Abstract
Three novel rhodium complexes, with l-tyrosine (l-Tyr), l-arginine (l-Arg), or 2,4-diamino-6-phenyl-1,3,5-triazine (Dpt) as a ligand, named as [Rh(cod)(l-Tyr)], [Rh(cod)(l-Arg)], and [Rh(cod)(Dpt)2], respectively, had been synthesized for catalyzing the polymerization of phenylacetylene. Their yields were 62.34, 54.87, and 58.21%, respectively, by the most suitable synthesis conditions at 25°C for 4 h. The structures and purity of these complexes were proved by 1H NMR, element analysis, and scanning electron microscope (SEM). It has been examined that phenylacetylene could be polymerized by the three complexes as catalysts with high degrees of polymerization (n = 368, 385, and 664, respectively) and yields (about 87.62, 88.39, and 59.67%, respectively). In conclusion, compared with traditional [Rh–N] type catalysts, the novel [N–Rh–N] type catalyst ([Rh(cod)(Dpt)2]) gained better catalytic performance. By comparing the yield, Mw, and degree of their polymerization, the polymerization mechanism was found under the [N–Rh–N] type rhodium catalyst system.
1 Introduction
Using catalysts to increase the reaction rate and selectivity was one of the most economical and effective methods in modern industrial production. Because of its high activity, high application, and high regularity, rhodium catalysts have been frequently employed in hydrogenation [1], oxidation [2], hydroformylation [3], oxidative coupling [4], and polymerization of alkynes [5]. Recently, the polymerization of phenylacetylene (PA) became a research hotspot. Therefore, it is of great significance to obtain high-performance polyphenylacetylene by rhodium catalysts.
In 1969, Kern [6] first reported that rhodium catalysts could catalyze the polymerization of PA. Diene ligands (cod, nbd, dcp, and tfb) as precursors of rhodium catalysts were studied by Saeed et al. [7], Furlani et al. [8], Kishimoto et al. [9], Bennett et al. [10], and Roe and Massey [11]. In 2009, Jimenez et al. [12] prepared and characterized a series of cationic complexes [Rh(diene){Ph2P(CH2) n Z}][BF4] containing functional phosphine ligands of the type Ph2P(CH2) n Z (n = 2 or 3; Z = OMe, NMe2 or SMe). The ligands were screened and optimized to obtain better catalytic activity for the polymerization of PA. Angoy et al. [13] synthesized the dinuclear [Rh2(diene){μ-NH(CH2)3PPh2}2] complexes with π-acceptors diene ligands, which exhibited remarkable catalytic activity in the stereoregular polymerization of PA.
In addition to phosphine ligands, rhodium catalysts with nitrogen-containing heterocycle ligands, such as pyridine, porphyrin, quinoline, and amino acid [14,15,16,17], were also used for polyphenylacetylene. Pan et al. [14] studied the reaction of self-made methyl-2-butyrate-pyridine (MBP) with [Rh(CO)2Cl]2 to obtain rhodium catalyst [Rh(CO)2(MBP)Cl], which was applied to the carbonylation of MeOH to AcOH. Stateman et al. [15] designed the azuliporphyrin ligand by azulene dialdehyde and tripyranes and then obtained the azuliporphyrin rhodium catalyst. Furlani et al. [16] found that rhodium complex with pyridine as a ligand showed high stereoregularity in the polymerization of PA. Kondo et al. [17] reported a catalyst with one 2-methyl-8-quinolinolate ligand (abbreviated to 2-Me-Q) and two –CO ligands on the Z-selective anti-Markovnikov addition of alcohols to terminal alkynes. Zhao et al. [4] synthesized rhodium catalysts with l-phenylalanine, l-valine, and l-proline as ligands and used them to catalyze the oxidative coupling reaction of PA and 2,4-dihydroxybenzaldehyde as substrates to obtain the flavonoid compounds.
In the present study, the novel catalysts, [Rh(cod)(l-Tyr)] (cod = 1,5-cyclooctadiene, l-Tyr = l-tyrosine), [Rh(cod)(l-Arg)] (l-Arg = l-arginine), and [Rh(cod)(Dpt)2] (Dpt = 2,4-diamino-6-phenyl-1,3,5-triazine), were synthesized, isolated, and purified by recrystallization. Especially, polyphenylacetylene catalyzed by [Rh(cod)(Dpt)2] had a higher molecular weight and degree of polymerization than that of other catalysts. Based on the polymerization results, it was discussed that the number of intramolecular Rh–N bonds affected the polymerization ability.
2 Results and discussion
Three novel rhodium catalysts with l-Tyr, l-Arg, and Dpt as ligands were synthesized (Scheme 1), namely, [Rh(cod)(l-Tyr)], [Rh(cod)(l-Arg)], and [Rh(cod)(Dpt)2].
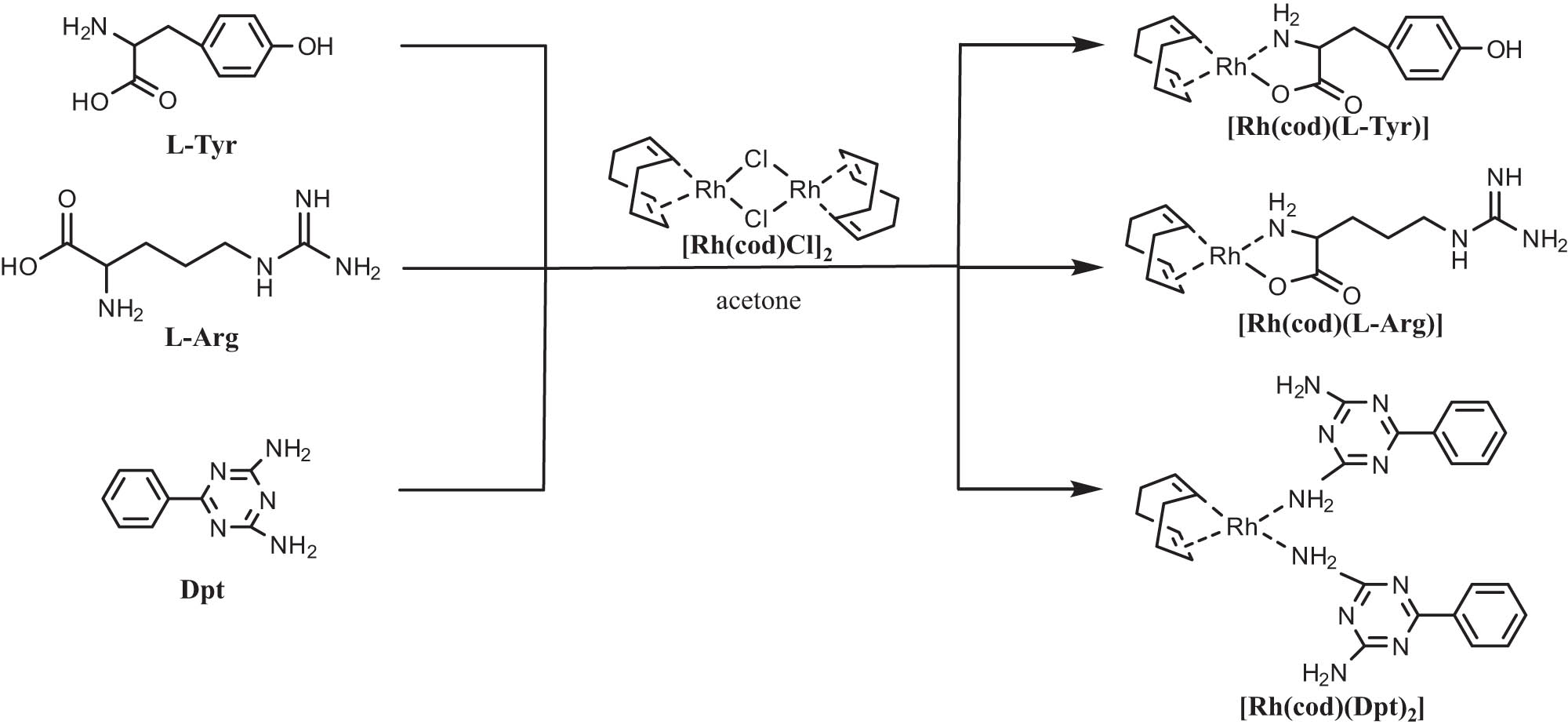
Synthesis of rhodium catalysts.
Rhodium catalysts, [Rh(cod)(l-Arg)], [Rh(cod)(l-Tyr)], and [Rh(cod)(Dpt)2], were successfully prepared in this study. 1H NMR spectrum of [Rh(cod)(Dpt)2] is shown in Figure 1. The peaks of e, f, and d correspond to hydrogen absorption on the phenyl ring. a1, a2, and b were the absorption peak of hydrogen position in cod. Due to the –CH2– conformation in cod and test solvent dimethyl sulfoxide (DMSO), the two hydrogen atoms were influenced differently by the coordinate bond of Rh·‖, so there were two groups (a1 and a2) of split peaks. The broad peak of c showed active hydrogen atoms of –NH2. 1H NMR spectra of [Rh(cod)(l-Arg)] and [Rh(cod)(l-Tyr)] were given in the supporting information (Figures A1 and A2).
![Figure 1
1H NMR spectrum of [Rh(cod)(Dpt)2] in DMSO.](/document/doi/10.1515/hc-2022-0014/asset/graphic/j_hc-2022-0014_fig_001.jpg)
1H NMR spectrum of [Rh(cod)(Dpt)2] in DMSO.
Elemental analysis of the synthesized rhodium catalysts was carried out. The results showed that although there was a little disparity between the measured data and the theoretical value, it could also prove the purity of the products obtained. The SEM images of the three synthesized rhodium catalysts are shown in Figure 2. [Rh(cod)(l-Tyr)] was presented with a loose random pore structure and stacked together (Figure 2a). [Rh(cod)(l-Arg)] was a lamellar structure (Figure 2b). [Rh(cod)(Dpt)2] presented a serried honeycomb structure (Figure 2c), which was stable, with a large specific surface area and many catalytic sites. It also manifested high purity. Image-D showed smaller gaps at 100 times, which proved a result of the strong π–π stacking interaction because of the triazine and phenyl ring in [Rh(cod)(Dpt)2].
![Figure 2
SEM images of the rhodium catalysts: (a) [Rh(cod)(l-Tyr)]; (b) [Rh(cod)(l-Arg)]; (c) [Rh(cod)(Dpt)2]; and (d) enlarged 100 times of image-C.](/document/doi/10.1515/hc-2022-0014/asset/graphic/j_hc-2022-0014_fig_002.jpg)
SEM images of the rhodium catalysts: (a) [Rh(cod)(l-Tyr)]; (b) [Rh(cod)(l-Arg)]; (c) [Rh(cod)(Dpt)2]; and (d) enlarged 100 times of image-C.
After determining the structures of novel catalysts, the influence of yield by reaction conditions was paid more attention. In addition to the factors of the catalysts, reaction time and temperature also had a certain impact on the reaction process (Table 1). By adjusting the reaction time and temperature, the optimum was obtained at 25°C for 4 h, namely, [Rh(cod)(l-Tyr)] (62.34%), [Rh(cod)(l-Arg)] (54.87%), and [Rh(cod)(Dpt)2] (58.21%). Due to the shorter reaction time (for 2 h in No. 1, 6, and 11 in Table 1) or lower temperature (at 10°C in No. 4, 9, and 14 in Table 1), the yields decreased, which may be because the reaction was carried out incompletely.
The yields of catalysts under different reaction times and temperature
| No. | Catalyst | Time (h) | Temperature (°C) | Yield (%) |
|---|---|---|---|---|
| 1 |
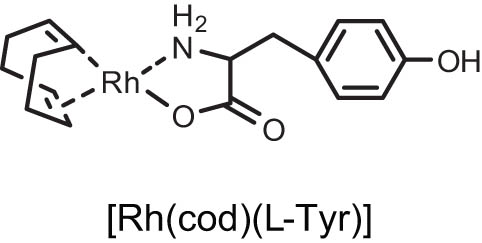
|
2 | 25 | 13.52 |
| 2 | [Rh(cod)(l-Tyr)] | 4 | 25 | 62.34 |
| 3 | 6 | 25 | 58.19 | |
| 4 | 4 | 10 | 20.43 | |
| 5 | 4 | 40 | 60.82 | |
| 6 |
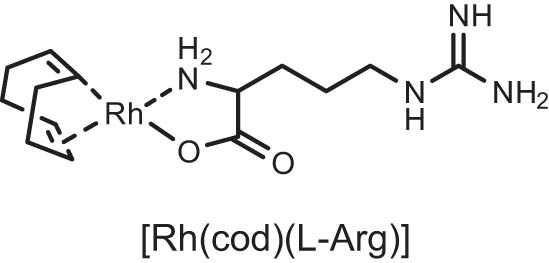
|
2 | 25 | 26.44 |
| 7 | [Rh(cod)(l-Arg)] | 4 | 25 | 54.87 |
| 8 | 6 | 25 | 54.17 | |
| 9 | 4 | 10 | 18.52 | |
| 10 | 4 | 40 | 52.14 | |
| 11 |
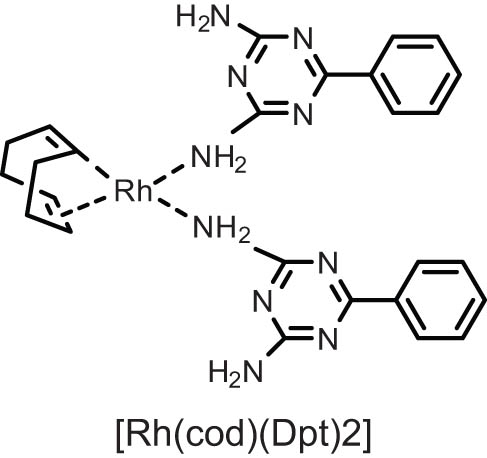
|
2 | 25 | 27.36 |
| 12 | [Rh(cod)(Dpt)2] | 4 | 25 | 58.21 |
| 13 | 6 | 25 | 53.64 | |
| 14 | 4 | 10 | 19.47 | |
| 15 | 4 | 40 | 57.32 |
Conversely, the longer reaction time (for 6 h in No. 3, 8, and 13 in Table 1) also caused the yields decreased, which may because of side reactions, such as decomposition of products. Therefore, it was believed that 4 h and 25°C were the best reaction conditions for the synthesis.
For detecting the catalytic polymerization property of the novel rhodium catalysts (Scheme 2), they were used to catalyze the polymerization of PA for 4 h at 25°C in different organic solvents (Table 2).
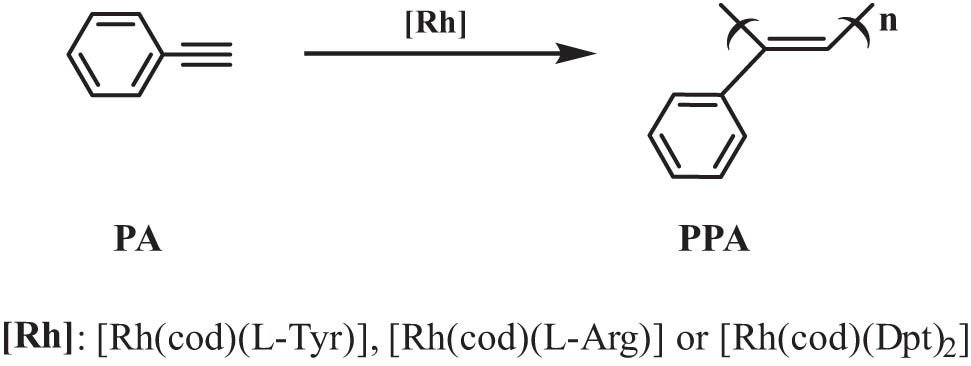
Polymerization of phenylacetylene.
Polymerization of PA by three novel rhodium catalysts in different solventsa
| No. | Catalyst | Solvent | Yield (%) | M w b (×104) | PDb | n c |
|---|---|---|---|---|---|---|
| 1 | [Rh(cod)(l-Tyr)] | Toluene | 87.62 | 8.83 | 2.35 | 368 |
| 2 | Acetone | 25.07 | 3.33 | 2.17 | 150 | |
| 3 | DMAC | 51.42 | 4.44 | 1.93 | 225 | |
| 4 | THF | 14.12 | 3.43 | 2.07 | 162 | |
| 5 | [Rh(cod)(l-Arg)] | Toluene | 88.39 | 9.55 | 2.43 | 385 |
| 6 | Acetone | 30.24 | 5.18 | 1.89 | 268 | |
| 7 | DMAC | 40.16 | 3.10 | 2.32 | 131 | |
| 8 | THF | 32.38 | 2.87 | 1.97 | 143 | |
| 9 | [Rh(cod)(Dpt)2] | Toluene | 59.67 | 16.01 | 2.36 | 664 |
| 10 | Acetone | 40.14 | 5.97 | 1.86 | 314 | |
| 11 | DMAC | 37.36 | 6.55 | 1.95 | 329 | |
| 12 | THF | 29.32 | 5.13 | 2.12 | 237 |
DMAC: N,N-Dimethylacetamide; THF: Tetrahydrofuran.
a[PA] = 0.01 mmol/L, [PA]/[Rh] = 100; at 25°C for 4 h.
bDetermined by gel permeation chromatography (GPC) in THF.
cDegree of polymerization, n = M n/M PA = (M w/PD)/M PA.
To compare the four organic solvents in Table 2, toluene was most suitable for the polymerization of PA. No matter which catalysts were used, the yields (87.62, 88.39, and 59.67%), the molecular weights (8.83 × 104, 9.55 × 104, and 16.01 × 104), and degrees of polymerization (n = 368, 385, and 664) were largest. The reasoning for toluene suitability was due to the similarity of the phenyl ring in toluene solvent and PA, which caused π–π stacking interaction to improve the regularity of the reaction system so that the catalysts could easily capture PA molecules and initiate free radical polymerization. Therefore, polyphenylacetylene (PPA) obtained under toluene had a higher yield, larger molecular weight, and degree of polymerization than those under other solvents. However, degree of polymerization (PD) (always ≈ 2.00) was given little effect in different solvents.
Viewed from the catalysts, there were opposite polymerization results in the same toluene solution. For No. 1 and 5 in Table 2, the yields of PPA were both above 87%, the molecular weights were both about 90,000, and the degrees of polymerization were both higher than 350. Conversely, for No. 9, the yield of PPA was under 60%, while the molecular weight was up to 160,000, and degree of polymerization was up to 664.
This brings a question: When choosing [Rh(cod)(Dpt)2] as the catalyst, why the yield of PPA was only about 2/3 as them under the other two, however, the molecular weight and degree of polymerization of PPA were nearly two times? It was because the catalytic mechanism of [N–Rh–N] type catalyst (as shown Scheme 3) was different from that of [Rh–N] type [5].
![Scheme 3
Polymerization mechanism of PA under [N–Rh–N] type rhodium catalyst system.](/document/doi/10.1515/hc-2022-0014/asset/graphic/j_hc-2022-0014_fig_007.jpg)
Polymerization mechanism of PA under [N–Rh–N] type rhodium catalyst system.
In the polymerization process, 2 was formed by the rhodium atom in 1 coordinate with one PA molecule. Then, the PA in 2 was restructured and produced a carbon-free radical as 3. Due to a PA–molecule couple replacing the nitrogen ligands in 3 to get 4, the carbon-free radical (C·) in 4 attacked the coordination bonds on both sides concurrently as 5. The two PA molecules were inserted and polymerized into the interior. In addition, α-C· was reserved as 6. Similarly, as the PA–molecule couples joined in the reaction on until the activity of carbon-free radical was too weak to support the subsequent reaction, the PPA macromolecule emerged out of the system. The residue backed to the state of 4 and entered another circle. It was worth mentioning that the double coordination pattern between carbon free radical and two PA molecules kept the activity of carbon-free radical for a longer time, leading to a larger molecular weight and degree of polymerization for PPA.
3 Conclusion
To sum up, three novel rhodium catalysts were successfully synthesized, [Rh(cod)(l-Tyr)], [Rh(cod)(l-Arg)], and [Rh(cod)(Dpt)2]. The amount of text results analysis showed that the best synthesis condition of them was at 25°C for 4 h, and the best polymerization condition of PA catalyzed by using them was in toluene at 25°C for 4 h. Furthermore, due to the particularity of the yield, molecular weight, and degree of polymerization for PPA polymerized from the [N–Rh–N] catalytic system, a new polymerization mechanism of PA was proposed by using [N–Rh–N]-type rhodium catalyst ([Rh(cod)(Dpt)2]. This pattern of N-dicoordination was rare. It allowed polymerization simultaneous on both sides of core Rh to obtain a higher degree of polymerization.
4 Experimental
4.1 Materials and equipment
All solvents were dried using standard methods. RhCl3·3H2O, 1,5-cyclooctadiene (cod), l-amino acids, 2,4-diamino-6-phenyl-1,3,5-triazine (Dpt), and PA were purchased from Aladdin Reagent (Shanghai) Co., Ltd. The molecular structures of catalysts were determined by 1H NMR (600 MHz, Bruker, Germany). Elemental analysis was recorded by PE2400 SERIES II CHNS/O (PerkinElmer, USA) instrument. SEM images were shown with S-4300 (Hitachi, Tokyo, Japan) apparatus. The molecular weight of the polymers was measured by GPC (Polymer Laboratory, UK).
[Rh(cod)Cl] 2 : This compound was synthesized and purified according to ref. [18].
[Rh(cod)(l-Tyr)]: At nitrogen atmosphere, the solution of l-Tyr (0.0456 mmol, 17.82 mg) and NaOH (0.130 mmol, 5.30 mg) in H2O (0.60 mL) was added to the suspension of [Rh(cod)Cl]2 (0.0593 mmol, 29.23 mg) in acetone (2.00 mL). The obtained clear yellow solution was stirred for 4 h at 25°C. After filtration, the filtrate was cooled at 5°C until precipitation out from CH2Cl2 (2.00 mL), and then sediment was separated and dried to obtain the product as a yellow powder (yield = 62.34%). 1H NMR (600 MHz, DMSO-d 6) (See Figure A1): 7.03 (d, 2H, J = 12 Hz, CH2Ph-2,6-H 2), 6.72 (d, 2H, J = 6 Hz, CH2Ph-3,5-H 2), 4.29 (m, 4H, = CH), 3.80 (s, 2H, NH 2 ), 3.26 (m, 1H, NHCH), 2.88&2.73 (ds, 2H, Ph-CH 2), 2.22 (t, 4H, J = 6 Hz, cod-CH 2(in)), 1.60 (t, 4H, J = 6 Hz, cod-CH 2(out)). Elemental analyses for C17H22NO3Rh: C, 52.19; H, 5.67; N, 3.58; O, 12.27. Found: C, 52.51; H, 5.27; N, 3.82; O, 12.61 (purity = 92.95%).
[Rh(cod)(l-Arg)]: It was prepared and purified by the similar method as that of Rh(cod)(l-Tyr). Yellow powder (yield = 54.87%). 1H NMR (600 MHz, CDCl3) (see Figure A2): 7.09 (m, 1H, NH2NH), 6.72 (d, 2H, J = 12 Hz, NH 2), 4.77 (m, 4H, = CH), 3.49 (m, 1H, COCH 2), 2.10 (m, 2H, NHCH 2), 1.25 (m, 8H, cod-CH 2), 0.89 (m, 4H, CHCH 2CH 2CH2). Elemental analyses for C14H24N4O2Rh: C, 43.87; H, 6.31; N, 14.62; O, 8.35. Found: C, 43.96; H, 6.53; N, 14.42; O, 8.26 (purity = 96.63%).
[Rh(cod)(Dpt) 2 ]: It was also prepared and purified by a similar method as that of Rh(cod)(l-Tyr). Yellow powder (yield = 58.21%). 1H NMR (600 MHz, DMSO-d 6) (see Figure 1): 8.25 (q, 4H, J = 6 Hz, Ph-3,5-H 2), 7.52 (q, 2H, J = 6 Hz, Ph-4-H), 7.46 (t, 4H, J = 12 Hz, Ph-2,6-H 2), 6.75 (s, 8H, NH 2), 4.46 (m, 4H, = CH), 2.43 (t, 4H, J = 6 Hz, cod-CH 2(in)), 1.98 (t, 4H, J = 6 Hz, cod-CH 2(out)). Elemental analyses for C26H30N10Rh: C, 53.34; H, 5.16; N, 23.92. Found: C, 53.62; H, 5.13; N, 24.11 (purity = 99.21%).
-
Funding information: This work was supported by Heilongjiang Provincial Natural Science Foundation of China (LH2019B032), Heilongjiang Provincial Leading Talent Echelon Infrastructure funds (2019-278), China, and The Fundamental Research Funds in Heilongjiang Provincial Universities (No: 135309503).
-
Conflict of interest: Authors state no conflict of interest.
-
Data availability statement: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Appendix
It was worth mentioning that –CH2– of cod produced two groups of split peaks in DMSO solution, which is inexistent in CDCl3.
![Figure A1
1H NMR spectrum of [Rh(cod)(l-Tyr)] in DMSO.](/document/doi/10.1515/hc-2022-0014/asset/graphic/j_hc-2022-0014_fig_003.jpg)
1H NMR spectrum of [Rh(cod)(l-Tyr)] in DMSO.
![Figure A2
1H NMR spectrum of [Rh(cod)(l-Arg)] in CDCl3.](/document/doi/10.1515/hc-2022-0014/asset/graphic/j_hc-2022-0014_fig_004.jpg)
1H NMR spectrum of [Rh(cod)(l-Arg)] in CDCl3.
References
[1] Sanchez A, Fang MF, Ahmed A, Sanchez-Delgado RA. Hydrogenation of arenes, N-heteroaromatic compounds, and alkenes catalyzed by rhodium nanoparticles supported on magnesium oxide. Appl Catal A: Gen. 2014;447:117–24.10.1016/j.apcata.2014.03.009Search in Google Scholar
[2] Mimoun H, Machirant M, Roch IS. Activation of molecular oxygen: Rhodium-catalyzed oxidation of olefins. J Am Chem Soc. 1978;100:5437–44.10.1021/ja00485a031Search in Google Scholar
[3] Yang J. Rhodium phosphine complex catalyzed hydroformylation of FCC light gasoline olefins. Qingdao: China University of Petroleum; 2018. p. 2–50.Search in Google Scholar
[4] Zhao XY, Jia HG, Song HM. Oxidation coupling of benzylene with 2,4-hydroxybenzaldehyde over rhodium catalyst with Amino acid as ligand. Chem Res. 2020;31:32–4.Search in Google Scholar
[5] Ke ZF, Abe S, Ueno T, Morokuma K. Rh-catalyzed polymerization of phenylacetylene: theoretical studies of the reaction mechanism, regioselectivity, and stereoregularity. J Am Chem Soc. 2011;133:7926–41.10.1021/ja2012565Search in Google Scholar
[6] Kern RJ. Preparation and properties of isomeric polyphenylacetylenes. J Polym Sci Part A-I: Polym Chem. 1969;7:621–31.10.1002/pol.1969.150070216Search in Google Scholar
[7] Saeed I, Shiotsuki M, Masuda T. Effect of diene ligands in the rhodium-catalyzed polymerization of phenylacetylene. Macromolecules. 2006;39:8977–81.10.1021/ma061689gSearch in Google Scholar
[8] Furlani A, Napoletano C, Russo MV, Camus A, Marsich N. The influence of the ligands on the catalytic activity of a series of Rh (I) complexes in reactions with phenylacetylene: Synthesis of stereoregular poly (phenyl) acetylene. J Polym Sci Part A: Polym Chem. 1989;27:75–86.10.1002/pola.1989.080270107Search in Google Scholar
[9] Kishimoto Y, Itou M, Miyatake T, Ikariya T, Noyori R. Polymerization of monosubstituted acetylenes with a zwitterionic rhodium (I) complex, Rh + (2,5-norbornadiene)[(η6-C6H5)B−(C6H5)3]. Macromolecules. 1995;28:6662–6.10.1021/ma00123a037Search in Google Scholar
[10] Bennett MA, McMahon IJ, Pelling S, Robertson GB, Wickramasinghe WA. Protonation of dicyclopentadiene complexes of ruthenium (0), osmium (0), rhodium (I), and iridium (I). Single-crystal X-ray study of [Os (2,3,5-η-C10H13)(η-C6H3Me3-1,3,5)] PF6, a complex containing an Os-H-C interaction. Organometallics. 1985;4:754–61.10.1021/om00123a024Search in Google Scholar
[11] Roe DM, Massey AG. Perfluorophenyl derivatives of the elements XXVI. Tetrafluorobenzobarrelene complexes of Mn, Co, Rh, Pd and Pt. J Organomet Chem. 1971;28:273–9.10.1016/S0022-328X(00)84576-3Search in Google Scholar
[12] Jimenez MV, Perez-Torrente JJ, Bartolome MI, Vispe E, Lahoz FJ, Oro LA. Cationic rhodium complexes with hemilabile phosphine ligands as polymerization catalyst for high molecular weight stereoregular poly (phenylacetylene). Macromolecules. 2009;42:8146–56.10.1021/ma901549gSearch in Google Scholar
[13] Angoy M, Jimenez MV, Orduna PG, Oro LA, Vispe E, Perez-Torrente JJ. Dinuclear phosphine-amido [Rh2(diene){μ-NH(CH2)3PPh2}2] complexes as efficient catalyst precursors for phenylacetylene polymerization. Organometallics. 2019;38:1991–2006.10.1021/acs.organomet.9b00078Search in Google Scholar
[14] Pan PL, Liu ZY, Huang MK, Yuan GQ. Synthesis and properties of rhodium complexes with cis dicarbonyl methyl 2-butyrate pyridine. Chem Bull. 1995;7:34–5.Search in Google Scholar
[15] Stateman LM, Ferrence GM, Lash TD. Rhodium (III) azuliporphyrins. Organometallics. 2015;45:3842–8.10.1021/acs.organomet.5b00433Search in Google Scholar
[16] Furlani A, Napoletano C, Russo MV, Feast WJ. Stereoregular polyphenylacetylene. Polym Bull. 1986;16:311–7.10.1007/BF00255002Search in Google Scholar
[17] Kondo M, Kochi T, Kakiuchi F. Rhodium-catalyzed anti-markovnikov intermolecular hydroalkoxylation of terminal acetylenes. J Am Chem Soc. 2011;133:32–4.10.1021/ja1097385Search in Google Scholar
[18] Paiaro G, Musco L, Diana G. Chemical and structural characterization of some π-allylicderivatives of rhodium (III). J Organomet Chem. 1965;4:466–74.10.1016/S0022-328X(00)88799-9Search in Google Scholar
© 2022 Rui Xu et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Sono and nano: A perfect synergy for eco-compatible Biginelli reaction
- Study of the reactivity of aminocyanopyrazoles and evaluation of the mitochondrial reductive function of some products
- “Click” assembly of novel dual inhibitors of AChE and MAO-B from pyridoxine derivatives for the treatment of Alzheimer’s disease
- Synthesis of 2,2-difluoro-2-arylethylamines as fluorinated analogs of octopamine and noradrenaline
- Cyclization of N-acetyl derivative: Novel synthesis – azoles and azines, antimicrobial activities, and computational studies
- Two independent and consecutive Michael addition of 1,3-dimethylbarbituric acid to (2,6-diarylidene)cyclohexanone: Flying-bird-shaped 2D-polymeric structure
- Ionic liquid-catalyzed synthesis of (1,4-benzoxazin-3-yl) malonate derivatives via cross-dehydrogenative-coupling reactions
- Synthesis of novel triiodide ionic liquid based on quaternary ammonium cation and its use as a solvent reagent under mild and solvent-free conditions
- Eelectrosynthesis of benzothiazole derivatives via C–H thiolation
- Synthesis of fluoro-rich pyrimidine-5-carbonitriles as antitubercular agents against H37Rv receptor
- Syntheses, crystal structure, thermal behavior, and anti-tumor activity of three ternary metal complexes with 2-chloro-5-nitrobenzoic acid and heterocyclic compounds
- Synthesis of enhanced lipid solubility of indomethacin derivatives for topical formulations
- Synthesis of newer substituted chalcone linked 1,2,3-triazole analogs and evaluation of their cytotoxic activities
- Novel benzodioxatriaza and dibenzodioxadiazacrown compounds carrying 1,2,4-oxadiazole moiety
- Synthesis of rhodium catalysts with amino acid or triazine as a ligand, as well as its polymerization property of phenylacetylene
- DABCO-based ionic liquid-promoted synthesis of indeno-benzofurans derivatives: Investigation of antioxidant and antidiabetic activities
- Design, synthesis, and biological activity of novel pomalidomide linked with diphenylcarbamide derivatives
- Study on effective synthesis of 7-hydroxy-4-substituted coumarins
- Review Article
- Chemical constituents of plants from the genus Carpesium
- Communication
- Reactions of 3-amino-1,2,4-triazine with coupling reagents and electrophiles
Articles in the same Issue
- Research Articles
- Sono and nano: A perfect synergy for eco-compatible Biginelli reaction
- Study of the reactivity of aminocyanopyrazoles and evaluation of the mitochondrial reductive function of some products
- “Click” assembly of novel dual inhibitors of AChE and MAO-B from pyridoxine derivatives for the treatment of Alzheimer’s disease
- Synthesis of 2,2-difluoro-2-arylethylamines as fluorinated analogs of octopamine and noradrenaline
- Cyclization of N-acetyl derivative: Novel synthesis – azoles and azines, antimicrobial activities, and computational studies
- Two independent and consecutive Michael addition of 1,3-dimethylbarbituric acid to (2,6-diarylidene)cyclohexanone: Flying-bird-shaped 2D-polymeric structure
- Ionic liquid-catalyzed synthesis of (1,4-benzoxazin-3-yl) malonate derivatives via cross-dehydrogenative-coupling reactions
- Synthesis of novel triiodide ionic liquid based on quaternary ammonium cation and its use as a solvent reagent under mild and solvent-free conditions
- Eelectrosynthesis of benzothiazole derivatives via C–H thiolation
- Synthesis of fluoro-rich pyrimidine-5-carbonitriles as antitubercular agents against H37Rv receptor
- Syntheses, crystal structure, thermal behavior, and anti-tumor activity of three ternary metal complexes with 2-chloro-5-nitrobenzoic acid and heterocyclic compounds
- Synthesis of enhanced lipid solubility of indomethacin derivatives for topical formulations
- Synthesis of newer substituted chalcone linked 1,2,3-triazole analogs and evaluation of their cytotoxic activities
- Novel benzodioxatriaza and dibenzodioxadiazacrown compounds carrying 1,2,4-oxadiazole moiety
- Synthesis of rhodium catalysts with amino acid or triazine as a ligand, as well as its polymerization property of phenylacetylene
- DABCO-based ionic liquid-promoted synthesis of indeno-benzofurans derivatives: Investigation of antioxidant and antidiabetic activities
- Design, synthesis, and biological activity of novel pomalidomide linked with diphenylcarbamide derivatives
- Study on effective synthesis of 7-hydroxy-4-substituted coumarins
- Review Article
- Chemical constituents of plants from the genus Carpesium
- Communication
- Reactions of 3-amino-1,2,4-triazine with coupling reagents and electrophiles

