Abstract
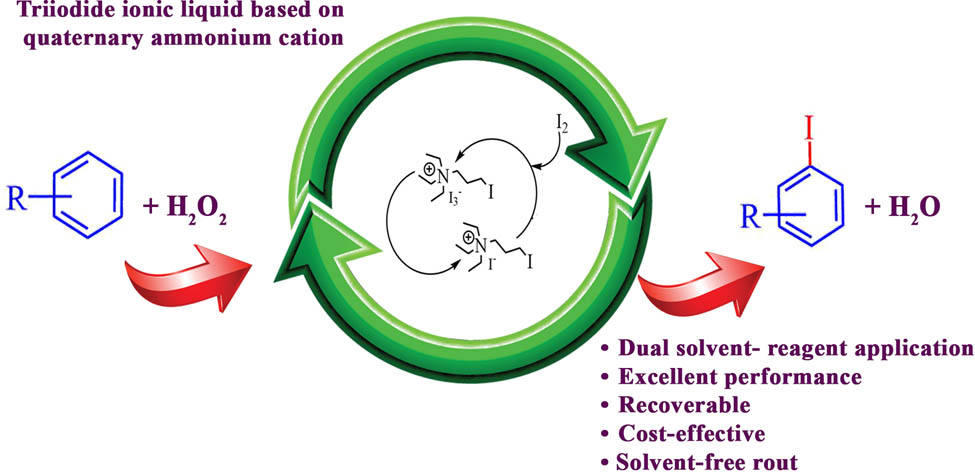
In this article, for the first time, N,N,N-triethyl-3-iodopropan-1-aminium triiodide [N2223I] [I3] was synthesized and utilized as both a reagent and a solvent in combination with H2O2 (35%) to convert aromatic compounds into their corresponding iodo derivatives. The iodination was accomplished in the absence of organic solvents, and in most instances, water was the sole extraction solvent used. The consumed reagent N,N,N-triethyl-3-iodopropan-1-aminium iodide was comfortably recycled.
Graphical abstract
1 Introduction
The goal of sustainable or green chemistry is to extend technical processes and their applications in utilizing chemicals with potentially eliminated or reduced hazards to the environment and humans [1,2]. The utilization of inherently safe or low-risk materials is favorable from an economic stand view and an ecological perspective [3]. Economic and social concerns make highly critical environmentally sustainable chemical processes, including clean organic reactions in the absence of noxious organic solvents [4]. Therefore, the industry has commenced embracing green chemistry practices, inclusive waste prevention rather than its treatment. Nowadays, the disposal of organic solvents in most industries, especially the pharmaceutical industry, is a significant problem and accounts for about 80% of their waste [5]. Therefore, solvents such as supercritical liquids, water, and ionic liquids in green synthesis have received much attention in recent times. Among the mentioned solvents, ionic liquids are a set of green solvents with unparalleled attributes, such as high thermal stability, scant vapor pressure, tunable polarity, recyclability, and immiscibility with some organic solvents compared to other solvents, which have a significant advantage [6,7,8,9,10,11].
One of the useful features of ionic liquids is their high polarity and ability to dissolve organic and inorganic compounds, elevating the rate of chemical processes and providing a higher selectivity than traditional solvents. For these reasons, they are a new alternative to volatile organic solvents in synthesizing organic compounds. Ionic liquids are inexpensive compounds that are straightforward to prepare and easy to recycle, and their attributes can be adjusted by switching ions or alkyl groups attached to cations. Due to the prominent benefits of room-temperature ionic liquids (RTILs) as an environmentally conducive reaction medium for chemical processes, much attention has now been paid to organic reactions consisting of ionic liquids [12,13,14,15,16,17,18,19], some of which halogenation reactions have been discussed [20,21,22,23,24,25,26]. Considering these leading properties, ionic liquids, especially RTILs, have become one of the most influential and widely used replacement solvents in industrial chemical processes. Furthermore, as they can be adjusted by switching the alkyl group or the anion connected to the cation, a wide range of ionic liquids with specific features can be synthesized [27,28,29,30].
Halogens are one of the most significant chemical and pharmaceutical compounds. Organohalogens are compounds generated by bacteria, fungi, diverse marine organisms, and plants and have been recognized as naturally occurring materials [31]. Many organohalogens have an exclusive place as solvents and pesticides, and play a critical role as intermediates in producing drugs, dyes, water treatment processes, dye-sensitized solar cells, and synthetic polymers, agrochemicals pigments, and photographic materials.
Regarding the value of green chemistry goals, here we introduce and synthesize a new and recoverable and inexpensive triiodide ionic liquid based on quaternary ammonium cation with extraordinary properties and excellent performance. Afterward, its application as a dual solvent reagent under mild conditions is investigated.
2 Experimental section
2.1 Materials and equipment
All chemicals used in this study were of the highest purity grade available from Merck. Distilled water was used throughout. Fourier transform infrared (FT-IR) spectra were afforded in the wavenumber range of 400–4,000 cm−1 using a Bruker IFS 66 FT-IR spectrometer. Nuclear magnetic resonance spectra were afforded with a Bruker Avance 300 spectrometer (1H NMR 300 or 500 MHz and 13C NMR 75 or 125 MHz) in pure deuterated chloroform and dimethyl sulfoxide, using tetramethylsilane as the internal standard in this procedure. A PG Instrument T80 was used to carry out the ultraviolet (UV)/Visible spectrophotometry.
2.2 Preparation of N,N,N-triethyl-3-iodopropan-1-aminium iodide 1
Triethylamine (1.2 mmol, 121.42 mg) and 1,3-diiodopropane (1 mmol, 295.89 mg) were refluxed for 45 min. The resultant solid was rinsed with ether (2 mL) and recrystallized from ethanol to yield (95%) N,N,N-triethyl-3-iodopropan-1-aminium iodide 1: cream solid; mp: 190–191°C. This salt is soluble in polar solvents (chloroform, methanol, water, and ethanol) but insoluble in nonpolar solvents (diethyl ether, ethyl acetate, hexane, and toluene).
1HNMR (CDCl3, 300 MHz): 1.12–1.07 (m, 9H), 2.06–2.00 (m, 2H), 3.23–3.09 (m, 10H); 13CNMR (DMSO, 125 MHz): 2.73, 7.77, 25.51, 52.78, 56.85.
2.3 Preparation of N,N,N-triethyl-3-iodopropan-1-aminium triiodide 2
Iodine (1 mmol, 253.8 mg) was added to solid crushed N,N,N-triethyl-3-iodopropan-1-aminium iodide 1 (1 mmol, 397.08 mg) under mechanical stirring immediately affording a viscous deep brown liquid N,N,N-triethyl-3-iodopropan-1-aminium triiodide 2. Yield: 100%, this liquid is soluble in chloroform, acetone, and acetonitrile, but insoluble in ethyl acetate, toluene, hexane, diethyl ether, and water.
1HNMR (CDCl3, 300 MHz): 1.12–1.07 (m, 9H), 2.06–2.00 (m, 2H), 3.23–3.09 (m, 10H); 13CNMR (DMSO, 125 MHz): 2.73, 7.77, 25.51, 52.78, 56.85.
2.4 N 1 ,N 1 ,N 1 ,N 3 ,N 3 ,N 3 -Hexaethylpropane-1,3-diaminium di iodide 3
To a round-bottomed flask containing 1,3-diiodopropane (1 mmol, 295.89 mg) in ethanol (5 mL) was added triethylamine (2.4 mmol, 242.85 mg). The reaction mixture was refluxed for 48 h and then cooled to ambient temperature. After filtration, the crystallized product (yield, 97%) was isolated: cream solid; mp: 118–120°C.
1HNMR (DMSO, 500 MHz): 1.19–1.14 (t, 18H), 2.06–2.00 (m, 2H), 3.29–3.16 (m, 16H); 13CNMR (D2O): 7.07, 15.21, 52.95, 53.34.
2.5 N 1 ,N 1 ,N 1 ,N 3 ,N 3 ,N 3 -Hexaethylpropane-1,3-diaminium di triiodide 4
To a solution of N 1 ,N 1 ,N 1 ,N 3 ,N 3 ,N 3 -hexaethylpropane-1,3-diaminium di iodide 3 (1 mmol, 498.27 mg) in ethanol (2 mL) was added iodine (2 mmol, 507.6 mg). After mechanical stirring for 30 min at ambient temperature, the ethanol was eliminated in vacuo to yield (100%) a black solid 4; mp: 271–273°C. This reagent is soluble in chloroform, acetone, and acetonitrile but insoluble in ethyl acetate, toluene, diethyl ether, hexane, methanol, and water.
1HNMR (DMSO, 500 MHz): 1.19–1.14 (t, 18H), 2.06–2.00 (m, 2H), 3.29–3.16 (m, 16H); 13CNMR (D2O): 7.07, 15.21, 52.95, 53.34.
2.6 Typical procedure for the iodination of 2,2′-(phenylazanediyl)diethanol
To a mixture of RTIL2 (1 mol, 650.89 mg) and 2,2′-(phenylimino)diethanol (1 mmol, 181.23 mg), H2O2 (1 mL) was added, and the mixture was stirred vigorously at 50°C. Thin-layer chromatography was used to monitor the progress of the reaction. After the accomplishment of the reaction, water (5 mL) was added. The resulting mixture was moved to a separating funnel and extracted with diethyl ether (2 × 5 L). In the further stage, the ethereal solution obtained from the extraction is first desiccated over anhydrous Na2SO4 and then filtered, and eventually, the evaporation of the solvent led to a pure favorable product.
2.7 4-Iodo-N,N-dimethylaniline (3a)
Blue solid, mp: 67–71°C; 1HNMR (CDCl3, 300 MHz): 2.94 (s, 6H), 6.54–6.51 (m, 2H), 7.46–7.43 (m, 2H); 13CNMR (CDCl3, 75 MHz): 40.51, 77.69, 114.60, 137.58, 149.89.
2.8 N,N-Diethyl-4-iodoaniline (3b)
Brown liquid; 1HNMR (CDCl3, 300 MHz): 1.31 (t, 6H), 3.34 (q, 4H), 6.49 (d, 2H), 7.46 (d, 2H); 13CNMR (CDCl3, 75 MHz): 12.40, 44.46, 77.69, 114.33, 137.77, 147.24.
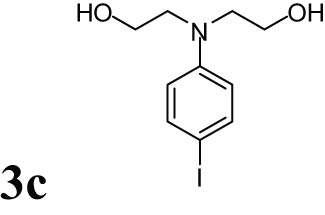
2.9 2,2′-(4-Iodophenylazanediyl)diethanol (3c)
Blue solid; 1HNMR (CDCl3, 300 MHz): 3.50 (t, 4H), 3.74 (t, 4H), 4.47 (s, 2H), 6.43–6.45 (d, 2H), 7.43–7.46 (d, 2H); 13CNMR (CDCl3, 75 MHz): 55.21, 60.30, 77.76, 114.80, 137.79, 147.23.
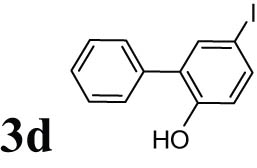
2.10 5-Iodobiphenyl-2-ol (3d)
Orange liquid; 1HNMR (CDCl3, 300 MHz): 5.66 (s, 1H), 6.74–7.72 (m, 8H); 13CNMR (CDCl3, 75 MHz): 86.25, 122.77, 128.13, 128.94, 129.11, 129.54, 131.21, 137.45, 138.26, 151.78.
2.11 4-(4-Iodophenyl)morpholine (3e)
Brown solid, mp: 151–154°C; 13CNMR (CDCl3, 75 MHz): 48.90, 66.67, 81.64, 117.70, 137.80, 150.97.
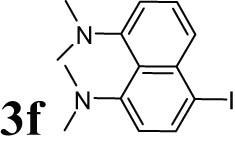
2.12 4-Iodo-N 1 ,N 1 ,N 8 ,N 8 -tetramethylnaphthalene-1,8-diamine (3f)
Dark brown solid; mp: 197–200°C; 1HNMR (CDCl3, 300 MHz): 2.78–2.88 (m, 12H), 6.91–7.33 (m, 5H); 13CNMR (CDCl3, 75 MHz): 44.49, 112.87, 120.24, 121.83, 125.16, 127.91, 137.32, 150.70.
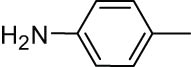
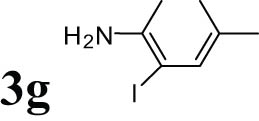
2.13 2-iodo-4-methylaniline (3g)
White solid, mp: 39–41°C; 1HNMR (CDCl3, 300 MHz): 2.10–2.27 (s, 3H), 3.96 (s, 2H), 6.58–7.40 (m, 3H); 13CNMR (CDCl3, 75 MHz): 19.84, 84.31, 114.68, 129.53, 130.05, 139.02, 144.33.
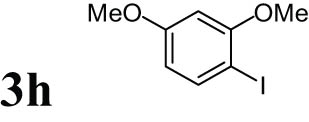
2.14 1-Iodo-2,4-dimethoxybenzene1 (3h)
Yellow solid, mp: 37–39°C; 1HNMR (CDCl3, 300 MHz): 3.70–3.73 (s, 3H), 3.76–3.79 (s, 3H), 6.21–6.35 (m, 2H), 7.51–7.54 (d, 1H); 13CNMR (CDCl3, 75 MHz): 55.58, 56.29, 74.80, 99.23, 107.07, 139.16, 158.85, 161.40.
2.15 13-(4-Iodophenyl)-1,4,7,10-tetraoxa-13-azacyclopentadecane2 (3j)
Brown liquid; 1HNMR (CDCl3, 300 MHz): 3.64 (t, 20H), 6.48 (d, 2H), 7.36 (d, 2H); 13CNMR (CDCl3, 75 MHz): 55.198, 71.172, 72.64, 74.04, 79.63, 120.16, 140.48, 150.11.
2.16 Reutilization of the tetraalkyl ammonium iodide salt (1)
After finishing the reaction, water (5 mL) was added. The resulting reaction mixture was moved to a separating funnel and extracted with diethyl ether (2 × 5 L). In the further stage, the ethereal solution obtained from the extraction is first desiccated over anhydrous Na2SO4 and then filtered, and eventually, the evaporation of the solvent led to a pure favorable product. The aqueous phase comprising highly water-soluble 1 was readily concentrated in vacuo to recycle 1, rinsed with diethyl ether (2 × 2.5 L), dried, and utilized to regenerate RTIL 2 with iodine. The ammonium iodide salt was reused four times without significant yield changes and reaction time.
3 Results and discussion
Iodinated organic compounds are of great importance and have many applications; for example, they can be used as precursors or synthons in the synthesis of organic compounds mainly to form C–C and C–N bonds [32,33,34] and can also be utilized as contrast agents in medical diagnosis or radioactively labeled markers [35,36]. In the past, many iodization methods have been developed in molecular solvents, while in recent years, more attention has been paid to “greener” protocols [37,38,39]. However, iodination in IL media has gained less attention relative to fluorination or bromination [35,36,37,38,39,40].
This article introduces the synthesis, characterization, and reactivity of nonaromatic cation-based salt, N,N,N-triethyl-3-iodopropan-1-aminium triiodide 2, which can act both as a solvent and as a reagent in combination with H2O2 (35%) for the iodination of arenes. N-Alkylammonium ionic liquid 2 is a proton-free RTIL iodine analog with no measurable vapor pressure. Furthermore, 2 is a rare example of RTIL, which integrates the function of solvent and reagent [36]. Our synthesis of [N2223I][I3] is outlined in Scheme 1, path A. In the first step, the needed N,N,N-triethyl-3-iodopropan-1-aminium iodide 1 was generated from inexpensive triethylamine and 1,3-diiodopropane pursuit a modified literature method [41]. Under the solvent-free condition, this N-alkylation reaction was easily obtained with a high isolated yield (95%). At room temperature, the second step took place immediately (1 s), adding one equivalent molecular iodine under mechanical stirring to powdered N,N,N-triethyl-3-iodopropan-1-aminium iodide 1 exothermically formed the brown liquid N,N,N-triethyl-3-iodopropan-1-aminium triiodide 2. From Scheme 1, path A, it can be perceived that this method needs approximately an hour, while conventional methods take several days, so the reaction time is greatly reduced. It is important to note that when triethylamine was heated in boiling ethanol in the presence of 1,3-diiodopropane (Scheme 1, Path B), the formation of the bis(trimethylammonium) salt 3 was observed. Surprisingly, no product containing N,N,N-triethyl-3-iodopropan-1-aminium iodide 1 was detected. Adding molecular iodine to a stirring mixture of N 1 ,N 1 ,N 1 ,N 3 ,N 3 ,N 3 -hexaethylpropane-1,3-diaminium diiodide in ethanol at room temperature formed the black solid N 1 ,N 1 ,N 1 ,N 3 ,N 3 ,N 3 -hexaethylpropane-1,3-diaminium di triiodide 4. Compounds have been characterized by FTIR, 1HNMR, 13CNMR, and UV-Vis spectroscopy (provided in the supplementary material).
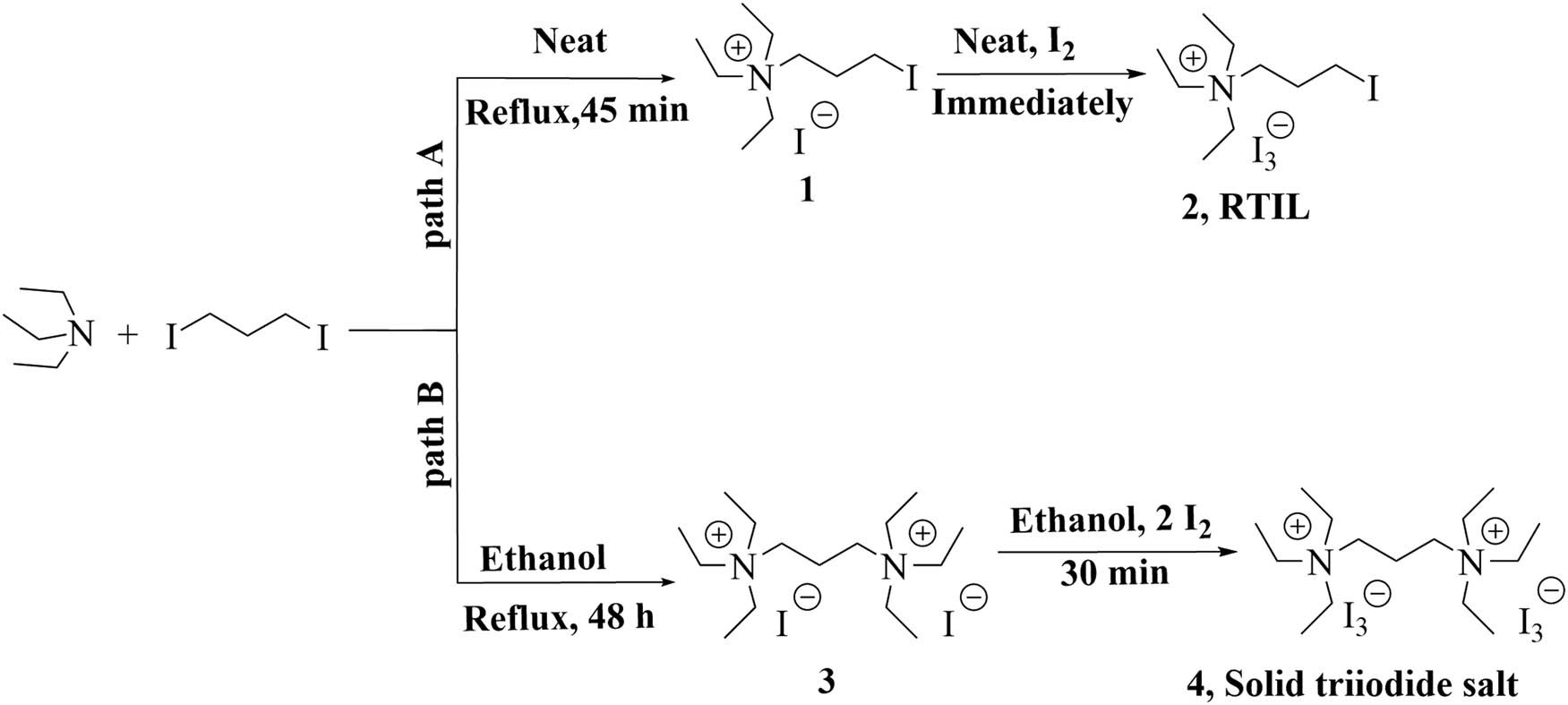
Synthesis pathway for the preparation of RTIL-2 and solid triiodide salt 4.
RTIL is miscible with polar organic solvents, such as chloroform, acetonitrile, and acetone, and insoluble in less polar solvents such as ethyl acetate, diethyl ether, toluene, and hexane. New ionic liquid, like ionic liquids containing PF6, is immiscible with water. On the other hand, its precursor N,N,N-triethyl-3-iodopropan-1-aminium iodide is highly soluble in water, thus facilitating the efficient recovery of N,N,N-triethyl-3-iodopropan-1-aminium iodide, and the separation of iodinated products. This feature affirms the supremacy of this reagent. RTIL 2 is stable for several months at ambient temperature without loss of its activity.
RTIL 2 was then examined as an iodinating agent for a set of aromatic compounds. In initial experiments, N,N-dimethylaniline was used as a model substrate to evaluate suitable reaction conditions to prepare iodo compounds. A wide range of oxidizing agents was examined in RTIL 2 as solvent and reagent. As shown in Table 1, the best results are referred to as ceric ammonium nitrate (CAN) and H2O (Entry 7).
Screening of various oxidants for iodination of N,N-dimethylanilinea
| Entry | Oxidant | Oxidant (mmol) | Time (min) | Yield (%)b |
|---|---|---|---|---|
| 1 | — | — | 480 | — |
| 2 | Oxone | 1 | 480 | — |
| 3 | Oxone/one drop of H2O | 1 | 480 | trace |
| 4 | CeCl3.7 H2O | 0.15 | 300 | 45 |
| 5 | CeCl3.7 H2O/one drop of H2O | 0.2 | 270 | 50 |
| 6 | CAN | 0.15 | 45 | 75 |
| 7 | CAN/one drop of H 2 O | 0.15 | 60 | 90 |
| 8 | CAN/one drop of H2O | 0.2 | 25 | 85 |
| 9 | CAN/one drop of H2O | 0.3 | 25 | 85 |
| 10 | NH4NO3 | 0.15 | 480 | Trace |
| 11 | NH4NO3/one drop of H2O | 0.15 | 480 | Trace |
| 12 | H2O2 | 1 ml | 120 | 60 |
a N,N-Dimethylaniline–RTIL 2 = 1:1, 25°C.
bIsolated yields.
The best results are referred to as ceric ammoniumnitrate (CAN) and H2O.
In green chemistry, the best materials for oxidants can be oxygen or hydrogen peroxide because water is their only by-product. [42,40]. In this project, according to the goals of green chemistry and considering that after the completion of the iodination of aromatic compounds, the only by-product of the reaction is N,N,N-triethyl-3-iodopropan-1-aminium iodide 1, and this salt dissolves easily in the water resulting from the oxidation of hydrogen peroxide and separates from the reaction product, so the best choice in this reaction is hydrogen peroxide as oxidant. Thus, we applied hydrogen peroxide as the oxidant (Table 1). We observed that the reaction of N,N-dimethylaniline (1.0 mmol) with 1 mL of H2O2 (35%) in 1 mmol of RTIL 2 at 50°C proceeded to completion within 45 min (Table 2, Entry 3).
The effect of temperature in the iodination of N,N-dimethylaniline using RTIL 2 as the reagent and solventa
| Entry | H2O2 (ml) | Time (min) | Temp. (°C) | Yield (%)b |
|---|---|---|---|---|
| 1 | 1 | 120 | 25 | 60 |
| 2 | 1 | 60 | 35 | 69 |
| 3 | 1 | 45 | 50 | 90 |
| 4 | 1 | 45 | 60 | 90 |
| 5c | — | 40 | 50 | 90 |
a N,N-Dimethylaniline–RTIL 2 = 1:1.
bYields refer to isolated products.
c N,N-Dimethylaniline–RTIL 2–CAN–H2O = 1:1:0.15:1 drop.
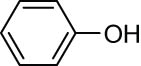
The progress of the reaction mixture led to the corresponding 4-iodo-N,N-dimethylaniline with excellent regioselectivity and excellent isolated yield (90%) (Table 3, Entry 1). This procedure worked successfully for the structurally different arenes. As can be perceived from the results listed in Table 3, all iodination substitution reactions with this system proceeded well. The reaction of 1,3-dimethoxybenzene was performed after 30 min to compose the mono-iodinated compound in a 95% isolated yield (Table 3, Entry 8). Anisole’s reaction needed more time, and 4-iodoanisole was the only reaction product obtained after 1 h (Table 3, Entry 11). Iodinations of N,N-dimethylaniline (Table 3, Entry 1) and N,N-diethylaniline (Table 3, Entry 2) were also performed in a short reaction time (∼50 min), and the mono-iodinated para isomers were generated in 90 and 88% isolated yields, respectively. The reaction of phenol took longer and was completed within 60 min to give 2,4,6-triiodophenol as the sole product of the reaction (Table 3, Entry 9). The iodination of N-phenylmorpholine produced 4-iodo-N-phenylmorpholine in 88% isolated yield as the only product after 40 min (Table 3, Entry 5). N 1 ,N 1 ,N 8 ,N 8 -Tetramethylnaphthalene-1,8-diamine was also iodinated within 1 h to produce the mono-iodinated product in 85% isolated yield, the only product of the reaction (Table 3, Entry 6). Afterward, we exerted these optimized conditions to N-phenyl-1-aza-15-crown-5. The reaction proceeded well and produced the mono-iodinated para isomer in excellent yield within a short reaction time (Table 3, Entry 10). Iodinations of p-toluidine, biphenyl-2-ol, and 2,2′-(phenylazanediyl)diethanol were also studied. Isolation of 2-iodo-4-methylaniline in 93% (Table 3, Entry 7), 5-iodobiphenyl-2-ol in 90% (Table 3, Entry 4), and 2,2′-(4-iodophenylazanediyl)diethanol in 80% (Table 3, Entry 3) from the reaction compounds as the exclusive products of the reaction shows the high regioselectivity of the procedure, and also excellent chemoselectivity was observed for substrates with OH and NH functionalities, providing mono-iodinated derivatives as the major products, and no significant oxidation took place. The iodination of furan-2-carbaldehyde and benzonitrile under resembling reaction conditions was unsuccessful, and the mentioned materials were isolated intact after 48 h. The outcomes of this perusal are compiled in Table 3. The reactions that generate water-tolerant and hydrophobic products are disrupted and quenched by adding water. This even results in precipitation of the products as solids or oils, easily separated and rinsed with water and desiccated (either with Na2SO4 or in vacuo). The aqueous phase comprising very water-soluble 1 was easily concentrated in vacuo to recover 1, which then could be utilized to renew RTIL 2 with iodine (Scheme 1, path A). Therefore, it was recycled several times after completing the reaction, and the results were very desirable (Table 4). We declare no organic solvent in this method, and water is the only solvent used to extract and quench the mixtures at the end of the reaction. In other words, reactions leading to water-miscible products (Entries 4, 6) were extracted with Et2O.
RTIL 2 as the reagent and solvent-assisted iodination of aromatic compounds in the attendance of H2O2 (35%) at 50°Ca
| Entry | Substrate | Product | Time (min) | Yield (%)b |
|---|---|---|---|---|
| 1 |
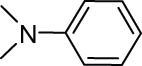
|

|
45 | 90 |
| 2 |
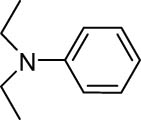
|

|
50 | 88 |
| 3 |

|
|
40 | 80 |
| 4 |

|
|
30 | 90 |
| 5 |

|

|
40 | 88 |
| 6 |
|
|
40 | 85 |
| 7 |
|
|
30 | 96 |
| 8 |

|
|
30 | 95 |
| 9 |
|
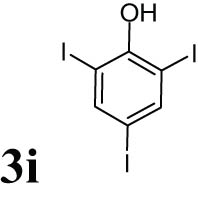
|
60 | 84 |
| 10 |
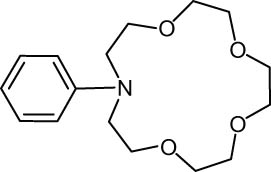
|
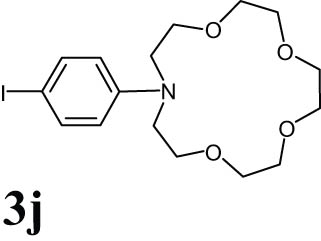
|
25 | 91 |
| 11 |

|
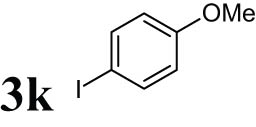
|
60 | 84 |
aAll products were designated by IR, 1HNMR, and 13CNMR spectroscopic data, and melting points.
bIsolated yields.
The reusability of the tetraalkylammonium iodide 1
| No. of recovery | Time (min) | Yield (%) |
|---|---|---|
| 1 | 40 | 80 |
| 2 | 44 | 80 |
| 3 | 47 | 78 |
| 4 | 53 | 76 |
The precise mechanism for this reaction is not clear. In the possible mechanism, hypoiodous acid (HOI) is produced by the reaction of RTIL 2 with hydrogen peroxide. Then, the electrophile (iodonium ion) attacks the aromatic ring to synthesize the iodinated product [43] (Scheme 2). The presence of tetraalkyl ammonium iodide salt 1 and brominated products was confirmed using FTIR, 1HNMR, and 13CNMR spectra at the end of the reaction.
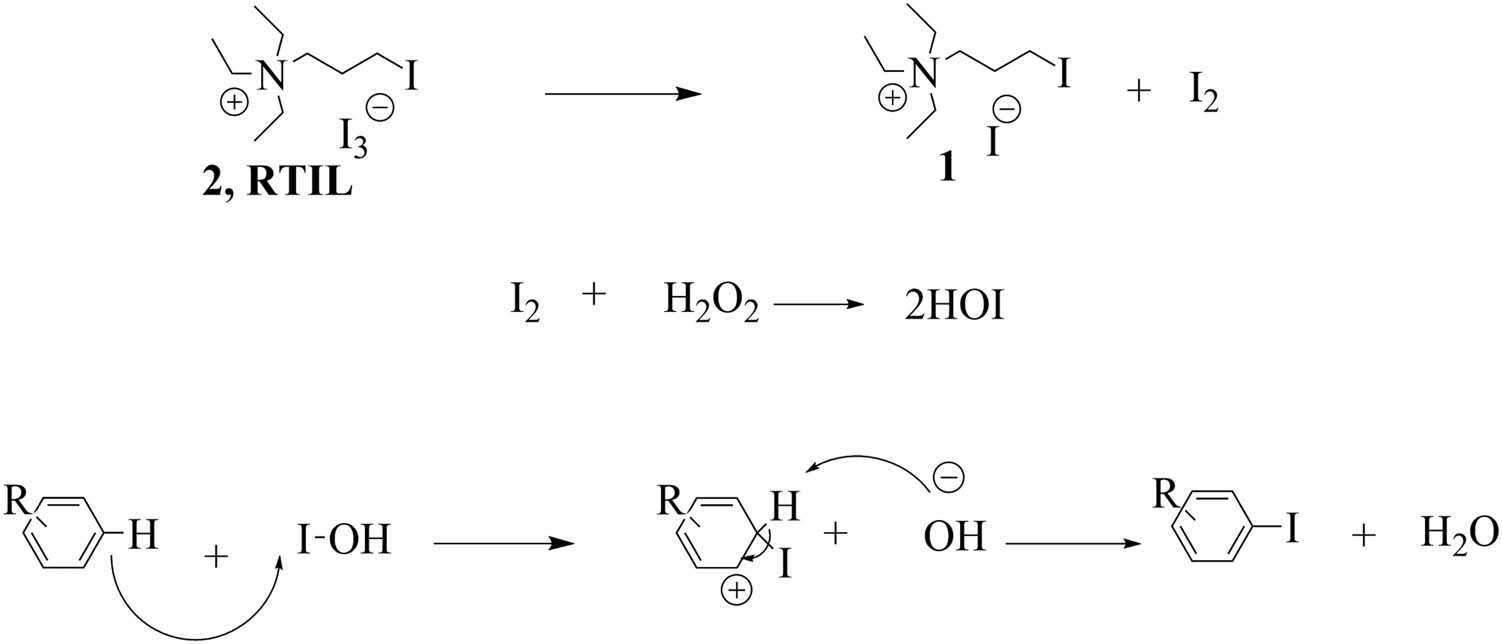
The plausible reaction mechanism for the iodination of aromatic compounds in the presence of RTIL 2 as the reagent and the solvent and H2O2 (35%).
4 Conclusion
According to the goals of green chemistry and its approach to using green solvents and compounds to reduce environmental pollution, this article presents a significant and mild method for iodination reaction utilizing ionic liquid 2 based on quaternary ammonium cation as a novel reagent and reaction medium. Based on data from the literature, we have, for the first time, reported a recoverable and inexpensive triiodide ionic liquid, and it is the first example that triiodide RTIL was used both as the green solvent and as a reagent in combination with H2O2 (35%) to produce corresponding iodo derivatives. The iodination was performed without the presence of any organic solvent, and water was the only extraction solvent. At the end of the reaction, the consumed reagent N,N,N-triethyl-3-iodopropan-1-aminium iodide was easily recycled.
The simplicity of the methodology, mild conditions, ease of product isolation, and the probability of IL recycling could make this process attainable in the future on the industrial scales. In addition to iodination, it can be utilized for various other organic transformations listed in due course.
Acknowledgments
The authors acknowledge the Semnan University Research Council for supporting this work.
-
Funding information: Research was funded by Semnan University. Grant Recipient: Dr. Nadiya Koukabi, Associate Professor.
-
Conflict of interest: Authors state no conflict of interest.
-
Data availability statement: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
[1] Anastas PT, Zimmerman JB. Design through the 12 principles of green engineering. Environ Sci Technol. 2003;37:94A–101.10.1109/EMR.2007.4296421Search in Google Scholar
[2] Ghanaat J, Khalilzadeh MA, Zareyee D. KF/CP NPs as an efficient nanocatalyst for the synthesis of 1,2,4-triazoles: the study of antioxidant and antimicrobial activity. Eurasian Chem Commun. 2020;2:202–12.10.33945/SAMI/ECC.2020.2.6Search in Google Scholar
[3] Stolte S, Matzke M, Arning J, Boschen A, Pitner W, Welz-Biermann U, et al. Effects of different head groups and functionalised side chains on the aquatic toxicity of ionic liquids. Green Chem. 2007;9:1170–9.10.1039/b711119cSearch in Google Scholar
[4] Khalafy J, Dilmaghani S, Etivand N, Marjani AP. A simplified green approach for synthesis of arylquinoxalines under solvent-free clay-catalysed conditions. Eurasian Chem Commun. 2019;1:257–67.Search in Google Scholar
[5] Constable DJC, Jimenez-Gonzalez C, Henderson RK. Perspective on Solvent Use in the Pharmaceutical Industry. Org Process Res Dev. 2007;11:133–7.10.1021/op060170hSearch in Google Scholar
[6] Das S, Ekka D, Roy MN. Conductance and FTIR spectroscopic study of triple-ion formation of tetrabutylphosphonium methanesulfonate in methylamine solution. Chem Methodolo. 2020;4:55–67.10.33945/SAMI/CHEMM.2020.1.5Search in Google Scholar
[7] Dupont J, de Souza RF, Suarez PAZ. Ionic liquid (molten salt) phase organometallic catalysis. Chem Rev. 2002;102:3667–3691.10.1021/cr010338rSearch in Google Scholar
[8] Islam MJ, Kumer A, Sarker MN, Paul S. The activity of alkyl groups in morpholinium cation on chemical reactivity, and biological properties of morpholinium tetrafluroborate ionic liquid using the DFT method. Chem Methodol. 2020;4:130–42.10.33945/SAMI/CHEMM.2020.2.3Search in Google Scholar
[9] Nikpassand M, Fekri LZ. Catalyst-free synthesis of mono and bis spiro pyrazolopyridines in DSDABCO as a novel media. Chem Methodolo. 2020;4:437–46.10.33945/SAMI/CHEMM.2020.4.6Search in Google Scholar
[10] Wasserscheid P, Keim W. Ionic liquids – new “solutions” for transition metal catalysis. Angew Chem Int Ed. 2000;39:3773–89.10.1002/1521-3773(20001103)39:21<3772::AID-ANIE3772>3.0.CO;2-5Search in Google Scholar
[11] Welton T. Room-temperature ionic liquids. solvents for synthesis and catalysis. Chem Rev. 1999;99:2071–83.10.1021/cr980032tSearch in Google Scholar
[12] Baj S, Chrobok A, Słupska R. The Baeyer–Villiger oxidation of ketones with bis(trimethylsilyl) peroxide in the presence of ionic liquids as the solvent and catalyst. Green Chem. 2009;11:279–82.10.1039/B814534BSearch in Google Scholar
[13] Cole AC, Jensen JL, Ntai I, Tran KLT, Weaver KJ, Forbes DC, et al. Novel brønsted acidic ionic liquids and their use as dual solvent−catalysts. J Am Chem Soc. 2002;124:5962–3.10.1021/ja026290wSearch in Google Scholar
[14] Gordon M. New developments in catalysis using ionic liquids. Appl Catal, A. 2001;222:101–17.10.1016/S0926-860X(01)00834-1Search in Google Scholar
[15] Jiang N, Ragauskas AJ. Selective aerobic oxidation of activated alcohols into acids or aldehydes in ionic liquids. J Org Chem. 2007;72:7030–3.10.1021/jo0707737Search in Google Scholar
[16] Peng J, Deng Y. Ionic liquids catalyzed Biginelli reaction under solvent-free conditions. Tetrahedron Lett. 2001;42:5917–9.10.1016/S0040-4039(01)01139-XSearch in Google Scholar
[17] Sheldon R. Catalytic reactions in ionic liquids. Chem Commun. 2001;2399–407.10.1039/b107270fSearch in Google Scholar PubMed
[18] Yadav LDS, Patel R, Srivastava VP. One-pot oxidative conjugate hydrothiocyanation-hydrosulfenylation of baylis-hillman alcohols promoted by a protic ionic liquid. Synlett. 2008;12:1789–92.10.1055/s-2008-1078549Search in Google Scholar
[19] Zang H, Wang M, Cheng B, Song J. Ultrasound-promoted synthesis of oximes catalyzed by a basic ionic liquid [bmIm]OH. Ultrason Sonochem. 2009;16:301–3.10.1016/j.ultsonch.2008.09.003Search in Google Scholar PubMed
[20] Jadhav VH, Kim JG, Park SH, Kim DW. Task-specific hexaethylene glycol bridged di-cationic ionic liquids as catalysts for nucleophilic fluorination using potassium fluoride. Chem Eng J. 2017;308:664–8.10.1016/j.cej.2016.09.118Search in Google Scholar
[21] Koguchi S, Shibuya Y, Igarashi Y, Takemura H. C–Te cross-coupling of diaryl ditellurides with arylboronic acids by using copper(i) thiophene-2-carboxylate under mild conditions. Synlett. 2019;30:943–46.10.1055/s-0037-1610324Search in Google Scholar
[22] Laali KK. Ionic liquids as novel media for electrophilic/onium ion chemistry and metal-mediated reactions: a progress summary. Arkivoc. 2016;2016:150–71.10.3998/ark.5550190.p009.490Search in Google Scholar
[23] Moosavi-Zare AR, Zolfigol MA, Rezanejad Z. The Synthesis of α,αʹ-bis(arylidene)Cycloalkanones using Sulfonic Acid Functionalized Pyridinium Chloride. Chem Methodolo. 2020;4:614–22.Search in Google Scholar
[24] Pavlinac J, Zupan M, Laali KK, Stavber S. Halogenation of organic compounds in ionic liquids. Tetrahedron. 2009;65:5625–62.10.1016/j.tet.2009.04.092Search in Google Scholar
[25] Ren YL, Wang P, Tian XZ, Li FW, Wang BY, Wang JJ. Ionic liquid/H2SO4 catalyzed aerobic iodination of alkoxyl-substituted benzenes and naphthalines. J Molecular. Catalysis. 2015;29:505–12.Search in Google Scholar
[26] Sajjadifar S, Amini I, Jabbari H, Pouralimardan O, Fekri MH, Pal K. An efficient facile and one-pot synthesis of 2-arylsubstituted benzimidazole derivatives using 1-methyl-3-(2-oxyethyl)-1H-imidazol-3-ium-borate sulfonic acid as a recyclable and highly efficient ionic liquid catalyst at green condition. Eurasian. Chem Commun. 2019;1:191–9.10.33945/SAMI/ECC.2019.2.7Search in Google Scholar
[27] Chemat F, Vian MA, Ravi HK, Khadhraoui B, Hilali S, Perino S, et al. Review of alternative solvents for green extraction of food and natural products: Panorama, principles, applications and prospects. Molecules 2019;24:3007.10.3390/molecules24163007Search in Google Scholar PubMed PubMed Central
[28] Huang J, Guo X, Xu T, Fan L, Zhou X, Wu S. Ionic deep eutectic solvents for the extraction and separation of natural products. J Chromatogr A. 2019;1598:1–19.10.1016/j.chroma.2019.03.046Search in Google Scholar PubMed
[29] Lee J, Jung D, Park K. TrAC,Trends. Hydrophobic deep eutectic solvents for the extraction of organic and inorganic analytes from aqueous environments. Anal Chem. 2019;118:853–68.10.1016/j.trac.2019.07.008Search in Google Scholar
[30] Pacheco-Fernández I, Pino V. Green solvents in analytical chemistry. Curr Opin Green Sustain Chem. 2019;18:42–50.10.1016/j.cogsc.2018.12.010Search in Google Scholar
[31] Gribble GW. Naturally Occurring Organohalogen Compounds. Acc Chem Res. 1998;31:141–52.10.1021/ar9701777Search in Google Scholar
[32] Arghan M, Koukabi N, Kolvari E. Cobalt supported on dendronized magnetic nanoparticles: A new highly efficient and recyclable catalyst for the Mizoroki–Heck cross-coupling reaction. Appl Organomet Chem. 2019;33, e5075. doi.org/10.1002/aoc.5075.10.1002/aoc.4823Search in Google Scholar
[33] Arghan M, Koukabi N, Kolvari E. Magnetic apple seed starch functionalized with 2,2′-furil as a green host for cobalt nanoparticles: Highly active and reusable catalyst for Mizoroki–Heck and the Suzuki–Miyaura reactions. Appl Organomet Chem. 2019;33, e4823. doi.org/10.1002/aoc.4823.10.1002/aoc.5075Search in Google Scholar
[34] Lam PYS, Deudon S, Averill KM, Li R, He MY, DeShong P, et al. Copper-promoted C−N bond cross-coupling with hypervalent aryl siloxanes and room-temperature N-arylation with aryl iodide. J Am Chem Soc. 2000;122:7600–1.10.1021/ja001305gSearch in Google Scholar
[35] Bailey L, Handy ST. Aromatic iodination using N-iodosaccharin in room temperature ionic liquids. Tetrahedron Lett. 2011;52:2413–4.10.1016/j.tetlet.2011.03.010Search in Google Scholar
[36] Pavlinac J, Zupan M, Laali KK, Stavber S. Halogenation of organic compounds in ionic liquids. Tetrahedron. 2009;65:5625–62.10.1016/j.tet.2009.04.092Search in Google Scholar
[37] Kolvari E, Ghorbani-Choghamarani A, Salehi P, Shirini F, Zolfigol MA. Application of N-halo reagents in organic synthesis. J Iran Chem Soc. 2007;4:126–74.10.1007/BF03245963Search in Google Scholar
[38] Kolvari E, Koukabi N, Khoramabadi-zad A, Shiri A, Zolfigol MA. Alternative methodologies for halogenation of organic compounds. Curr Org Synth. 2013;10:837–63.10.2174/157017941006140206102541Search in Google Scholar
[39] Stavber S, Jereb M, Zupan M. Electrophilic iodination of organic compounds using elemental iodine or iodides. Synthesis. 2008;2008:1487–1513.10.1055/s-2008-1067037Search in Google Scholar
[40] Podgoršek A, Zupan M, Iskra J. Oxidative halogenation with “green” oxidants: oxygen and hydrogen peroxide. Angew Chem Int Ed. 2009;48:8424–50.10.1002/anie.200901223Search in Google Scholar PubMed
[41] Kavala V, Naik S, Patel BK. A new recyclable ditribromide reagent for efficient bromination under solvent free condition. J Org Chem. 2005;70:4267–71.10.1021/jo050059uSearch in Google Scholar PubMed
[42] Gunasekaran N. Aerobic oxidation catalysis with air or molecular oxygen and ionic liquids. Adv Synth Catal. 2015;357:1990–2010.10.1002/adsc.201400989Search in Google Scholar
[43] Singhal S, Jain SL, Sain B. A simple and improved regioselective bromination of aromatic compounds using N-methylpyrolidin-2-one hydrotribromide and aqueous hydrogen peroxide under mild reaction conditions. Catal A: Chem. 2006;258:198–202.10.1016/j.molcata.2006.05.042Search in Google Scholar
© 2022 Mahnaz Sakhdari et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Sono and nano: A perfect synergy for eco-compatible Biginelli reaction
- Study of the reactivity of aminocyanopyrazoles and evaluation of the mitochondrial reductive function of some products
- “Click” assembly of novel dual inhibitors of AChE and MAO-B from pyridoxine derivatives for the treatment of Alzheimer’s disease
- Synthesis of 2,2-difluoro-2-arylethylamines as fluorinated analogs of octopamine and noradrenaline
- Cyclization of N-acetyl derivative: Novel synthesis – azoles and azines, antimicrobial activities, and computational studies
- Two independent and consecutive Michael addition of 1,3-dimethylbarbituric acid to (2,6-diarylidene)cyclohexanone: Flying-bird-shaped 2D-polymeric structure
- Ionic liquid-catalyzed synthesis of (1,4-benzoxazin-3-yl) malonate derivatives via cross-dehydrogenative-coupling reactions
- Synthesis of novel triiodide ionic liquid based on quaternary ammonium cation and its use as a solvent reagent under mild and solvent-free conditions
- Eelectrosynthesis of benzothiazole derivatives via C–H thiolation
- Synthesis of fluoro-rich pyrimidine-5-carbonitriles as antitubercular agents against H37Rv receptor
- Syntheses, crystal structure, thermal behavior, and anti-tumor activity of three ternary metal complexes with 2-chloro-5-nitrobenzoic acid and heterocyclic compounds
- Synthesis of enhanced lipid solubility of indomethacin derivatives for topical formulations
- Synthesis of newer substituted chalcone linked 1,2,3-triazole analogs and evaluation of their cytotoxic activities
- Novel benzodioxatriaza and dibenzodioxadiazacrown compounds carrying 1,2,4-oxadiazole moiety
- Synthesis of rhodium catalysts with amino acid or triazine as a ligand, as well as its polymerization property of phenylacetylene
- DABCO-based ionic liquid-promoted synthesis of indeno-benzofurans derivatives: Investigation of antioxidant and antidiabetic activities
- Design, synthesis, and biological activity of novel pomalidomide linked with diphenylcarbamide derivatives
- Study on effective synthesis of 7-hydroxy-4-substituted coumarins
- Review Article
- Chemical constituents of plants from the genus Carpesium
- Communication
- Reactions of 3-amino-1,2,4-triazine with coupling reagents and electrophiles
Articles in the same Issue
- Research Articles
- Sono and nano: A perfect synergy for eco-compatible Biginelli reaction
- Study of the reactivity of aminocyanopyrazoles and evaluation of the mitochondrial reductive function of some products
- “Click” assembly of novel dual inhibitors of AChE and MAO-B from pyridoxine derivatives for the treatment of Alzheimer’s disease
- Synthesis of 2,2-difluoro-2-arylethylamines as fluorinated analogs of octopamine and noradrenaline
- Cyclization of N-acetyl derivative: Novel synthesis – azoles and azines, antimicrobial activities, and computational studies
- Two independent and consecutive Michael addition of 1,3-dimethylbarbituric acid to (2,6-diarylidene)cyclohexanone: Flying-bird-shaped 2D-polymeric structure
- Ionic liquid-catalyzed synthesis of (1,4-benzoxazin-3-yl) malonate derivatives via cross-dehydrogenative-coupling reactions
- Synthesis of novel triiodide ionic liquid based on quaternary ammonium cation and its use as a solvent reagent under mild and solvent-free conditions
- Eelectrosynthesis of benzothiazole derivatives via C–H thiolation
- Synthesis of fluoro-rich pyrimidine-5-carbonitriles as antitubercular agents against H37Rv receptor
- Syntheses, crystal structure, thermal behavior, and anti-tumor activity of three ternary metal complexes with 2-chloro-5-nitrobenzoic acid and heterocyclic compounds
- Synthesis of enhanced lipid solubility of indomethacin derivatives for topical formulations
- Synthesis of newer substituted chalcone linked 1,2,3-triazole analogs and evaluation of their cytotoxic activities
- Novel benzodioxatriaza and dibenzodioxadiazacrown compounds carrying 1,2,4-oxadiazole moiety
- Synthesis of rhodium catalysts with amino acid or triazine as a ligand, as well as its polymerization property of phenylacetylene
- DABCO-based ionic liquid-promoted synthesis of indeno-benzofurans derivatives: Investigation of antioxidant and antidiabetic activities
- Design, synthesis, and biological activity of novel pomalidomide linked with diphenylcarbamide derivatives
- Study on effective synthesis of 7-hydroxy-4-substituted coumarins
- Review Article
- Chemical constituents of plants from the genus Carpesium
- Communication
- Reactions of 3-amino-1,2,4-triazine with coupling reagents and electrophiles

