Abstract
A simple and effective method for synthesis of indeno-benzofurans derivatives using polyphenols and ninhydrins is explored using an acidic catalyst based on DABCO (1,4-diaza bicycle [2.2.2] octane)-based ionic liquid. The products of these types of reactions have very low yields without catalysts, but with DABCO-AIL, the yields are excellent, reaction times are reduced, and the media are cleaner. Using infrared (IR), proton nuclear magnetic resonance (1H NMR), Carbon-13 nuclear magnetic resonance (13C NMR), and mass spectrometry, the structures of products can be confirmed. There is evidence that oxidative stress plays a role in the pathophysiology of numerous diseases, including diabetes. Therapeutic antioxidants are promising candidates for the prevention and treatment of such diseases. To investigate the antioxidant properties of all synthesized derivatives, the 2,2-diphenyl-1-picrylhydrazylhydrazyl-hydrate (DPPH) assay was used. Derivatives 3d and 4 with the highest antioxidant effect (with IC50 value of 0.015 µmol/mL) were selected to evaluate the anti-diabetic effect using the Bernfeld method. The best result was seen at 0.8 mg/mL of 4 derivative and results of the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) test revealed that 4 at this concentration lacked cellular toxicity, too.
1 Introduction
The unique properties of ionic liquids (ILs), which include their non-flammability, recyclability, and lack of measurable vapor pressure, have drawn considerable attention as green solvents in recent years. When compared to conventional solvents, they have a higher polarity, which allows them to dissolve both nonpolar and polar materials, which can lead to increased chemical rates.
In chemical transformations, ILs have been used as solvents and catalysts because of their green credentials and potential to enhance rate and selectivity [1,2,3,4].
Biological properties of oxygen-containing heterocyclic compounds make them a very important class of compounds in organic chemistry [5,6,7]. Among them, benzofuran is the main unit that is ubiquitous in different natural products [8]. In recent years, many natural and synthetic benzofuran derivatives have attracted pharmacologists because of their biological activities and potential applications [9,10]. In the synthesis of benzofurans, ninhydrin is commonly combined with aromatic rings containing electrons. An acetic acid-catalyzed reaction between ninhydrin and amino, C-alkyl, hydroxy, and alkoxy phenols has been studied, and its mechanisms of action have been hypothesized [11]. The acid-catalyzed reaction of ninhydrin with various aromatic and non-aromatic substrates using mild and micro oven reactions has been reported [12]. Many natural and synthetic benzofuran derivatives possess several therapeutic properties such as antimicrobial, antitumor activities, and antidiabetic [13,14,15,16,17,18,19]. Diabetes is a common metabolic disease characterized by elevated plasma sugar levels (hyperglycemia) [20]. According to the International Diabetes Federation, by 2045, the number of people with this disease will reach 693 million [20,21]. According to the above-mentioned statistics, research on new diabetes drugs is needed.
Alpha amylase (EC 3.2.1.1) catalyzes the hydrolysis of the (a-1,4) glycosidic linkages in starch as well as various other oligosaccharides. In several areas of disease control and prevention, enzyme inhibitors can be considered potential targets. The blood glucose level in diabetes is maintained by delaying the breakdown of starch with amylase inhibitors [22,23]. In patients with diabetes, hyperglycemia induces free radicals while impairing their endogenous antioxidant defense system. Both experimental and clinical evidence suggests that diabetes is closely associated with reactive oxygen species (ROS) and diabetic complications such as retinopathy, nephropathy, neuropathy, accelerated coronary artery disease, and diabetic Wounds are associated with increased reactive oxygen species [24,25]. Antioxidant compounds can scavenge free radicals and delay diabetic complications [26]. Therefore, the synthesis of new alpha-amylase inhibitors, which also have antioxidant properties, can be useful in the production of new drugs against diabetes.
Several research groups are now focusing on various methods for synthesis and structural modification of benzofuran derivatives because of their biological significance [27,28,29]. Continuing our previous research [30] on new heterocyclic compounds, we report the production of new benzofuran derivatives in excellent yields and relatively short reaction times by reacting ninhydrin 1 with polyphenol 2 using the solvent and catalyst 1-(3-sulfopropyl)-1,4-diazabicyclo[2.2.2]octan-1-ium as green solvent and catalyst (Table 1). Furthermore, the antioxidant effect, inhibition of alpha-amylase, and toxicity of the synthetic derivatives were examined.
Reactions of ninhydrin and polyphenoles

|
||||
|---|---|---|---|---|
| Entry | Compound 1 | Compound 2 | Product | Yield of (%) of 3a |
| 1 |

|

|

|
96 |
| 2 |

|

|

|
93 |
| 3 |

|

|

|
90 |
| 4 |

|

|

|
87 |
Reaction conditions: ninhydrin (2 mmol); polyphenol (2 mmol); [DABCOC4H8SO3H]HSO4 (2 ml); the temperature is 70–80°C.
aThe isolated yields.
2 Results and discussion
2.1 Synthesis
In this work, a new ionic liquid based on DABCO was used for the preparation of benzofuran derivatives from the reaction of ninhydrin 1 and polyphenol 2 at 70–80°C (Table 1). The reaction of ninhydrin and pyrogallel at 100–101°C for 2 h produced bisadduct 4 as the thermodynamic product (Scheme 1), but other polyols did not produce dimers when the temperature was raised. To characterize the structures of benzofuran 3 and 4, spectroscopic techniques such as infrared (IR), proton nuclear magnetic resonance (1H NMR), Carbon-13 nuclear magnetic resonance (13C NMR), and mass spectral data were used. For example, the 1H NMR spectrum of 4 revealed two broad singlets at 6.35 and 7.79 ppm for alcoholic hydrogen and one singlet at 8.73 ppm for phenolic hydrogen along with signals for aromatic moiety. In the 13C NMR spectrum, the signal corresponding to two same carbonyl groups of 4 was observed at 199.8ppm. The IR spectrum of 4 displayed characteristic C═O bands.

Synthesis of ninhydrin-pyrogallol bisadduct 4.
While no detailed information is available about the mechanism, the reaction can be described by the proposed mechanism in Scheme 2.

Proposed mechanism for synthesis of benzofuran derivatives 3.
Although the mechanism of the earlier reaction is not known, a tentative model is shown here. It is likely that the electron-rich polyphenoles react with the carbonyl group at the 2-position of ninhydrin and that the intermediate 5 is formed in the presence of an ionic liquid (Scheme 2).
2.2 Evaluation of antioxidant properties using diphenyl-2-picrylhydrazyl (DPPH)
Based on the DPPH radical scavenging method, compounds 3a–3d, 4 were tested for antioxidant activities. The reduction of free radical DPPH removes its violet color, which indicates the presence of free radical-scavenging compounds in the mixture [31]. The radical scavenging of DPPH at different concentrations of synthesized compounds and BHT as a control is shown in Figure 1.
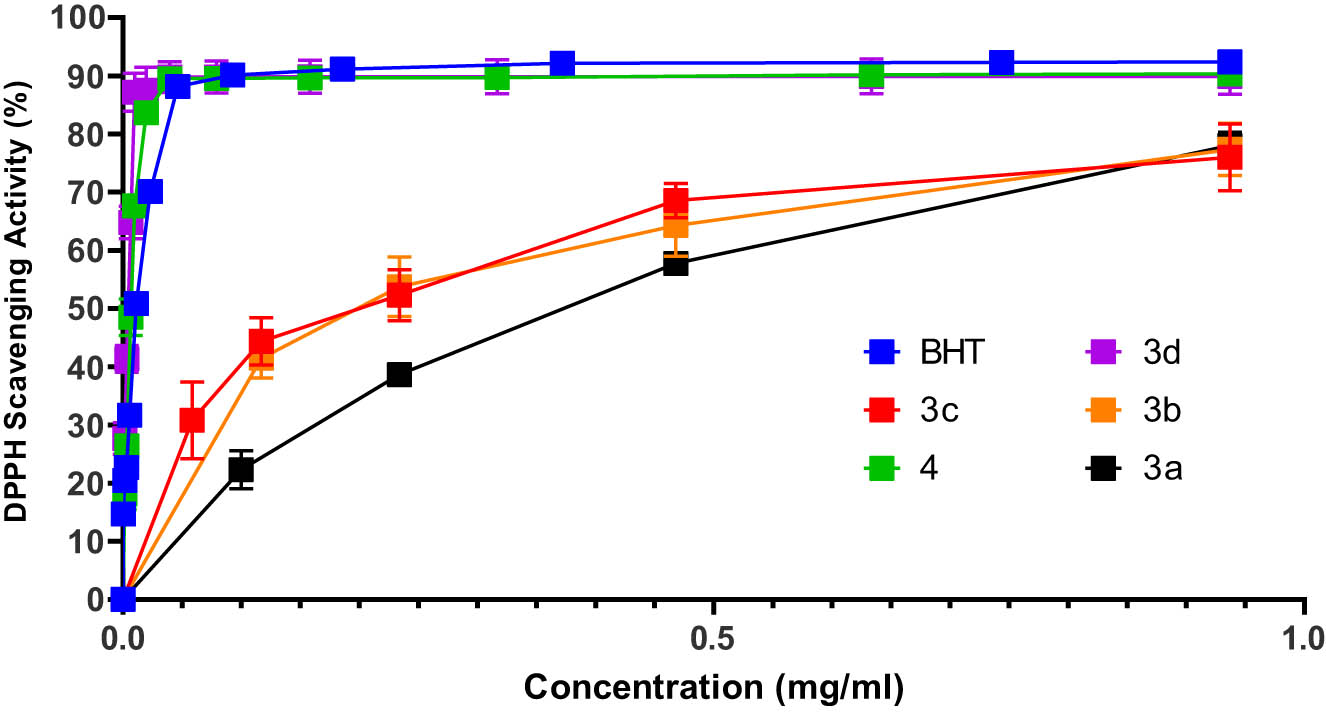
In vitro antioxidant activity of indeno-benzofurans derivatives and BHT.
A linear relationship between the compound concentration and the probability of DPPH radical scavenging was used to calculate the IC50 (Figure 2). The IC50 value indicates the concentration of the sample compound that is capable of scavenging 50% of free radicals in the reaction mixture. Therefore, low IC50 values indicate a high antioxidant activity. The best antioxidant effect was seen for 3d and 4 with an IC50 value of 0.015 µmol/mL, using BHT as a standard (0.051 µmol/mL). The power of DPPH trapping was obtained in the following order: 3d, 4 > BHT > 3b > 3c > 3a.

IC50 of indeno-benzofurans derivatives and BHT, **** indicates P-value < 0.0001.
Due to the role of hydroxyl phenolic groups in antioxidant activity [32], increasing the number of hydroxy phenolic groups in compound 3d compared to other synthetic compounds may be the reason for the decrease in IC50. In the case of compound 4, good antioxidant activity is probably related to the expanded resonance structure of this polycyclic compound along with the hydroxyl phenolic group. In the case of structures 3b and 3c, the presence of oxygen and sulfur heteroatoms probably increases the electron density and stability of the free radical, while such a feature does not exist in structure 3a.
2.3 Investigation of enzymatic inhibition of synthesized compounds
In this research, the Bernfeld method was employed for measuring the inhibition of α-amylase enzyme. Since compounds with antioxidant properties are effective in the treatment of diabetes [33], two samples 3d and 4 with high antioxidant properties were selected for measurement α-amylase inhibition (Figure 3).

Inhibition of α-amylase by indeno-benzofurans derivatives and acarbose.
In the present study, indeno-benzofurans derivatives displayed α-amylase inhibition in a concentration-dependent manner. The results show that among the two samples, indeno-benzofurans 4 had a better inhibition than indenobenzofurans 3d. Increasing the concentration of 4 caused the percentage of its inhibition close to acarbose (positive control) amount. The best result was seen at a sample concentration of 0.8 mg/mL. In this concentration, the enzyme was inhibited by 4 at a rate of 69.266 ± 1.190 and acarbose as a positive control at a concentration of 0.8 mg/mL was able to inhibit the enzyme at a rate of 83.246 ± 1.080.
2.4 The cytotoxicity results of compounds 3d and 4
Because chemical compounds can cause toxicity in cells by different mechanisms [34], evaluation of toxicity of chemical compounds with pharmaceutical purposes is of special importance. Despite the existence of various methods for determining cytotoxicity in the field of pharmacology, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) has become one of the most popular colorimetric methods for studying substance toxicity in cells [35]. In this case, two samples with high antioxidant effects were examined by the MTT method (Figure 4).

Percentage of cell viability of PBMCs. *** indicates P-value < 0.0002, **** indicates P-value < 0.0001.
The results show that the indeno-benzofurans 3d and 4 not only did not have toxicity on the PBMCs, but also increases the concentration of them increases the number of the PBMCs. The cell viability percentage increase is dependent on concentration, and statistical calculations show a significant relationship between the increase in the concentration of both indeno-benzofurans and cell viability percentage (P-value
2.5 Conclusion
Overall, we report the preparation of indenobenzofurans 3 and 4 using ninhydrin and polyphenoles in the DABCO ionic liquid in good yields. Also, the power of synthesized indeno-benzofuran (3a-4, 4) as an antioxidant was determined by the radical trapping of DPPH. The indenobenzofurans 3d and 4 display excellent radical trapping of DPPH (IC50 value of 0.015 µmol/mL) relative to BHT (IC50 value of 0.051 µmol/mL) as a synthetic antioxidant. α-Amylase enzyme inhibition is an effective therapeutic decorum in the treatment of type 2 diabetes mellitus. Sample 4 at a concentration of 0.8 mg/mL was able to inhibit enzyme activity at a rate of 69.266 ± 1.190. Given the importance of hydrogen bonding in inhibiting alpha-amylase [36], it is likely that the increased inhibitory effect of sample 4 compared to sample 3d is due to its greater potential for hydrogen interaction. In the MTT test, these two compounds did not cause any toxic effects on PBMC cells. Accordingly, these substances may be able to be used in the clinic after toxicity tests have been conducted in animal cell cultures and animal models. Therefore, the synthesis of indenobenzofurans derivatives with pharmaceutical applications in a manner with short reaction time characteristics, high efficiency and cleaning conditions is remarkable.
3 Experimental
3.1 Materials
All chemical reagents were purchased from Sigma chemical company and used without further purification. Analyses of synthesized indeno-Benzofurans were conducted on Shimadzu IR-460 spectrometers in KBr medium. As an internal standard, the 1H-NMR and 13C-NMR spectra were obtained using a Bruker DRX-500 AVANCE spectrometer operating at 500 MHz and 125 MHz, respectively. A Finnigan MAT 8430 mass spectrometer with an electron impact ionization potential of 70 eV was used for obtaining mass spectra. A CHN–O-Rapid analyzer was used by Heraeus to analyze the elemental composition of products. In order to measure the absorption of samples in different wavelengths, the BioTeck plate reader (Epoch model, BioTeck company) was used.
3.2 Methods
3.2.1 Synthesis of 1-(3-sulfopropyl)-1,4-diazabicyclo[2.2.2]octan-1-ium hydrogen sulfate (Dabco-AIL)
The synthesis of DABCO-based ionic liquid used in this research was carried out according to the Menshutkin reaction [37].
To a stirred solution of DABCO (5.6 g, 0.05 mol) in toluene under reflux conditions was added 1,3-propanesultone (6.1 g, 0.05 mol) for 24 h at 75°C. The white precipitate was produced after the completion of the reaction. Then, the precipitate was separated by filtration and placed in an ice bath and wet with dichloromethane. Then, sulfuric acid (2.8 mL, 0.05 mol) was added to the precipitate and placed in an oil bath at 75°C for 4 h (Scheme 3). After the formation of the Dabco-AIL product, it was recrystallized in ethanol for further purification. The structure of synthesized ionic liquid was confirmed using 1HNMR, 13CNMR, and mass spectra.

Synthesis of Dabco-AIL.
3.2.1.1 1-(3-Sulfopropyl)-1,4-diazabicyclo[2.2.2]octan-1-ium hydrogen sulfate
C9H20S2N2O7 (332): white powder, m.p. 148–151°C; yield: 86%. IR (KBr): 2,930, 2,500–2,700, 1,450, 1,353, 1,250, 1,158 cm−1. 1H NMR (D2O): δ = 2.17 (2 H, quintet, 3 J = 7.4 Hz, CH2), 2.90 (2 H, t, 3 J = 7.2 Hz, CH2), 3.62 (2 H, t, 3 J = 6.8 Hz, CH2), 3.76 (6 H, t, 3 J = 6.4 Hz, 3 CH2), 3.87 (6 H, t, 3 J = 6.4 Hz, 3 CH2) ppm.13C NMR (D2O): δ 17.57 (CH2), 43.6 (CH2), 46.7 (CH2), 50.9 (3 CH2), 63.0 (3 CH2) ppm.
3.2.2 Common method for generation of 3a–d
The ninhydrin 1 (2 mmol) and polyphenoles 2 (2 mmol) were mixed and stirred in 1-(3-sulfopropyl)-1,4-diazabicyclo[2.2.2]octan-1-ium (2 mL) as green solvent and catalyst at 70–80°C. After completion of the reaction (1 h), the solvent extracted from the mixture of the reaction and the solid was purified by washing with Et2O to afford pure title compound 3 (Table 1).
3.2.2.1 4b,7,9b-Trihydroxy-4b,9b-dihydro-10H-indeno[1,2-b]benzofuran-10-one (3a) [9]
Yellow crystals, m.p. 234–237°C; yield: 96%. IR (KBr): 3,439, 1,732, 1,624, 1,456, 1,230, 1,198 cm−1. 1H NMR (CHCl3): δ = 6.20 (1 H, br-s, OH–alcoholic), 6.64 (1 H, s, CH), 6.85 (1 H, d, 3 J = 7.6 Hz, CH), 7.40 (1 H, t, 3 J = 7.6 Hz, CH-arom), 7.58 (1 H, d, 3 J = 7.6 Hz, CH), 7.68 (1 H, t, 3 J = 7.7 Hz, CH), 7.75 (1 H, d, 3 J = 7.7 Hz, CH), 7.83 (1 H, d, 3 J = 7.7 Hz, CH), 8.81 (1H, br-s, OH–alcoholic), 11.54 (1H, br-s, OH-phenolic) ppm. 13C NMR (DMSO): δ 88.0 (C–OH), 92.4 (CH), 110.9 (CH), 114.5 (C–OH), 118.9 (C), 122.6 (CH), 126.9 (CH), 128.6 (CH), 132.8 (CH), 135.9 (C), 139.02 (CH), 153.1 (C), 156.2 (C), 158.3 (C), 182.6 (C═O) ppm.
3.2.2.2 4b,6,9b-trihydroxy-3-methoxy-4b,9b-dihydro-10H-indeno[1,2-b]benzofuran-10-one (3b)
Yellow crystals, m.p. 221–223°C; yield: 93%. IR (KBr): 3,427, 1,732, 1,626, 1,585, 1,469, 1,227 cm−1. 1H NMR (CHCl3): δ = 3.87 (3 H, s, MeO), 6.35 (1 H, br-s, OH–alcoholic), 6.63 (1 H, d, 3 J = 7.6 Hz, CH), 6.83 (1 H, d, 3 J = 7.6 Hz, CH), 7.06 (1 H, d, 3 J = 7.6 Hz, CH), 7.14 (1 H, t, 3 J = 7.7 Hz, CH), 7.32 (1 H, s, CH), 7.85 (1 H, d, 3 J = 7.7 Hz, CH), 8.80 (1H, br-s, OH–alcoholic), 11.62 (1 H, br-s, OH-phenolic) ppm. 13C NMR (DMSO): δ 55.6 (MeO), 83.8 (C–OH), 115.7 (CH), 117.8 (C–OH), 118.6 (CH), 119.7 (CH), 120.6 (CH), 123.7 (C), 126.4 (CH), 129.9 (CH), 133.8 (C), 140.9 (C), 145.1(C), 158.3 (C), 159.6 (C) 182.6(C═O) ppm.
3.2.2.3 4b,6,9b-Trihydroxy-3-(methylthio)-4b,9b-dihydro-10H-indeno[1,2-b]benzofuran-10-one (3c)
Yellow crystals, m.p. 208–210°C; yield: 90%. IR (KBr): 3,446, 1,719, 1,628, 1,597, 1,461, 1,359, 1,257 cm−1. 1H NMR (CHCl3): δ = 2.36 (3 H, s, MeS), 6.42 (1 H, br-s, OH–alcoholic), 6.65 (1 H, d, 3 J = 7.6 Hz, CH), 6.87 (1 H, d, 3 J = 7.6 Hz, CH), 7.14 (1 H, d, 3 J = 7.6 Hz, CH), 7.22 (1 H, t, 3 J = 7.7 Hz, CH), 7.34 (1 H, s, CH),7.72 (1 H, d, 3 J = 7.7 Hz, CH), 8.74 (1H, br-s, OH–alcoholic), 11.58 (1H, br-s, OH-phenolic) ppm. 13C NMR (DMSO): δ 15.9 (MeS), 88.9 (C–OH), 93.4 (CH), 106.5 (CH), 113.2 (C), 114.5 (C–OH), 118.1 (CH), 122.1 (CH), 124.3 (CH), 126.2 (CH), 129.8 (C), 146.5 (C), 154.2 (C), 156.3 (C), 157.4 (C), 183.9 (C═O) ppm.
3.2.2.4 4b,6,7,9b-Tetrahydroxy-4bH-indeno[1,2-b]benzofuran-10(9bH)-one (3d)
Colorless crystals, m.p. 265–266°C; yield: 0.27 g (96%). IR (KBr): 3,387, 3,324, 3,200, 1,713, 1,635, 1,603, 1,225, 1,138 cm−1. 1H NMR (DMSO): δ = 6.31 (1 H, d, 3 J 8.1, CH), 6.35 (1 H, br-s, OH–alcoholic), 6.62 (1 H, d, 3 J 8.1, CH), 7.57 (1 H, t, 3 J 7.5, CH), 7.89 (1 H, d, 3 J 7.5, CH), 7.79 (1 H, br-s, OH–alcoholic), 7.82 (1 H, t, 3 J 7.5, CH), 7.89 (1 H, d, 3 J 7.5, CH), 8.53 (1 H, br-s, OH-phenolic), 9.12 (1 H, br-s, OH-phenolic) ppm. 13C NMR (DMSO): δ 82.7 (C–OH), 109.3 (C–OH), 110.9 (CH), 115.1 (CH), 117.3 (C), 122.7 (CH), 125.1 (CH), 129.3 (CH), 130.8 (CH), 134.0 (C), 136.3 (C), 145.9 (Carom–O), 148.4 (Carom–OH), 149.1 (Carom–OH), 199.3 (C═O) ppm. EI-MS: m/z (%) = 286 (M+, 76), 268 (21), 184 (22), 153 (95), 126 (43), 104 (100), 76 (78). Anal. Calcd. for C15H10O6 (286.0): C, 62.44; H, 3.52. Found: C, 62.22; H, 3.85.
3.2.3 Common method for generation of 4
The ninhydrin 1 (712 mg, 4 mmol) and pyrogallol 2d (252, 2 mmol) were mixed and stirred in 1-(3-sulfopropyl)-1,4-diazabicyclo[2.2.2]octan-1-ium (2 mL) as green solvent and catalyst at 100–110°C. After completion of the reaction (2 h), 10 mL of water was added, and the white solid was deposited in the reaction mixture. The product by washing with Et2O to afford pure title compound 4 (Scheme 1). The ionic liquid can be reused after extraction from the aqueous phase.
3.2.3.1 6,12a,13b,4b,7a-Pentahydroxy-12a,13b,4b,7a-tetrahydroindano[1,2-b]indano[2″,1″-5′,4′]furano[2′,3′-5,4]benzo[d]furan-12,14-dione (4)
Colorless crystals, m.p. 231–233°C; yield: 0.40 g (90%). IR (KBr): 3,431, 3,330, 3,071, 1,726, 1,605, 1,499, 1,233, 1,108 cm−1. 1H NMR (DMSO): δ = 6.90 (1 H, s, CH), 6.32–6.35 (2 H, br-s, 2 OH–alcoholic), 7.55–7.57 (2 H, m, 2 CH), 7.87–7.89 (2 H, m, 2 CH), 7.77–7.79 (2 H, br-s, 2 OH–alcoholic), 7.82 (2 H, m, 2 CH), 7.89–8.02 (2 H, m, 2 CH), 8.73 (1 H, br-s, OH-phenolic) ppm. 13C NMR (DMSO): δ 80.2 (2 C–OH), 102.6 (2 C–OH), 108.2 (CHp-arom), 119.2 (2 Cm-arom), 123.8 (2 CH), 127.1 (2 CH), 128.8 (2 CH), 129.3 (2 CH), 133.5 (2 C), 139.6 (2 C), 163.2 (2 Carom–O), 168.3 (Carom–OH), 197.9 (2 C═O) ppm. Anal. Calcd. for C24H14O9 (446.3): C, 64.98; H, 3.16. Found: C, 65.02; H, 3.25.
3.3 Biological activities evaluation
3.3.1 DPPH radical scavenging assay
This study used a slightly different method of determining the free radical scavenging capacity of DPPH than previous studies [38]. Diluted DMSO solutions of the samples were prepared at different concentrations (0–1 mg/mL). With the above-mentioned solution, 150 µl of freshly prepared (100 µg/mL) DPPH methanol solution was added. After 30 minutes of mixing the contents of the microplate in the dark, the absorbance of the samples was measured at 517 nm.
Butylated hydroxytoluene (BHT), a substance known for its antioxidant properties, was used as a positive control [39,40].
Inhibition curves were used to derive line equations. IC50 was determined by determining the concentration required to inhibit 50% of free radicals. The following formula was used to calculate the inhibition percentage at each concentration.
3.3.2 Investigation of alpha-amylase inhibition of synthesized compounds
The inhibitory activity of α-amylase of the samples was estimated using the modified Bernfeld method [41] using acarbose as standard. This method calculates sugar based on the hydrolysis of starch. Briefly, a series of concentrations of samples (0–0.8 mg/mL) was prepared, and 200 µL of samples was mixed with 200 µL of α-amylase solution (1 mg/mL) in phosphate buffer (pH 5 with 0.1 N sodium chloride) and incubated for 30 min at 37 C. After preincubation, the reaction was initiated with the addition of starch solution (200 µL, 1% w/v). The reaction was terminated by adding 600 μL of DNS reagent, followed by water bath treatment for 15 min at 95°C and the absorbance was measured at 540 nm using a microplate reader (Epoch model, BioTeck company). The following formula was used to calculate the inhibition percentage at each concentration.
3.3.3 Study of cell toxicity in peripheral blood mononuclear cells (PBMCs)
Blood samples from healthy individuals were used to collect environmental mononuclear cells. Separating blood cells by density and size was done using a centrifuge and histopaque 1077. An equal volume of trypan-blue dye was mixed with the cellular precipitate obtained from this stage after two washes in the sterile RPMI 1640 culture medium. Neubauer chambers were used to count live cells after 5 minutes of treatment. A culture medium containing fetal calf serum (inactivated) and penicillin–streptomycin was used to dilute the cell suspension to 106 cells per milliliter of culture medium based on the mean of the three counts. Incubation was performed at 37°C with 5% CO2 for 6 h with 150 μL of cell suspension and 50 μL of sterile dilutions of each sample added to each well. In each well, 20 μL of sterile MTT solution containing 0.5 mg/mL was added, and the solution was heated for another three hours. Based on the absorption rate measured by the plate reader (Epoch model, BioTeck company) at wavelengths of 540 nm and reference 620 nm, the percentage of bioavailability and cytotoxicity of each sample was calculated from the following equation [42]:
3.4 Statistical analyses
The determination of antioxidant activity for each sample was performed in triplicate. The obtained results were presented as means ± standard deviation (SD). Statistical analysis was performed employing GraphPad Prism software version 9.2.0. Comparison between three groups was analyzed via one-way ANOVA (analysis of variance) followed by Dunnett’s multiple comparisons test.
Acknowledgements
We would like to thank the Islamic Azad University, Yadegar-e-Imam Khomeini (RAH) shahr-e-Rey Branch, for its spiritual support.
-
Funding information: Authors state no funding involved.
-
Conflict of interest: Authors state no conflict of interest.
-
Data availability statement: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
[1] Wasserscheid P, Welton T. Ionic liquids in synthesis; First published. KGaA: Wiley‐VCH Verlag GmbH & Co.; 2007.10.1002/9783527621194Search in Google Scholar
[2] Yavari I, Kowsari E. Ionic liquids as novel and recyclable reaction media for N-alkylation of amino-9, 10-anthraquinones by trialkyl phosphites. Tetrahedron Lett. 2007;48(21):3753–6.10.1016/j.tetlet.2007.03.077Search in Google Scholar
[3] Sandaroos R, Goldani MT, Damavandi S. One-pot synthesis of acenaphtho [1, 2-b] pyrroles in the presence of supported ionic liquid catalyst. Heterocycl Comm. 2012;18(3):157–60.10.1515/hc-2011-0126Search in Google Scholar
[4] Sharifi A, Moazami M, Abaee MS, Mirzaei M. Ionic liquid-catalyzed synthesis of (1, 4-benzoxazin-3-yl) malonate derivatives via cross-dehydrogenative-coupling reactions. Heterocycl Comm. 2022;28(1):51–7.10.1515/hc-2022-0007Search in Google Scholar
[5] Zarei F, Soleimani-Amiri S, Azizi Z. Heterogeneously catalyzed Pechmann condensation employing the HFe(SO4)2.4H2O-Chitosan nano-composite: ultrasound-accelerated green synthesis of coumarins. Polycyclic Aromat Compd. 2022;42(9):6072–89.10.1080/10406638.2021.1973520Search in Google Scholar
[6] Soleimani‐Amiri S, Shafaei F, Varasteh Moradi A, Gholami‐Orimi F, Rostami Z. A novel synthesis and antioxidant evaluation of functionalized [1,3] oxazoles using Fe3O4‐magnetic nanoparticles. J Heterocycl Chem. 2019;56(10):2744–52.10.1002/jhet.3640Search in Google Scholar
[7] Foroush MP, Ahmadi R, Yousefi M, Najafpour J. In Silico study of adsorption of penicillin antibiotic on the surface of single walled nitride boron nanotubes (SBNNT). S Afr J Chem Eng. 2021;37:135–40.10.1016/j.sajce.2021.05.007Search in Google Scholar
[8] Dawood KM. An update on benzofuran inhibitors: a patent review. Expert Opin Ther Pat. 2019;29(11):841–70.10.1080/13543776.2019.1673727Search in Google Scholar PubMed
[9] Prabhakar KR, Veerapur VP, Bansal P, Vipan KP, Reddy KM, Barik A, et al. Identification and evaluation of antioxidant, analgesic/anti-inflammatory activity of the most active ninhydrin–phenol adducts synthesized. Bioorg Med Chem. 2006;14(21):7113–20.10.1016/j.bmc.2006.06.068Search in Google Scholar PubMed
[10] Firoozi N, Roshan Z, Mohammadizadeh MR. Facile Chemoselective synthesis of 2‐(2‐(Methoxycarbonyl)‐3‐oxo‐2, 3‐dihydrobenzofuran‐2‐yl) benzoic acids and 3H, 3’H‐Spiro [benzofuran‐2, 1′‐isobenzofuran]‐3, 3′‐dione derivatives. Appl Organomet Chem. 2018;32(1):e3963.10.1002/aoc.3963Search in Google Scholar
[11] Klumpp DA, Fredrick S, Lau S, Jin KK, Bau R, Surya Prakash GK, et al. Acid-catalyzed condensations of ninhydrin with aromatic compounds. Preparation of 2,2-diaryl-1,3-indanediones and 3-(diarylmethylene) isobenzofuranones1. J Org Chem Res. 1999;64(14):5152–5.10.1021/jo990197hSearch in Google Scholar PubMed
[12] Kundu SK, Das S, Pramanik A. 6-(α-Hydroxy-α-aryl/naphthyl) methyl-3,4-dihydro-2,5-benzodiazocin-1 (2H)-ones and diphenylmethanes from C-2 arylated 1, 3-indanediones. J Chem Res. 2004;11:781–3.10.3184/0308234043431654Search in Google Scholar
[13] Habtemariam S. Antiinflammatory activity of the antirheumatic herbal drug, gravel root (Eupatorium purpureum): further biological activities and constituents. Phytother Res. 2001;15(8):687–90.10.1002/ptr.887Search in Google Scholar PubMed
[14] Pauletti PM, Araújo AR, Young MC, Giesbrecht AM, da Silva Bolzani V. nor-Lignans from the leaves of Styrax ferrugineus (Styracaceae) with antibacterial and antifungal activity. Phytochemistry. 2000;55(6):597–601.10.1016/S0031-9422(00)00225-9Search in Google Scholar PubMed
[15] Masubuchi M, Kawasaki KI, Ebiike H, Ikeda Y, Tsujii S, Sogabe S, et al. Design and synthesis of novel benzofurans as a new class of antifungal agents targeting fungal N-myristoyltransferase. Part 1. Bioorganic Med Chem Lett. 2001;11(14):1833–7.10.1016/S0960-894X(01)00319-5Search in Google Scholar
[16] Wrobel JE, Dietrich AJ, Antane MM. inventors; American Home Products Corp, assignee. Benzothiophenes, benzofurans, and indoles useful in the treatment of insulin resistance and hyperglycemia. United States patent US. 2001;6(251):936.Search in Google Scholar
[17] Kayser O, Chen M, Kharazmi A, Kiderlen AF. Aurones interfere with Leishmania major mitochondrial fumarate reductase. Z Naturforsch C. 2002;57(7–8):717–20.10.1515/znc-2002-7-828Search in Google Scholar PubMed
[18] Hayakawa I, Shioya R, Agatsuma T, Furukawa H, Naruto S, Sugano Y. 4-Hydroxy-3-methyl-6-phenylbenzofuran-2-carboxylic acid ethyl ester derivatives as potent anti-tumor agents. Bioorganic Med Chem Lett. 2004;14(2):455–8.10.1016/j.bmcl.2003.10.039Search in Google Scholar PubMed
[19] Ashok D, Kumar RS, Gandhi DM, Sarasija M, Jayashree A, Adam S. Solvent-free microwave-assisted synthesis and biological evaluation of 2, 2-dimethylchroman-4-one based benzofurans. Heterocycl Comm. 2016;22(6):363–8.10.1515/hc-2016-0147Search in Google Scholar
[20] World Health Organization. World Health Organization Global Report on Diabetes. Geneva: World Health Organization; 2016.Search in Google Scholar
[21] Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW, et al. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271–81.10.1016/j.diabres.2018.02.023Search in Google Scholar PubMed
[22] Alagesan K, Raghupathi PK, Sankarnarayanan S. Amylase inhibitors: potential source of anti-diabetic drug discovery from medicinal plants. Int J Pharm Life Sci. 2012;3(2):1407–12.Search in Google Scholar
[23] Khalil-Moghaddam S, Ebrahim-Habibi A, Pasalar P, Yaghmaei P, Hayati-Roodbari N. Reflection on design and testing of pancreatic alpha-amylase inhibitors: an in silico comparison between rat and rabbit enzyme models. DARU J Pharm Sci. 2012;20(1):1–9.10.1186/2008-2231-20-77Search in Google Scholar PubMed PubMed Central
[24] Matough FA, Budin SB, Hamid ZA, Alwahaibi N, Mohamed J. The role of oxidative stress and antioxidants in diabetic complications. Sultan Qaboos Univ Med J. 2012;12(1):5.10.12816/0003082Search in Google Scholar PubMed PubMed Central
[25] Deng L, Du C, Song P, Chen T, Rui S, Armstrong DG, et al. The role of oxidative stress and antioxidants in diabetic wound healing. Oxid Med Cell Longev. 2021;2021:8852759.10.1155/2021/8852759Search in Google Scholar PubMed PubMed Central
[26] Mehrabi M, Esmaeili S, Ezati M, Abassi M, Rasouli H, Nazari D, et al. Antioxidant and glycohydrolase inhibitory behavior of curcumin-based compounds: Synthesis and evaluation of anti-diabetic properties in vitro. Bioorg Chem. 2021;110:104720.10.1016/j.bioorg.2021.104720Search in Google Scholar PubMed
[27] Kadieva MG, Oganesyan ET. Methods for the synthesis of benzofuran derivatives. Chem Heterocycl Compd. 1997;33(11):1245–58.10.1007/BF02320323Search in Google Scholar
[28] Sidhu PS. One-pot synthesis protocol for highly functionalized benzofuran scaffold. Postdoc J. 2013;31:36.10.14304/SURYA.JPR.V1N12.4Search in Google Scholar
[29] Rossy C, Fouquet E, Felpin FX. Practical synthesis of indoles and benzofurans in water using a heterogeneous bimetallic catalyst. Beilstein J Org Chem. 2013;9(1):1426–31.10.3762/bjoc.9.160Search in Google Scholar PubMed PubMed Central
[30] Shahvelayati AS, Yavari I, Delbari AS. Formation of thiazol-2 (3H)-imines by reaction of α-amino acids, aroylisothiocyanates, and α-bromoketones in an ionic liquidChin. Chem Lett. 2014;25(1):119–22.10.1016/j.cclet.2013.11.009Search in Google Scholar
[31] Baliyan S, Mukherjee R, Priyadarshini A, Vibhuti A, Gupta A, Pandey RP, et al. Determination of Antioxidants by DPPH Radical Scavenging Activity and Quantitative Phytochemical Analysis of Ficus religiosa. Molecules. 2022;27(4):1326.10.3390/molecules27041326Search in Google Scholar PubMed PubMed Central
[32] AlNeyadi SS, Amer N, Thomas TG, Al Ajeil R, Breitener P, Munawar N. Synthesis, characterization, and antioxidant activity of some 2-methoxyphenols derivatives. Heterocycl Comm. 2020;26(1):112–22.10.1515/hc-2020-0112Search in Google Scholar
[33] Chen J, Yang J, Ma L, Li J, Shahzad N, Kim CK. Structure-antioxidant activity relationship of methoxy, phenolic hydroxyl, and carboxylic acid groups of phenolic acids. Sci Rep. 2020;10(1):1–9.10.1038/s41598-020-59451-zSearch in Google Scholar
[34] Ishiyama M, Tominaga H, Shiga M, Sasamoto K, Ohkura Y, Ueno K. A combined assay of cell vability and in vitro cytotoxicity with a highly water-soluble tetrazolium salt, neutral red and crystal violet. Biol Pharm Bull. 1996;19(11):1518–20.10.1248/bpb.19.1518Search in Google Scholar PubMed
[35] Riss TL, Moravec RA, Niles AL, Duellman S, Benink HA, Worzella TJ, et al. Cell viability assays. Assay Guidance Manual [Internet]. Bethesda (MD): Eli Lilly & Company and the National center for advancing translational sciences; 2004.Search in Google Scholar
[36] Lee HB, Ha H, King GL. Reactive oxygen species and diabetic nephropathy. J Am Soc Nephrol. 2003;14(3):209–10.10.1097/01.ASN.0000077403.06195.D2Search in Google Scholar
[37] Sola M, Lledos A, Duran M, Bertran J, Abboud JL. Analysis of solvent effects on the Menshutkin reaction. J Am Chem Soc. 1991;113(8):2873–9.10.1021/ja00008a013Search in Google Scholar
[38] Khalil-moghaddam S, Shahvelayati AS. Aliahmadi A. Synthesis and Antioxidant Properties of Two New Derivatives of Indeno-Benzofuran. J Chem Health Risks. 2020;10(1):75–80.Search in Google Scholar
[39] Yehye WA, Abdul Rahman NA, Alhadi A, Khaledi H, Ng SW, Ariffin A. Butylated hydroxytoluene analogs: Synthesis and evaluation of their multipotent antioxidant activities. Molecules. 2012;17(7):7645–65.10.3390/molecules17077645Search in Google Scholar PubMed PubMed Central
[40] Celestino MT, Magalhaes UD, Fraga AG, Carmo FA, Lione V, Castro HC, et al. Rational use of antioxidants in solid oral pharmaceutical preparations. Braz J Pharm Sci. 2012;48:405–15.10.1590/S1984-82502012000300007Search in Google Scholar
[41] Bernfeld P. Amylases, α and β. Methods in enzymology. Cambridge, US: Academic Press; 1955.10.1016/0076-6879(55)01021-5Search in Google Scholar
[42] Khalil-moghaddam S, Aliahmadi A, Jalilian N, Aref Tabad M. The study of antioxidant and cellular toxicity effects of methanol, ethyl acetate, aqueous and n-hexane extracts of symphytum kurdicum plant. J Chem Health Risks. 2021;11:105–11.Search in Google Scholar
© 2022 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Sono and nano: A perfect synergy for eco-compatible Biginelli reaction
- Study of the reactivity of aminocyanopyrazoles and evaluation of the mitochondrial reductive function of some products
- “Click” assembly of novel dual inhibitors of AChE and MAO-B from pyridoxine derivatives for the treatment of Alzheimer’s disease
- Synthesis of 2,2-difluoro-2-arylethylamines as fluorinated analogs of octopamine and noradrenaline
- Cyclization of N-acetyl derivative: Novel synthesis – azoles and azines, antimicrobial activities, and computational studies
- Two independent and consecutive Michael addition of 1,3-dimethylbarbituric acid to (2,6-diarylidene)cyclohexanone: Flying-bird-shaped 2D-polymeric structure
- Ionic liquid-catalyzed synthesis of (1,4-benzoxazin-3-yl) malonate derivatives via cross-dehydrogenative-coupling reactions
- Synthesis of novel triiodide ionic liquid based on quaternary ammonium cation and its use as a solvent reagent under mild and solvent-free conditions
- Eelectrosynthesis of benzothiazole derivatives via C–H thiolation
- Synthesis of fluoro-rich pyrimidine-5-carbonitriles as antitubercular agents against H37Rv receptor
- Syntheses, crystal structure, thermal behavior, and anti-tumor activity of three ternary metal complexes with 2-chloro-5-nitrobenzoic acid and heterocyclic compounds
- Synthesis of enhanced lipid solubility of indomethacin derivatives for topical formulations
- Synthesis of newer substituted chalcone linked 1,2,3-triazole analogs and evaluation of their cytotoxic activities
- Novel benzodioxatriaza and dibenzodioxadiazacrown compounds carrying 1,2,4-oxadiazole moiety
- Synthesis of rhodium catalysts with amino acid or triazine as a ligand, as well as its polymerization property of phenylacetylene
- DABCO-based ionic liquid-promoted synthesis of indeno-benzofurans derivatives: Investigation of antioxidant and antidiabetic activities
- Design, synthesis, and biological activity of novel pomalidomide linked with diphenylcarbamide derivatives
- Study on effective synthesis of 7-hydroxy-4-substituted coumarins
- Review Article
- Chemical constituents of plants from the genus Carpesium
- Communication
- Reactions of 3-amino-1,2,4-triazine with coupling reagents and electrophiles
Articles in the same Issue
- Research Articles
- Sono and nano: A perfect synergy for eco-compatible Biginelli reaction
- Study of the reactivity of aminocyanopyrazoles and evaluation of the mitochondrial reductive function of some products
- “Click” assembly of novel dual inhibitors of AChE and MAO-B from pyridoxine derivatives for the treatment of Alzheimer’s disease
- Synthesis of 2,2-difluoro-2-arylethylamines as fluorinated analogs of octopamine and noradrenaline
- Cyclization of N-acetyl derivative: Novel synthesis – azoles and azines, antimicrobial activities, and computational studies
- Two independent and consecutive Michael addition of 1,3-dimethylbarbituric acid to (2,6-diarylidene)cyclohexanone: Flying-bird-shaped 2D-polymeric structure
- Ionic liquid-catalyzed synthesis of (1,4-benzoxazin-3-yl) malonate derivatives via cross-dehydrogenative-coupling reactions
- Synthesis of novel triiodide ionic liquid based on quaternary ammonium cation and its use as a solvent reagent under mild and solvent-free conditions
- Eelectrosynthesis of benzothiazole derivatives via C–H thiolation
- Synthesis of fluoro-rich pyrimidine-5-carbonitriles as antitubercular agents against H37Rv receptor
- Syntheses, crystal structure, thermal behavior, and anti-tumor activity of three ternary metal complexes with 2-chloro-5-nitrobenzoic acid and heterocyclic compounds
- Synthesis of enhanced lipid solubility of indomethacin derivatives for topical formulations
- Synthesis of newer substituted chalcone linked 1,2,3-triazole analogs and evaluation of their cytotoxic activities
- Novel benzodioxatriaza and dibenzodioxadiazacrown compounds carrying 1,2,4-oxadiazole moiety
- Synthesis of rhodium catalysts with amino acid or triazine as a ligand, as well as its polymerization property of phenylacetylene
- DABCO-based ionic liquid-promoted synthesis of indeno-benzofurans derivatives: Investigation of antioxidant and antidiabetic activities
- Design, synthesis, and biological activity of novel pomalidomide linked with diphenylcarbamide derivatives
- Study on effective synthesis of 7-hydroxy-4-substituted coumarins
- Review Article
- Chemical constituents of plants from the genus Carpesium
- Communication
- Reactions of 3-amino-1,2,4-triazine with coupling reagents and electrophiles

