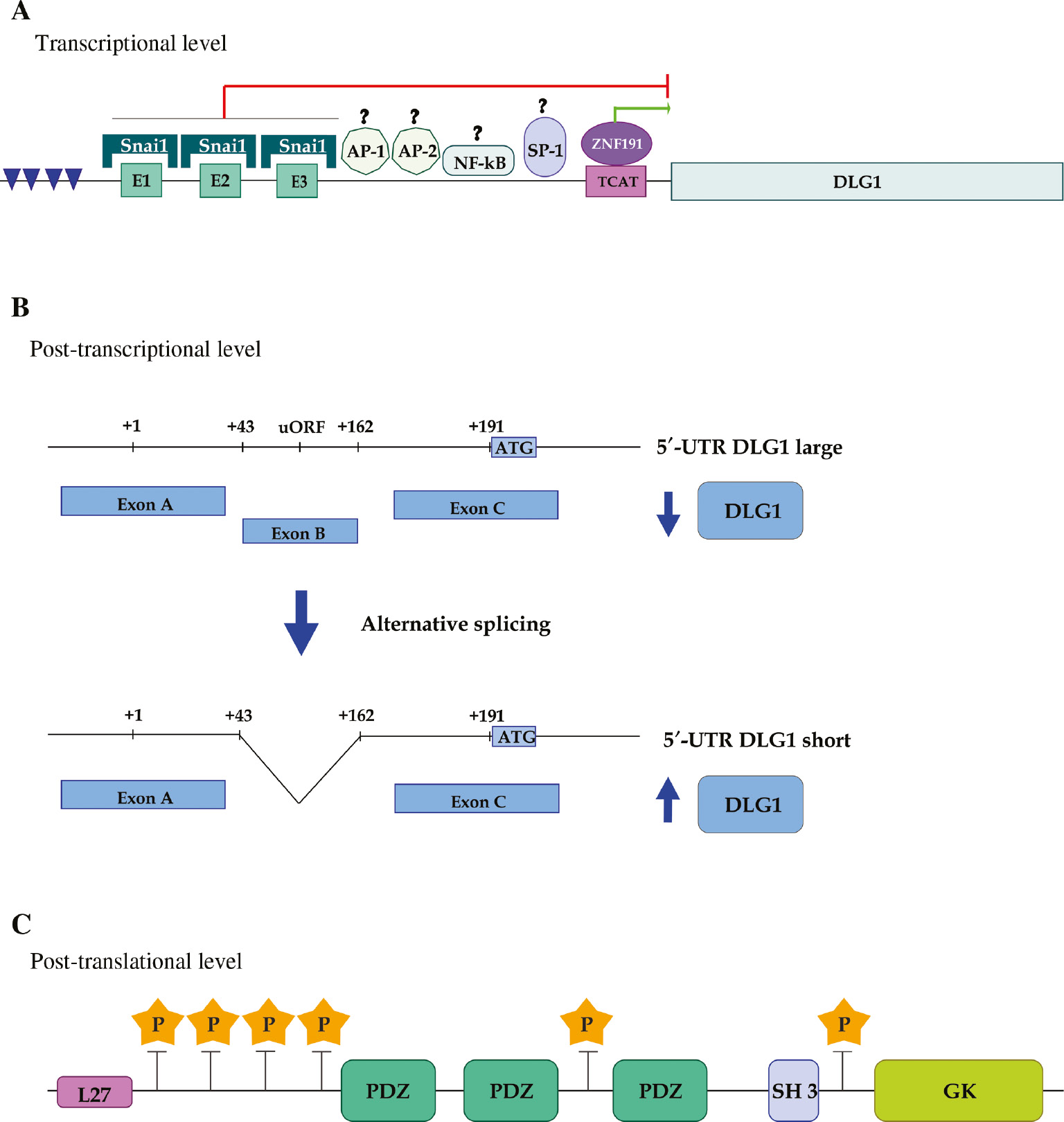
Mechanisms controlling DLG1 expression at different levels.
(A) Transcriptional level. A schematic representation of DLG1 promoter region shows both putative (AP-1, AP-2, NF-kB and SP-1) and demonstrated interacting transcription factors (Snai1 and ZNF191). The green and red lines indicate transcriptional activation or repression, respectively. Regions enriched with CpG dinucleotides are indicated by blue arrowheads. (B) Post-transcriptional level. The 5′UTR of DLG1 mRNA includes the non-coding exons A, B and part of exon C. The alternative splicing of exon B, that gives rise to the short mRNA variant, is represented. The translational efficiency of unspliced and spliced variants is indicated by blue arrows. (C) Post-translational level. The relative localization of phosphorylation sites relative to modular interacting domains are indicated by yellow stars.
Transcriptional level
The first attempt to identify elements regulating DLG1 expression was carried out by cloning the minimal region with promoter activity upstream from the transcription start site initially reported by Lue and collaborators (Lue et al., 1994; Cavatorta et al., 2008). A detailed analysis of such regions revealed the presence of several consensus binding sites for transcription factors like nuclear factor kappa B, AP-1, AP-2, SP-1, Snai1 (Cavatorta et al., 2008). More recently, a study has added the human zinc finger 191 (ZNF191) factor to the list (Wu et al., 2016). Although their functions have been well studied, the influence on DLG1 promoter has only been ascertained for Snai1 and ZNF191 (Figure 2A).
Snai1 is a transcription factor member of the Snail family which participates actively in the epithelial-mesenchymal transition (EMT) during embryonic development, driving epithelial cell migration. Reactivation of EMT is one of the most striking features of epithelial tumor cells in advanced stages of malignancy. This can be triggered by the tumor environment through secretion of cytokines like epithermal growth factor, transforming growth factor-beta, Notch, Wnt and tumor necrosis factor-alpha. In response to such signals, epithelial tumor cells upregulate the EMT promoting factor Snai1 (Wang et al., 2013). Interestingly, Snai1 was shown to downregulate DLG1 expression by interacting with E-box-like elements present in DLG1 promoter and repressing gene transcription (Cavatorta et al., 2008). Therefore, the observed decrease in DLG1 levels frequently reported in advanced stages of epithelial tumors may be, in part, a consequence of EMT program reactivation triggered by Snai1.
DLG1 expression was recently found to also be regulated by the transcription factor ZNF191, which plays a role in mammal embryonic development as well as in adult organs (Li et al., 2009). In hepatocytes, ZNF191 inhibits migration by downregulating the Hippo-Yes-associated protein (YAP) signaling pathway. More specifically, ZNF191 promotes the cytoplasmic retention of the signaling activator YAP through phosphorylation. Strikingly, ZNF191-mediated YAP phosphorylation was shown to depend on DLG1 expression, suggesting a functional link between ZNF191 and DLG1 during HIPO-YAP signaling regulation. This relationship was clarified having found that ZNF191 interacts with TCAT repetitive motifs on DLG1 promoter and activates its transcription (Wu et al., 2016). Importantly, ZNF191 was found to be downregulated in invasive hepatocellular carcinoma. Hence, this fact could strongly contribute to understanding the loss of DLG1 expression found in such stages of malignancy (Wu et al., 2016).
Finally, the promoter region of DLG1 presents sequences enriched with CpG dinucleotides. Such elements allow transcription from different start sites giving rise to pools of mRNA for DLG1 with high variability in the 5′ extremes, however, the usage of particular sites under different biological contexts has not yet been studied (Cavatorta et al., 2011). In addition, CpG dinucleotides allow epigenetic control by hypo or hyper-DNA methylation. Nevertheless, to date no studies have deeply analyzed how the epigenetic status of DLG1 promoter relates to DLG1 protein levels. This knowledge, as well as the identification of epigenetic regulators, could be particularly important for the neurological disorders explained before.
Post-transcriptional level
Genes that must be precisely regulated, such as oncogenes and genes encoding transcription factors, receptors, transduction pathway regulators and tumor suppressors, often give rise to transcripts with variable 5′ untranslated regions (5′UTR) (Hughes, 2006; Willimott and Wagner, 2010; Ishii and Sakuma, 2011). DLG1 could be placed in such category as a 5′UTR alternative splicing event was identified in that region (Figure 2B). Such a mechanism was detected in different cell types, highlighting its conserved nature. Of note, the spliced exon contains two elements that regularly have a negative influence on translation efficiency: a short upstream open reading frame (uORF) and stable stem loops. uORF are genetic elements reported to decrease the translation rate of main ORFs by reducing the availability of ribosomes. On the same hand, stem loops within 5′UTR provoke ribosome stalling and may also create binding sites for cellular factors that affect translation efficiency (Smith, 2008; Hinnebusch et al., 2016). Importantly, the negative impact of both elements on DLG1 expression was confirmed having found a higher translation efficiency for the spliced variant (Cavatorta et al., 2011). These findings opened the possibility of 5′UTR splicing as a mechanism to regulate DLG1 levels in biological contexts in which DLG1 plays an important role. In this regard, an upregulation of 5′UTR spliced version relative to the unspliced form was observed during adherens-junction formation of colon epithelial cells, where an increase in DLG1 levels has been reported (Marziali et al., 2015). However, a less significant role of this mechanism was found in DLG1 decreased levels observed during cell cycle progression from G1 to the S phase (Marziali et al., 2015). Therefore, these data revealed that the extent of 5′UTR splicing as a mechanism to control DLG1 protein levels is highly dependent on specific cellular behaviors. Thus, it is tempting to speculate that interferences in this 5´UTR splicing mechanism could have potential to contribute to oncogenic processes by increasing or decreasing DLG1 levels depending on the tumoral context.
Post-translational level
DLG1 plays an important role in the organization of signaling pathways recruiting several kinases at specific cell regions and supporting the availability of substrates near the kinase active sites. In turn, phosphorylation is a major post-translational modification that controls DLG1 activities, interacting partners and cellular localization. Many different phosphorylation events by diverse kinases were described for DLG1 with different outcomes in DLG1 functions (Figure 2C).
DLG1 is important for cell regulation and it was shown to be phosphorylated in cells exposed to different stress signals. Under hyperosmotic stress eukaryotic cells may shrink and cells may develop responses to restore cell integrity, volume and homeostasis. For this, several signalling pathways are activated in order to reinforce and modulate the cytoskeletal network. Particularly, the p38 mitogen-activated protein kinases (MAPKs) are stimulated and specifically, SAPK3/p38gamma activation is rapid and strong compared to other p38s (Sabio et al., 2004). It was shown that DLG1 is a substrate of this kinase and this phosphorylation prompts the dissociation of DLG1 from the guanylate kinase-associated protein (GKAP). GKAP interacts with intermediate filaments and is able to associate with DLG1 and recruit it to the cytoskeleton. Phosphorylated DLG1 by osmotic shock may suffer conformational changes that prevent its interaction with GKAP and induce its release from the cytoskeletal fraction, probably for regulating intercellular complexes and polarity under changes in the extracellular environment (Sabio et al., 2005). In line with this study, Massimi and colleagues demonstrated that the osmotic shock induces the phosphorylation of DLG1 in parallel with its accumulation in the cells’ borders, at sites of cell-to-cell junctions, suggesting that the release of DLG1 from the cytoskeleton could contribute to the maintenance of cell contacts under stress conditions (Massimi et al., 2006). This idea was also supported by the fact, that under osmotic shock, DLG1 moves to the insoluble fraction of the protein cell extracts, most likely because of its participation in molecular complexes with membrane-associated proteins. In this study, the phosphorylation sites were mapped within the first 185 amino acids, and it was shown that the Jun N-terminal kinase (JNK) was responsible for DLG1 relocalization. Moreover, the redistribution of DLG1 to the cell junctions renders this protein more susceptible to proteasome degradation in the presence of HVP E6 oncoprotein; hence, phosphorylation may not only control DLG1 cell distribution but also its stability (Massimi et al., 2006).
It was shown that phosphorylation can also control particular DLG1 functions in specific cell types. As described before, in T cells DLG1 localizes at the IS and controls TCR signal transduction by interacting with cytoskeletal and signaling regulators through its several interacting protein domains (Xavier et al., 2004). In CD8+ T cells, DLG1 directs TCR stimulation to the activation of pro-inflammatory cytokine gene expression and cytotoxic granule release, processes necessary for destroying infected or transformed cells which require the activation of different signal networks. In these cells, DLG1AB, an alternative spliced isoform of DLG1, is tyrosine phosphorylated (Y222) by LCK kinase after TCR activation (Silva et al., 2015). This specific modification renders DLG1 able to modulate p38 activation and the subsequent cytokine expression, highlighting the importance of this posttranslational modification as a key control for the DLG1 activities involved in lymphocyte biology. Moreover, the blocking of DLG1AB phosphorylation was proposed as a novel therapeutic tool to specifically block proinflammatory cytokine production (Crocetti et al., 2014)
DLG1 is able to interact with some tumor suppressors, such as adenomatous polyposis coli and PTEN, and it was shown to regulate cell proliferation in many contexts. Hereafter, the regulation of DLG1 during the cell cycle should be important in order to strictly control cell growth.
The first report about DLG1 in association with kinases during cell replication showed that the PDZ binding kinase (PBK) interacts with DLG1 PDZ domains and this interaction should be important for the association of this kinase with the central spindle and the promotion of cytokinesis. Both PBK and DLG1 were shown to be phosphorylated at mitosis in HeLa cells, and the mitotic phosphorylation of PBK by cyclin B/cdc2 was required for its activity. These initial findings suggested a link for DLG1 to signal transduction pathways regulating the cell cycle or cellular proliferation (Gaudet et al., 2000). Afterwards, an analysis of DLG1 expression during the cell cycle identified that DLG1 localized at the cell junctions in G1, in the cytoplasm in S phase, relocating to the mitotic spindle in M phase and finally accumulating in the midbody during cytokinesis, demonstrating a cell cycle-dependent distribution of DLG1 that may impact on the protein activities. It was also shown that both cyclin dependent kinase 1 and 2 (CDK1 and CDK2) phosphorylate DLG1 on Ser158 and Ser442. These phosphorylation events modulate DLG1 nuclear localization, and particularly, protein stability, together with the redistribution of DLG1 to the mitotic spindle. These findings demonstrated the importance of cell cycle phosphorylation in the regulation of DLG1 localization and abundance during the different phases with potential consequences on its oncosuppressor functions (Narayan et al., 2009).
Other authors have also reported the phosphorylation of DLG1 at multiple sites during mitosis, like Ser102, Ser122 and Ser158. However, using specific phospho-antibodies the authors showed that, while DLG1 is accumulated at the midbody, phospo-DLG1 is absent from such structures, suggesting that phosphorylation may induce the dissociation of DLG1, likely by changing the protein binding pattern. Moreover, these authors also demonstrated that DLG1 phosphorylation in M phase is regulated by the ERK pathway which is involved in cell cycle control, potentially linking DLG1 to the regulation of entry and progression of mitosis (Inesta-Vaquera et al., 2010).
These data remark on the presence of several sites in DLG1 that could be phosphorylated by diverse kinases, either independently or simultaneously, depending on the activated signal pathways, cell type and extracellular microenvironment.
As described already, DLG1 seems to have a dual role in oncogenesis with both oncogenic or tumor suppressor activities, according to the biological context. It is likely to hypothesize that the phosphorylation status could be a way of controlling such activities as the activation of many kinases depends on the stimulation of signal translation pathways involved in the regulation of tumor development or prevention. In line with this, protein kinase C α isoform (PKCα) interacts with DLG1 in a PDZ-dependent manner and is able to phosphorylate DLG1 in the Thr-656 site. This phosphorylation correlates with increased invasiveness in non-small cell lung cancer lines and this event was considered a marker of PKC-mediated invasion, showing the requirement of DLG1 for PKCα to promote cellular migration. PKCα and DLG1 colocalize at the leading edge of the migrating cells and this depends on PKCα activity. These findings emphasize the involvement of DLG1 phosphorylation in both DLG1 cell distribution and functions, in this particular case, contributing to PKCα mediated tumor progression and metastasis (O’Neill et al., 2011).
Conclusions
MAGUKs proteins are structurally and functionally conserved in many organisms, highlighting the relevance of their biological functions. Interestingly, the bulk of evidence summarized in this revision strengthen the hypothesis of a close association of changes in DLG1 expression or DLG1 gene mutations with diverse illnesses of high impact in public health, such as tumor development and neurological or immunological disorders.
The identification of multiple cell partners of DLG1 contributed to understanding DLG1 functions and its participation in key signal transduction pathways and diverse biological processes such as cell polarity maintenance, cell junction formation, cell proliferation and apoptosis (Roberts et al., 2012; Stephens et al., 2018). Hence, the finding of DLG1 misexpression or DLG1 genetic alterations in tissues and cells derived from different illnesses opens a new and promising scenario for a better comprehension of the molecular basis underlying the above pathological conditions. There is, however, an urgent need for more studies to deeply understand (i) how DLG1 abundance and cell distribution are deregulated, (ii) which specific functions or interactions are altered and (iii) how these changes impact on the pathogenesis. A more complete knowledge on the transcriptional control, especially the role of unexplored transcription factors, and epigenetic and posttranscriptional regulation will provide new insights about the control of DLG1 abundance. This knowledge, added to new data on signal transduction pathways that may post-translationally modify DLG1 localization, would aid in clarifing specific pathogenic mechanisms and, eventually, to define therapeutic targets or diagnosis biomarkers. In addition, further high-throughput studies are necessary for a much clearer comprehension of the real incidence and relevance of DLG1 gene mutations, as underlying genetic changes of specific neurological disorders and probably autoimmune diseases.
In summary, the understanding gained during the last years opened a plethora of new possibilities for dissecting mechanisms involved in the development of significant pathologies, with not only uncertain biological etiology, but also with the lack of a rapid diagnosis and effective therapies. Further analysis in the area would be extremely valuable to overcome these global health concerns.
Acknowledgments
Federico Marziali and Maria Paula Dizanzo were supported by CONICET. We are grateful to all members of the Tumor Virology laboratory, past and present, and the funding agencies: Provincia de Santa Fe, Instituto Nacional del Cancer, Fundación Bunge y Born, Agencia de Promoción Científica y Tecnológica.
References
Araya, N., Sato, T., Yagishita, N., Ando, H., Utsunomiya, A., Jacobson, S., and Yamano, Y. (2011). Human T-lymphotropic virus type 1 (HTLV-1) and regulatory T cells in HTLV-1-associated neuroinflammatory disease. Viruses. 3, 1532–1548.10.3390/v3091532Search in Google Scholar PubMed PubMed Central
Araya, N., Sato, T., Ando, H., Tomaru, U., Yoshida, M., Coler-Reilly, A., Yagishita, N., Yamauchi, J., Hasegawa, A., Kannagi, M., et al. (2014). HTLV-1 induces a Th1-like state in CD4+CCR4+ T cells. J. Clin. Invest. 124, 3431–3442.10.1172/JCI75250Search in Google Scholar PubMed PubMed Central
Byeon, S., Youn Kim, W., Yeol Lee, K., Sook Hwang, T., Ho Kim, Y., and Soo Chang, M. (2011). Expression of the human homolog of discs, large homolog 1 (Drosophila) in normal epithelium, nodule, papilloma and invasive squamous cell carcinoma of larynx. Basic Appl. Pathol. 4, 105–109.10.1111/j.1755-9294.2011.01119.xSearch in Google Scholar
Cavatorta, A.L., Di Gregorio, A., Bugnon Valdano, M., Marziali, F., Cabral, M., Bottai, H., Cittadini, J., Nocito, A.L., and Gardiol, D. (2017). DLG1 polarity protein expression associates with the disease progress of low-grade cervical intraepithelial lesions. Exp. Mol. Pathol. 102, 65–69.10.1016/j.yexmp.2016.12.008Search in Google Scholar PubMed
Cavatorta, A.L., Fumero, G., Chouhy, D., Aguirre, R., Nocito, A.L., Giri, A.A., Banks, L., and Gardiol, D. (2004). Differential expression of the human homologue of Drosophila discs large oncosuppressor in histologic samples from human papillomavirus-associated lesions as a marker for progression to malignancy. Int. J. Cancer. 111, 373–380.10.1002/ijc.20275Search in Google Scholar PubMed
Cavatorta, A.L., Giri, A.A., Banks, L., and Gardiol, D. (2008). Cloning and functional analysis of the promoter region of the human Disc large gene. Gene. 424, 87–95.10.1016/j.gene.2008.07.040Search in Google Scholar PubMed
Cavatorta, A.L., Facciuto, F., Valdano, M.B., Marziali, F., Giri, A.A., Banks, L., and Gardiol, D. (2011). Regulation of translational efficiency by different splice variants of the Disc large 1 oncosuppressor 5′-UTR. FEBS J. 278, 2596–2608.10.1111/j.1742-4658.2011.08188.xSearch in Google Scholar PubMed
Crocetti, J., Silva, O., Humphries, L.A., Tibbs, M.D., and Miceli, M.C. (2014). Selective phosphorylation of the Dlg1AB variant is critical for TCR-induced p38 activation and induction of proinflammatory cytokines in CD8+ T cells. J. Immunol. 193, 2651–2660.10.4049/jimmunol.1401196Search in Google Scholar PubMed PubMed Central
Elsum, I., Yates, L., Humbert, P.O., and Richardson, H.E. (2012). The Scribble–Dlg–Lgl polarity module in development and cancer: from flies to man. Essays Biochem. 53, 141–168.10.1042/bse0530141Search in Google Scholar PubMed
Enose-Akahata, Y., Vellucci, A., and Jacobson, S. (2017). Role of HTLV-1 Tax and HBZ in the pathogenesis of HAM/TSP. Front. Microbiol. 8, 2563–2573.10.3389/fmicb.2017.02563Search in Google Scholar PubMed PubMed Central
Fernández-Jaén, A., Castellanos, M.C., Fernández-Perrone, A.L., Fernández-Mayoralas, D.M., de la Vega, A.G., Calleja-Pérez, B., Fernandez, E.C., Albert, J., and Hombre, M.C.S. (2014). Cerebral palsy, epilepsy, and severe intellectual disability in a patient with 3q29 microduplication syndrome. Am. J. Med. Genet. A. 164A, 2043–2047.10.1002/ajmg.a.36559Search in Google Scholar PubMed
Fourie, C., Li, D., and Montgomery, J.M. (2014). The anchoring protein SAP97 influences the trafficking and localisation of multiple membrane channels. Biochim. Biophys. Acta 1838, 589–594.10.1016/j.bbamem.2013.03.015Search in Google Scholar PubMed
Fromer, M., Pocklington, A.J., Kavanagh, D.H., Williams, H.J., Dwyer, S., Gormley, P., Georgieva, L., Rees, E., Palta, P., Ruderfer, D.M., et al. (2014). De novo mutations in schizophrenia implicate synaptic networks. Nature 506, 179–184.10.1038/nature12929Search in Google Scholar PubMed PubMed Central
Fuja, T.J., Lin, F., Osann, K.E., and Bryant, P.J. (2004). Somatic mutations and altered expression of the candidate tumor suppressors CSNK1 epsilon, DLG1, and EDD/hHYD in mammary ductal carcinoma. Cancer Res. 64, 942–951.10.1158/0008-5472.CAN-03-2100Search in Google Scholar
Gardiol, D., Kuhne, C., Glaunsinger, B., Lee, S.S., Javier, R., and Banks, L. (1999). Oncogenic human papillomavirus E6 proteins target the discs large tumour suppressor for proteasome-mediated degradation. Oncogene 18, 5487–5496.10.1038/sj.onc.1202920Search in Google Scholar PubMed
Gardiol, D., Galizzi, S., and Banks, L. (2002). Mutational analysis of the discs large tumour suppressor identifies domains responsible for human papillomavirus type 18 E6-mediated degradation. J. Gen. Virol. 83, 283–289.10.1099/0022-1317-83-2-283Search in Google Scholar PubMed
Gardiol, D., Zacchi, A., Petrera, F., Stanta, G., and Banks, L. (2006). Human discs large and scrib are localized at the same regions in colon mucosa and changes in their expression patterns are correlated with loss of tissue architecture during malignant progression. Int. J. Cancer. 119, 1285–1290.10.1002/ijc.21982Search in Google Scholar PubMed
Gardoni, F., Mauceri, D., Marcello, E., Sala, C., Di Luca, M., and Jeromin, A. (2007). SAP97 directs the localization of Kv4.2 to spines in hippocampal neurons. J. Biol. Chem. 282, 28691–28699.10.1074/jbc.M701899200Search in Google Scholar PubMed
Gaudet, S., Branton, D., and Lue, R.A. (2000). Characterization of PDZ-binding kinase, a mitotic kinase. Proc. Natl. Acad. Sci. USA. 97, 5167–5172.10.1073/pnas.090102397Search in Google Scholar PubMed PubMed Central
Glassford, M.R., Rosenfeld, J.A., Freedman, A.A., Zwick, M.E., and Mulle, J.G. (2016). Novel features of 3q29 deletion syndrome: results from the 3q29 registry. Am. J. Med. Genet. Part A. 170, 999–1006.10.1002/ajmg.a.37537Search in Google Scholar PubMed PubMed Central
Golub, O., Wee, B., Newman, R.A., Paterson, N.M., and Prehoda, K.E. (2017). Activation of Discs large by aPKC aligns the mitotic spindle to the polarity axis during asymmetric cell division. Elife. 6, e32137.10.7554/eLife.32137.018Search in Google Scholar
Habu, K., Nakayama-Yamada, J., Asano, M., Saijo, S., Itagaki, K., Horai, R., Yamamoto, H., Sekiguchi, T., Hatanaka, M., and Iwakura, Y. (1999). The human T cell leukemia virus type I-tax gene is responsible for the development of both inflammatory polyarthropathy resembling rheumatoid arthritis and noninflammatory ankylotic arthropathy in transgenic mice. J. Immunol. 162, 2956–2963.10.4049/jimmunol.162.5.2956Search in Google Scholar
Hinnebusch, A.G., Ivanov, I.P., and Sonenberg, N. (2016). Translational control by 5′-untranslated regions of eukaryotic mRNAs. Science 352, 1413–1416.10.1126/science.aad9868Search in Google Scholar PubMed PubMed Central
Hirata, A., Higuchi, M., Niinuma, A., Ohashi, M., Fukushi, M., Oie, M., Akiyama, T., Tanaka, Y., Gejyo, F., and Fujii, M. (2004). PDZ domain-binding motif of human T-cell leukemia virus type 1 Tax oncoprotein augments the transforming activity in a rat fibroblast cell line. Virology 318, 327–336.10.1016/j.virol.2003.10.006Search in Google Scholar PubMed
Hughes, T.A. (2006). Regulation of gene expression by alternative untranslated regions. Trends Genet. 22, 119–122.10.1016/j.tig.2006.01.001Search in Google Scholar PubMed
Inesta-Vaquera, F.A., Campbell, D.G., Arthur, J.S., and Cuenda, A. (2010). ERK5 pathway regulates the phosphorylation of tumour suppressor hDlg during mitosis. Biochem. Biophys. Res. Commun. 399, 84–90.10.1016/j.bbrc.2010.07.046Search in Google Scholar PubMed
Ishii, H. and Sakuma, Y. (2011). Complex organization of the 5′-untranslated region of the mouse estrogen receptor α gene: identification of numerous mRNA transcripts with distinct 5′-ends. J. Steroid Biochem. Mol. Biol. 125, 211–218.10.1016/j.jsbmb.2011.03.004Search in Google Scholar PubMed
James, C. and Roberts, S. (2016). Viral interactions with PDZ domain-containing proteins – an oncogenic trait? Pathogens 5, 8.10.3390/pathogens5010008Search in Google Scholar PubMed PubMed Central
Kirov, G., Pocklington, A.J., Holmans, P., Ivanov, D., Ikeda, M., Ruderfer, D., Moran, J., Chambert, K., Toncheva, D., Georgieva, L., et al. (2012). De novo CNV analysis implicates specific abnormalities of postsynaptic signalling complexes in the pathogenesis of schizophrenia. Mol. Psychiatry. 17, 142–153.10.1038/mp.2011.154Search in Google Scholar PubMed PubMed Central
Kitagawa, Y. and Sakaguchi, S. (2017). Molecular control of regulatory T cell development and function. Curr. Opin. Immunol. 49, 64–70.10.1016/j.coi.2017.10.002Search in Google Scholar PubMed
Knoblich, J.A. (2008). Mechanisms of asymmetric stem cell division. Cell. 132, 583–597.10.1016/j.cell.2008.02.007Search in Google Scholar PubMed
Kong, K., Kumar, M., Taruishi, M., and Javier, R.T. (2014). The human adenovirus E4-ORF1 protein subverts discs large 1 to mediate membrane recruitment and dysregulation of phosphatidylinositol 3-kinase. PLoS Pathog. 10, e1004102.10.1371/journal.ppat.1004102Search in Google Scholar PubMed PubMed Central
Laprise, P., Viel, A., and Rivard, N. (2004). Human homolog of disc-large is required for adherens junction assembly and differentiation of human intestinal epithelial cells. J. Biol. Chem. 279, 10157–10166.10.1074/jbc.M309843200Search in Google Scholar PubMed
Lasserre, R., Charrin, S., Cuche, C., Danckaert, A., Thoulouze, M.I., de Chaumont, F., Duong, T., Perrault, N., Varin-Blank, N., Olivo-Marin, J.C., et al. (2010). Ezrin tunes T-cell activation by controlling Dlg1 and microtubule positioning at the immunological synapse. EMBO J. 29, 2301–2314.10.1038/emboj.2010.127Search in Google Scholar PubMed PubMed Central
Li, M.O., and Rudensky, A.Y. (2016). T cell receptor signalling in the control of regulatory T cell differentiation and function. Nat. Rev. Immunol. 16, 220–233.10.1038/nri.2016.26Search in Google Scholar PubMed PubMed Central
Li, J., Chen, X., Gong, X., Liu, Y., Feng, H., Qiu, L., Hu, Z., and Zhang, J. (2009). A transcript profiling approach reveals the zinc finger transcription factor ZNF191 is a pleiotropic factor. BMC Genomics. 10, 241.10.1186/1471-2164-10-241Search in Google Scholar PubMed PubMed Central
Lue, R.A., Marfatia, S.M., Branton, D., and Chishti, A.H. (1994). Cloning and characterization of hdlg: the human homologue of the Drosophila discs large tumor suppressor binds to protein 4.1. Proc. Natl. Acad. Sci. USA. 91, 9818–9822.10.1073/pnas.91.21.9818Search in Google Scholar PubMed PubMed Central
Marziali, F., Bugnon Valdano, M., Brunet Avalos, C., Moriena, L., Cavatorta, A.L., and Gardiol, D. (2017). Interference of HTLV-1 tax protein with cell polarity regulators: defining the subcellular localization of the tax-DLG1 interaction. Viruses 9, 355.10.3390/v9120355Search in Google Scholar PubMed PubMed Central
Marziali, F., Cavatorta, A.L., Valdano, M.B., Facciuto, F., and Gardiol, D. (2015). Transcriptional and translational mechanisms contribute to regulate the expression of Discs Large 1 protein during different biological processes. Biol. Chem. 396, 893–902.10.1515/hsz-2014-0286Search in Google Scholar PubMed
Massimi, P., Gammoh, N., Thomas, M., and Banks, L. (2004). HPV E6 specifically targets different cellular pools of its PDZ domain-containing tumour suppressor substrates for proteasome-mediated degradation. Oncogene 23, 8033–8039.10.1038/sj.onc.1207977Search in Google Scholar PubMed
Massimi, P., Narayan, N., Cuenda, A., and Banks, L. (2006). Phosphorylation of the discs large tumour suppressor protein controls its membrane localisation and enhances its susceptibility to HPV E6-induced degradation. Oncogene 25, 4276–4285.10.1038/sj.onc.1209457Search in Google Scholar PubMed
McKinney, B., Ding, Y., Lewis, D.A., and Sweet, R.A. (2017). DNA methylation as a putative mechanism for reduced dendritic spine density in the superior temporal gyrus of subjects with schizophrenia. Transl. Psychiatry. 7, e1032–e1032.10.1038/tp.2016.297Search in Google Scholar PubMed PubMed Central
Mulle, J.G., Dodd, A.F., McGrath, J.A., Wolyniec, P.S., Mitchell, A.A., Shetty, A.C., Sobreira, N.L., Valle, D., Rudd, M.K., Satten, G., et al. (2010). Microdeletions of 3q29 confer high risk for schizophrenia. Am. J. Hum. Genet. 87, 229–236.10.1016/j.ajhg.2010.07.013Search in Google Scholar PubMed PubMed Central
Narayan, N., Massimi, P., and Banks, L. (2009). CDK phosphorylation of the discs large tumour suppressor controls its localisation and stability. J. Cell Sci. 122, 65–74.10.1242/jcs.024554Search in Google Scholar PubMed
Nash, J.E., Johnston, T.H., Collingridge, G.L., Garner, C.C., and Brotchie, J.M. (2005). Subcellular redistribution of the synapse-associated proteins PSD-95 and SAP97 in animal models of Parkinson’s disease and L-DOPA-induced dyskinesia. FASEB J. 19, 583–585.10.1096/fj.04-1854fjeSearch in Google Scholar PubMed
O’Neill, A.K., Gallegos, L.L., Justilien, V., Garcia, E.L., Leitges, M., Fields, A.P., Hall, R.A., and Newton, A.C. (2011). Protein kinase Cα promotes cell migration through a PDZ-dependent interaction with its novel substrate discs large homolog 1 (DLG1). J. Biol. Chem. 286, 43559–43568.10.1074/jbc.M111.294603Search in Google Scholar PubMed PubMed Central
Purcell, S.M., Moran, J.L., Fromer, M., Ruderfer, D., Solovieff, N., Roussos, P., O’Dushlaine, C., Chambert, K., Bergen, S.E., Kähler, A., et al. (2014). A polygenic burden of rare disruptive mutations in schizophrenia. Nature 506, 185–190.10.1038/nature12975Search in Google Scholar PubMed PubMed Central
Quintero-Rivera, F., Sharifi-Hannauer, P., and Martinez-Agosto, J.A. (2010). Autistic and psychiatric findings associated with the 3q29 microdeletion syndrome: case report and review. Am. J. Med. Genet. Part A. 152A, 2459–2467.10.1002/ajmg.a.33573Search in Google Scholar PubMed
Rabinovici, G.D., and Miller, B.L. (2010). Frontotemporal lobar degeneration. CNS Drugs 24, 375–398.10.2165/11533100-000000000-00000Search in Google Scholar PubMed PubMed Central
Roberts, S., Delury, C., and Marsh, E. (2012). The PDZ protein discs-large (DLG): the “Jekyll and Hyde” of the epithelial polarity proteins. FEBS J. 279, 3549–3558.10.1111/j.1742-4658.2012.08729.xSearch in Google Scholar PubMed
Round, J.L., Tomassian, T., Zhang, M., Patel, V., Schoenberger, S.P., and Miceli, M.C. (2005). Dlgh1 coordinates actin polymerization, synaptic T cell receptor and lipid raft aggregation, and effector function in T cells. J. Exp. Med. 201, 419–430.10.1084/jem.20041428Search in Google Scholar PubMed PubMed Central
Sabio, G., Reuver, S., Feijoo, C., Hasegawa, M., Thomas, G.M., Centeno, F., Kuhlendahl, S., Leal-Ortiz, S., Goedert, M., et al. (2004). Stress- and mitogen-induced phosphorylation of the synapse-associated protein SAP90/PSD-95 by activation of SAPK3/p38gamma and ERK1/ERK2. Biochem. J. 380, 19–30.10.1042/bj20031628Search in Google Scholar PubMed PubMed Central
Sabio, G., Arthur, J.S., Kuma, Y., Peggie, M., Carr, J., Murray-Tait, V., Centeno, F., Goedert, M., Morrice, N., and Cuenda, A. (2005). p38gamma regulates the localisation of SAP97 in the cytoskeleton by modulating its interaction with GKAP. EMBO J. 24, 1134–1145.10.1038/sj.emboj.7600578Search in Google Scholar PubMed PubMed Central
Saito, Y., Desai, R., and Muthuswamy, S. (2018). Reinterpreting polarity and cancer: the changing landscape from tumor suppression to tumor promotion. Biochim. Biophys. Acta Rev. Cancer 1869, 103–116.10.1016/j.bbcan.2017.12.001Search in Google Scholar PubMed
Sandoval, G.J., Graham, D.B., Gmyrek, G.B., Akilesh, H.M., Fujikawa, K., Sammut, B., Bhattacharya, D., Srivatsan, S., Kim, A., Shaw, A.S., et al. (2013). Novel mechanism of tumor suppression by polarity gene discs large 1 (DLG1) revealed in a murine model of pediatric B-ALL. Cancer Immunol. Res. 1, 426–437.10.1158/2326-6066.CIR-13-0065Search in Google Scholar PubMed PubMed Central
Sato, J., Shimazu, D., Yamamoto, N., and Nishikawa, T. (2008). An association analysis of synapse-associated protein 97 (SAP97) gene in schizophrenia. J. Neural. Transm. 115, 1355–1365.10.1007/s00702-008-0085-9Search in Google Scholar PubMed
Silva, O., Crocetti, J., Humphries, L.A., Burkhardt, J.K., and Miceli, M.C. (2015). Discs large homolog 1 splice variants regulate p38-dependent and -independent effector functions in CD8+ T cells. PLoS One 10, e0133353.10.1371/journal.pone.0133353Search in Google Scholar PubMed PubMed Central
Smith, L. (2008). Post-transcriptional regulation of gene expression by alternative 5′-untranslated regions in carcinogenesis. Biochem. Soc. Trans. 36, 708–711.10.1042/BST0360708Search in Google Scholar PubMed
Soler, J., Fañanás, L., Parellada, M., Krebs, M.O., Rouleau, G.A., and Fatjó-Vilas, M. (2018). Genetic variability in scaffolding proteins and risk for schizophrenia and autism-spectrum disorders: a systematic review. J. Psychiatry Neurosci. 43, 223–244.10.1503/jpn.170066Search in Google Scholar PubMed
Stephens, R., Lim, K., Portela, M., Kvansakul, M., Humbert, P.O., and Richardson, H.E. (2018). The scribble cell polarity module in the regulation of cell signaling in tissue development and tumorigenesis. J. Mol. Biol. 430, 3585–3612.10.1016/j.jmb.2018.01.011Search in Google Scholar PubMed
Sugihara, T., Nakagawa, S., Sasajima, Y., Ichinose, T., Hiraike, H., Kondo, F., Uozaki, H., Fukusato, T., and Ayabe, T. (2016). Loss of the cell polarity determinant human Discs-large is a novel molecular marker of nodal involvement and poor prognosis in endometrial cancer. Br. J. Cancer. 114, 1012–1018.10.1038/bjc.2016.24Search in Google Scholar PubMed PubMed Central
Sun, A.G., Wang, J., Shan, Y.Z., Yu, W.J., Li, X., Cong, C.H., and Wang, X. (2014). Identifying distinct candidate genes for early Parkinson’s disease by analysis of gene expression in whole blood. Neuro. Endocrinol. Lett. 35, 398–404.Search in Google Scholar
Szymanowska-Narloch, A., Jassem, E., Skrzypski, M., Muley, T., Meister, M., Dienemann, H., Taron, M., Rosell, R., Rzepko, R., Jarząb, M., et al. (2013). Molecular profiles of non-small cell lung cancers in cigarette smoking and never-smoking patients. Adv. Med. Sci. 58, 196–206.10.2478/ams-2013-0025Search in Google Scholar PubMed
Taskesen, E., Mishra, A., van der Sluis, S., Ferrari, R., International FTD-Genomics Consortium, Veldink, J.H., van Es, M.A., Smit, A.B., Posthuma, D., and Pijnenburg, Y. (2017). Susceptible genes and disease mechanisms identified in frontotemporal dementia and frontotemporal dementia with amyotrophic lateral sclerosis by DNA-methylation and GWAS. Sci. Rep. 7, 8899.10.1038/s41598-017-09320-zSearch in Google Scholar PubMed PubMed Central
Toyooka, K., Iritani, S., Makifuchi, T., Shirakawa, O., Kitamura, N., Maeda, K., Nakamura, R., Niizato, K., Watanabe, M., Kakita, A., et al. (2002). Selective reduction of a PDZ protein, SAP-97, in the prefrontal cortex of patients with chronic schizophrenia. J. Neurochem. 83, 797–806.10.1046/j.1471-4159.2002.01181.xSearch in Google Scholar PubMed
Uezato, A., Kimura-Sato, J., Yamamoto, N., Iijima, Y., Kunugi, H., and Nishikawa, T. (2012). Further evidence for a male-selective genetic association of synapse-associated protein 97 (SAP97) gene with schizophrenia. Behav. Brain Funct. 8, 2.10.1186/1744-9081-8-2Search in Google Scholar PubMed PubMed Central
Walch, L. (2013). Emerging role of the scaffolding protein Dlg1 in vesicle trafficking. Traffic 14, 964–973.10.1111/tra.12089Search in Google Scholar PubMed
Wang, Y., Shi, J., Chai, K., Ying, X., and Zhou, B.P. (2013). The role of snail in EMT and tumorigenesis. Curr. Cancer Drug Targets 13, 963–972.10.2174/15680096113136660102Search in Google Scholar PubMed PubMed Central
Watson, R.A., Rollason, T.P., Reynolds, G.M., Murray, P.G., Banks, L., and Roberts, S. (2002). Changes in expression of the human homologue of the Drosophila discs large tumour suppressor protein in high-grade premalignant cervical neoplasias. Carcinogenesis 23, 1791–1796.10.1093/carcin/23.11.1791Search in Google Scholar PubMed
Willimott, S. and Wagner, S.D. (2010). Post-transcriptional and post-translational regulation of Bcl2. Biochem. Soc. Trans. 38, 1571–1575.10.1042/BST0381571Search in Google Scholar PubMed
Won, S., Levy, J.M., Nicoll, R.A., and Roche, K.W. (2017). MAGUKs: multifaceted synaptic organizers. Curr. Opin. Neurobiol. 43, 94–101.10.1016/j.conb.2017.01.006Search in Google Scholar PubMed PubMed Central
Wu, D., Liu, G., Liu, Y., Saiyin, H., Wang, C., Wei, Z., Zen, W., Liu, D., Chen, Q., Zhao, Z., et al. (2016). Zinc finger protein 191 inhibits hepatocellular carcinoma metastasis through discs large 1-mediated yes-associated protein inactivation. Hepatology 64, 1148–1162.10.1002/hep.28708Search in Google Scholar PubMed
Xavier, R., Rabizadeh, S., Ishiguro, K., Andre, N., Ortiz, J.B., Wachtel, H., Morris, G., Lopez-Ilasaca, M., Shaw, A.C., Swat, W., et al. (2004). Discs large (Dlg1) complexes in lymphocyte activation. J. Cell. Biol. 166, 173–178.10.1083/jcb.200309044Search in Google Scholar PubMed PubMed Central
Xing, J., Kimura, H., Wang, C., Ishizuka, K., Kushima, I., Arioka, Y., Yoshimi, A., Nakamura, Y., Shiino, T., Oya-Ito, T., et al. (2016). Resequencing and association analysis of six PSD-95-related genes as possible susceptibility genes for schizophrenia and autism spectrum disorders. Sci. Rep. 6, 27491.10.1038/srep27491Search in Google Scholar PubMed PubMed Central
Yakova, M., Lézin, A., Dantin, F., Lagathu, G., Olindo, S., Jean-Baptiste, G., Arfi, S., and Césaire, R. (2005). Increased proviral load in HTLV-1-infected patients with rheumatoid arthritis or connective tissue disease. Retrovirology 2, 4.10.1186/1742-4690-2-4Search in Google Scholar PubMed PubMed Central
Zanin-Zhorov, A., Lin, J., Scher, J., Kumari, S., Blair, D., Hippen, K.L., Blazar, B.R., Abramson, S.B., Lafaille, J.J., and Dustin, M.L. (2012). Scaffold protein Disc large homolog 1 is required for T-cell receptor-induced activation of regulatory T-cell function. Proc. Natl. Acad. Sci. USA. 109, 1625–1630.10.1073/pnas.1110120109Search in Google Scholar PubMed PubMed Central
Zanin-Zhorov, A., Kumari, S., Hippen, K.L., Merkel, S.C., MacMillan, M.L., Blazar, B.R., and Dustin, M.L. (2017). Human in vitro-induced regulatory T cells display Dlgh1 dependent and PKC-θ restrained suppressive activity. Sci. Rep. 7, 4258.10.1038/s41598-017-04053-5Search in Google Scholar PubMed PubMed Central
Zhu, G.D., OuYang, S., Liu, F., Zhu, Z.G., Jiang, F.N., and Zhang, B. (2017). Elevated expression of DLG1 is associated with poor prognosis in patients with colorectal cancer. Ann. Clin. Lab. Sci. 47, 657–662.Search in Google Scholar
©2019 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- Reviews
- Unforgettable force – crosstalk and memory of mechanosensitive structures
- Differential expression of DLG1 as a common trait in different human diseases: an encouraging issue in molecular pathology
- The effects of oxidative stress on the development of atherosclerosis
- Research Articles/Short Communications
- Protein Structure and Function
- Kinetically selective and potent inhibitors of HDAC8
- Assay of β-glucosidase 2 (GBA2) activity using lithocholic acid β-3-O-glucoside substrate for cultured fibroblasts and glucosylceramide for brain tissue
- Cell Biology and Signaling
- Changqin NO. 1 inhibits neuronal apoptosis via suppressing GAS5 expression in a traumatic brain injury mice model
- Nm23-H1 inhibits hypoxia induced epithelial-mesenchymal transition and stemness in non-small cell lung cancer cells
- Nodal promotes the malignancy of non-small cell lung cancer (NSCLC) cells via activation of NF-κB/IL-6 signals
- MCT1, MCT4 and CD147 expression and 3-bromopyruvate toxicity in colorectal cancer cells are modulated by the extracellular conditions
- Proteolysis
- Metalloprotease inhibitor profiles of human ADAM8 in vitro and in cell-based assays
Articles in the same Issue
- Frontmatter
- Reviews
- Unforgettable force – crosstalk and memory of mechanosensitive structures
- Differential expression of DLG1 as a common trait in different human diseases: an encouraging issue in molecular pathology
- The effects of oxidative stress on the development of atherosclerosis
- Research Articles/Short Communications
- Protein Structure and Function
- Kinetically selective and potent inhibitors of HDAC8
- Assay of β-glucosidase 2 (GBA2) activity using lithocholic acid β-3-O-glucoside substrate for cultured fibroblasts and glucosylceramide for brain tissue
- Cell Biology and Signaling
- Changqin NO. 1 inhibits neuronal apoptosis via suppressing GAS5 expression in a traumatic brain injury mice model
- Nm23-H1 inhibits hypoxia induced epithelial-mesenchymal transition and stemness in non-small cell lung cancer cells
- Nodal promotes the malignancy of non-small cell lung cancer (NSCLC) cells via activation of NF-κB/IL-6 signals
- MCT1, MCT4 and CD147 expression and 3-bromopyruvate toxicity in colorectal cancer cells are modulated by the extracellular conditions
- Proteolysis
- Metalloprotease inhibitor profiles of human ADAM8 in vitro and in cell-based assays


