Abstract
The oxidation of 1,4-diacetylbenzene using several oxidizing agents gave 1,4-phenylene-bis-glyoxal in 61–85% yields. A convenient and efficient synthesis of bis-quinoxaline and bis-pyrido[2,3-b]pyrazine derivatives involves the double condensation of 1,2-diamines with 1,4-phenylene-bis-glyoxal in ethanol under reflux conditions. The structures of the new products were defined by proton nuclear magnetic resonance (1H NMR), carbon-13 nuclear magnetic resonance (13C NMR), Fourier-transform infrared spectroscopy (FT-IR) and mass spectrometry (MS).
Introduction
Glyoxals are important building blocks in organic synthesis, particularly in the synthesis of biologically active heterocyclic compounds [1], [2], [3], [4], [5], [6], [7], [8], [9] including quinoxaline derivatives [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26]. Synthesis of a quinoxaline by the reaction of a glyoxal with a 1,2-diamine is part of the general strategy involving the reaction of 1,2-dicarbonyl compounds with 1,2-diamines [27], [28], [29], [30]. In continuation of our studies on the development of new synthetic routes to heterocyclic compounds using arylglyoxals, herein we report the synthesis of new bis-quinoxaline derivatives 3a–f in a 68–94% yield via condensation of 1,4-phenylene-bis-glyoxal as its hydrate 1 (structure in Scheme 1) and 1,2-diamines 2a–f in ethanol under reflux conditions.
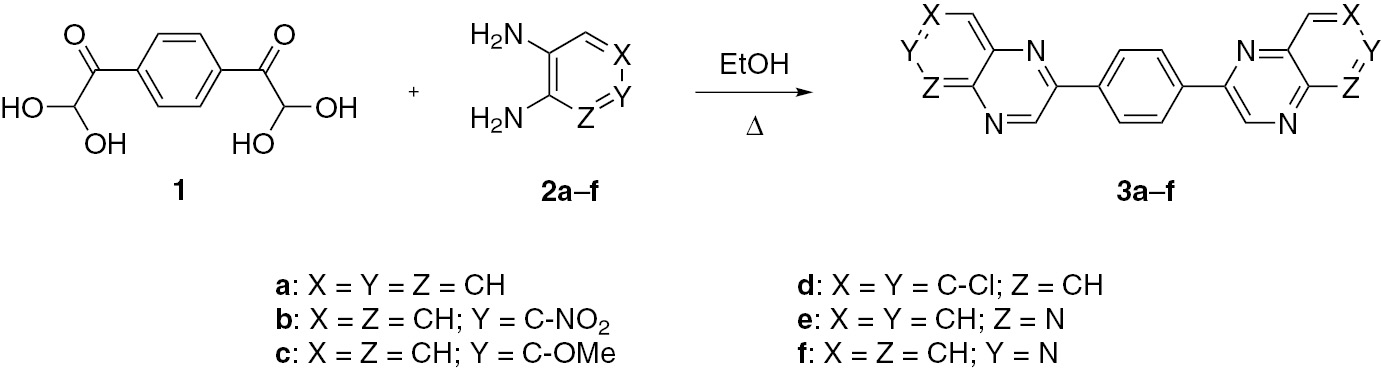
Results and discussion
The glyoxal hydrate 1 was synthesized by oxidation of 1,4-diacetylbenzene using different oxidizing agents in a 61–85% yield. The SeO2/dioxane/H2O system is preferred to other oxidizing agents as its use provides product 1 in an 85% yield after crystallization from water. The use of other oxidizing systems described in the literature, namely HBr/DMSO/H2O, CuCl2/DMSO/H2O and I2/CuO/DMSO, furnished compound 1 in the respective yields of 73%, 65% and 61%.
The condensation of compound 1 with 1,2-diamines 2a–f in ethanol under reflux gave the desired bis-quinoxalines and bis-pyrido[2,3-b]pyrazines under catalyst-free conditions in a 68–89% yield (Scheme 1). The use of unsymmetrical aromatic diamines 2b, 2e and 2f could in principle lead to isomeric products, but the formation of a single product in each case shows that the reactions are regioselective. As can be seen, in the condensation of bis-glyoxal 1 with 4-nitro-1,2-diaminobenzene (2b) and aminopyridines 2e or 2f, the more nucleophilic 1-amino group attacks the formyl group in the first step, and the condensation of the less reactive 2-amino group with the keto groups occurs in the second step, leading to the formation of bis-quinoxaline 3b. The reactions of aminopyridines 2e and 2f with 1 follow a similar pattern and furnish regioselectively the respective bis-pyrido[2,3-b]pyrazines 3e and 3f. This reactivity pattern is in full agreement with previous mechanistic studies on the construction of fused pyrazines [27], [28], [29], [30].
Conclusion
A double condensation of 1,4-phenylene-bis-glyoxal with various 1,2-diamines furnished bis-quinoxaline derivatives 3a–f in high to excellent yields. The simplicity of operation, high yields and regioselectivity are the key advantages of this method.
Experimental
Melting points were measured on an Electrothermal 9200 apparatus and are uncorrected. Infrared spectra were measured on a Spectrum RXI, Perkin Elmer, UK Fourier-transform infrared (FT-IR) instrument using KBr disks. The 1H (300 MHz) and 13C (75 MHz) nuclear magnetic resonance (NMR) spectra were recorded on a Bruker DRX-300 Avance spectrometer in DMSO-d6, CDCl3 and C6D6 relative to tetramethylsilane (TMS) as the internal reference. Thin layer chromatography (TLC) was carried out on a pre-coated aluminum sheet with silica gel 60F 254 obtained from Merck, and detection was made with the help of an ultraviolet (UV) lamp (λ 254 nm). Mass analysis was performed on an Agilent Technology (HP) 5973 Network Mass Selective Detector and high-resolution mass spectra were recorded on a Kratos mass spectrometry (MS) 25RF spectrometer.
Synthesis of 1,1′-(1,4-phenylene)bis(2,2-dihydroxyethanone) (1)
A solution of selenium dioxide (1.55 g, 14 mmol) in 90% aqueous dioxane (10 mL) was treated at 100°C with a solution of 1,4-diacetylbenzene (1.62 g, 10 mmol) in dioxane (12 mL). The mixture was heated under reflux for 14 h and the precipitated selenium was removed by filtration. The solution was cooled and the resultant pale yellow precipitate of product 1 was collected and crystallized from water: yield 1.92 g (85%); mp 141–142°C; IR: νmax 3428, 3377, 2975, 1670, 1505, 1446, 1407, 1297, 1121, 1037, 962, 873, 801, 696, 584, 517 cm−1; 1H NMR (DMSO-d6): δ 8.15 (s, 4H), 6.90 (d, J=7.2 Hz, 4H, 4×OH, exchanged by D2O addition), 5.68 (bt, J=7.2 Hz, 2H, changed to a singlet after D2O addition); 13C NMR (DMSO-d6): δ 196.5, 137.4, 129.7, 89.9; MS: m/z (%) 226 ([M]+, 59), 184 (100), 163 (58), 149 (65), 133 (76), 105 (59), 91 (52), 77 (56), 55 (75). ESI-HRMS: Calcd. for C10H10O6, [M]+: m/z 226.0477. Found: m/z 226.0462.
General procedure for the synthesis of bis-quinoxalines and bis-pyrido[2,3-b]pyrazines (3a–f)
A mixture of 1,4-phenylene-bis-glyoxal (1, 1 mmol) and a 1,2-diamine (2a–f, 2 mmol) in absolute ethanol (10 mL) was heated under reflux for 10–15 h, then cooled and the resultant precipitate of 3a–f was filtered, washed with ethanol and crystallized from ethanol.
1,4-Bis-(quinoxalin-2-yl)benzene (3a)
Reaction time 12 h; yield 82% of light yellow powder; mp 264–266°C; IR: νmax 3054, 1678, 1609, 1574, 1545, 1490, 1462, 1426, 1370, 1320, 1262, 1231, 1208, 1130, 1054, 1014, 956, 847, 756, 674, 630, 601, 566 cm−1; 1H NMR (CDCl3): δ 9.45 (s, 2H), 8.45 (s, 4H), 8.24–8.16 (m, 4H), 7.85–7.80 (m, 4H); 13C NMR (CDCl3): δ 150.9, 142.4, 141.8, 138.3, 130.5, 129.9, 129.7, 129.2, 128.2; EI-MS: m/z (%) 335 ([M+1]+, 28), 334 [M]+ (100), 307 (11), 306 (17), 204 (12), 76 (19). ESI-HRMS. Calcd. for C22H14N4, [M]+: 334.1218. Found: m/z 334.1205.
1,4-Bis(6-nitroquinoxalin-2-yl)benzene (3b)
Reaction time 15 h; yield 68% of orange powder; mp 235–236°C; IR: νmax 3047, 1578, 1554, 1348, 1320, 1280, 1193, 1078, 1050, 964, 844, 831, 792, 742 cm−1; 1H NMR (C6D6): δ 9.16 (s, 1H), 9.13 (s, 1H), 8.20 (s, 4H), 8.10 (d, J=9 Hz, 2H), 7.98 (d, J=9 Hz, 2H), 7.10 (bs, 2H); 13C NMR (C6D6): δ 138.5, 137.7, 136.1, 135.7, 133.7, 127.6, 126.9, 124.6, 124.0, 123.8; EI-MS: m/z (%) 425 ([M+1]+, 27), 424 ([M]+, 100), 394 (25), 382 (11), 351 (14), 75 (13). ESI-HRMS. Calcd. for C22H12N6O4, [M]+: m/z 424.0920. Found: m/z 424.0907.
1,4-Bis(6-methoxyquinolin-2-yl)benzene (3c)
Reaction time 10 h; yield 89% of brown powder; mp 267–268°C; IR: νmax 3047, 1616, 1498, 1374, 1321, 1261, 1217, 1201, 1173, 1122, 1060, 1026, 958, 846, 830, 779 cm−1; 1H NMR (DMSO-d6): δ 9.52 (s, 2H), 8.55(s, 4H), 8.03 (d, J=8.7 Hz, 2H), 7.54 (s, 2H), 7.51 (d, J=8.7 Hz, 2H), 3.86 (s, 6H); 13C NMR spectrum could not be recorded due to low solubility of the sample; EI-MS: m/z (%) 395 ([M+1]+, 29), 394 ([M]+, 100), 262 (10), 197 (7), 106 (12), 63 (10). ESI-HRMS. Calcd. for C24H18N4O2, [M]+: m/z 394.1430. Found: m/z 394.1416.
1,4-Bis(6,7-dichloroquinoxalin-2-yl)benzene (3d)
Reaction time 12 h; yield 78% of gray powder; mp 190–191°C; the 1H NMR and 13C NMR spectra could not be recorded due to low solubility of the sample; IR: νmax 3066, 3045, 1544, 1459, 1418, 1317, 1274, 1177, 1111, 1058, 1016, 945, 928, 900, 878, 840, 810, 660, 624 cm−1; EI-MS: m/z (%) 478 ([M+6]+, 14), 474 ([M+4]+, 52), 472 ([M+2]+, 100), 470 ([M]+, 80), 300 (17), 272 (15), 237 (11), 170 (13), 146 (32), 144 (50), 109 (30), 74 (12). ESI-HRMS. Calcd. for C22H10Cl4N4, [M]+: m/z 469.9660. Found: m/z 469.9648.
1,4-Bis(pyrido[2,3-b]pyrazine-2-yl)benzene (3e)
Reaction time 10 h; yield 80% of brown powder; mp 320–321°C; IR: νmax 3413, 3067, 2372, 1546, 1461, 1310, 209, 1125, 1061, 952, 848, 794 cm−1; 1H NMR (CDCl3): δ 9.29 (s, 2H), 9.25 (bs, 2H) 8.82 (s, 4H), 8.54 (d, J=7.8 Hz, 2H), 7.77 (dd, J1=8.4 Hz, J2=4.5 Hz, 2H); 13C NMR spectrum could not be recorded due to low solubility of the sample; EI-MS: m/z (%) 337 ([M+1]+, 24), 336 ([M]+, 100), 308 (9), 233 (20), 205 (9), 104 (10), 77 (14). ESI-HRMS. Calcd. for C20H12N6, [M]+m/z 336.1123. Found: m/z 336.1111.
1,4-Bis(pyrido[3,4-b]pyrazine-2-yl)benzene (3f)
Reaction time 10 h; yield 78% of creamy powder; mp 248–249°C; IR: νmax 3216, 2373, 1547, 1419, 1317, 1235, 1054, 960, 854, 837, 584 cm−1; 1H NMR (CDCl3): δ 9.62 (s, 2H), 9.55 (s, 2H), 8.90 (d, J=5.7 Hz, 2H), 8.52 (s, 4H), 8.04 (d, J=6 Hz, 2H); 13C NMR (CDCl3+DMSO-d6): δ 154.2, 147.7, 145.2, 144.8, 136.8, 128.9, 128.5, 121.9, 121.5; EI-MS: m/z (%) 337 ([M+1]+, 22), 336 ([M]+, 100), 309 (13), 308 (15), 233 (11), 77 (11), 50 (32). ESI-HRMS. Calcd. for C20H12N6, [M]+: m/z 336.1123. Found: m/z 336.111.
Acknowledgments
We are grateful to Urmia University for the financial support and we also thank Professor R. H. Prager (Flinders University, Australia) for language editing and proofreading of this article.
References
[1] Zaliani, V.; Cocconcelli, G.; Fantini, M.; Ghiron, C.; Rivara, M. A practical synthesis of 2,4(5)-diarylimidazoles from simple building blocks. J. Org. Chem.2007, 72, 4551–4553.10.1021/jo070187dSuche in Google Scholar PubMed
[2] Juspin, T.; Terme, T.; Vanelle, P. Rapid access to diphenyl- and acenaphthoquinoline derivatives. Synfacts2009, 9, 1485–1489.10.1055/s-0029-1216741Suche in Google Scholar
[3] Fischer, B.; Kabha, E.; Gendron, F. P.; Beaudoin, A. R. Analytical ancestry: “firsts” in fluorescent labeling of nucleosides, nucleotides, and nucleic acids. J. Med. Chem. 2000, 19, 1033–1034.10.1080/15257770008033041Suche in Google Scholar
[4] Prashanthukumar, B. R.; Sharma, G. K.; Srinath, S.; Noor, M.; Suresh, B.; Srinivasa, B. R. Synthesis and biological evaluation of newer benzofuran derivatives as potential anticancer and anathematic agents. J. Heterocycl. Chem. 2009, 46, 278.10.1002/jhet.68Suche in Google Scholar
[5] Robjohn, N. Selenium dioxide oxidation. Org. React. 1949, 5, 331.10.1002/0471264180.or024.04Suche in Google Scholar
[6] Robjohn, N. Selenium dioxide oxidation. Org. React. 1976, 24, 261.10.1002/0471264180.or024.04Suche in Google Scholar
[7] Kornblum, N.; Powers, J. W.; Anderson, G. J.; Jones, W. J.; Larson, H. O.; Weaver, W. M. Conversion of aldehydes to amides via dimethyl sulfoxide oxidation of the corresponding α-aminonitriles. J. Am. Chem. Soc. 1957, 79, 6562.10.1021/ja01581a057Suche in Google Scholar
[8] Schaefer, J. P. Selenium dioxide oxidations. I. studies on the mechanism of oxidation of 1,2-dibenzoylethane. J. Am. Chem. Soc. 1962, 84, 713–716.10.1021/ja00864a005Suche in Google Scholar
[9] Zhiling, C.; Dahua, Sh.; Ying, Q.; Chuanzhou, T.; Weiwei, L.; Guowei, Y. A new method for synthesis of 3,6-diacetyl-9-ethylcarbazole and its oxidation to the corresponding diglyoxal using several oxidizing agents. Molecules2013, 18, 15717–15723.10.3390/molecules181215717Suche in Google Scholar
[10] Zarranz, B.; Jaso, A.; Aldana, I.; Monge, A. Synthesis and anticancer activity evaluation of new 2-alkylcarbonyl and 2-benzoyl-3-trifluoromethylquinoxaline 1,4-di-N-oxide derivatives. J. Med. Chem.2004, 12, 3711–3721.10.1016/j.bmc.2004.04.013Suche in Google Scholar
[11] Grande, F.; Aiello, F.; Grazia, O. D.; Brizzi, A.; Garofalo, A.; Neamati, N. Synthesis and antitumor activities of a series of novel quinoxalin hydrazides. J. Med. Chem. 2005, 15, 288–294.10.1016/j.bmc.2006.09.073Suche in Google Scholar
[12] Ali, M. M.; Ismail, M. M. F.; EI-Gaby, M. S. A.; Zahran, M. A.; Ammar, T. A. Synthesis and anti-microbial activity of some novel quinoxaline derivatives. Molecules2000, 5, 864–873.10.3390/50600864Suche in Google Scholar
[13] Seitz, L. E.; Suling, W. J.; Reynolds, R. C. Synthesis and antimycobacterial activity of pyrazine and quinoxaline derivatives. J. Med. Chem. 2002, 45, 5604–5606.10.1021/jm020310nSuche in Google Scholar PubMed
[14] Jaso, A.; Zarranz, B.; Aldana, I.; Monge, A. Synthesis of new quinoxaline-2-carboxylate 1,4-dioxide derivatives as anti-mycobacterium tuberculosis agents. J. Med. Chem. 2005, 48, 2019–2025.10.1021/jm049952wSuche in Google Scholar PubMed
[15] Bailly, C.; Echepare, S.; Gago, F.; Waring, M. Recognition elements that determine affinity and sequence-specific binding to DNA of 2QN, a biosynthetic bis-quinoline analogue of echinomycin. J. Med. Chem. 1999, 14, 291–303.Suche in Google Scholar
[16] Gomtsyan, A.; Bayburt, E. K.; Schmidt, R. G.; Zheng, G. Z.; Perner, R. J.; Didomenico, S.; Koeni, J. R.; Turner, S.; Jinkerson, T.; Drizin, I.; et al. Novel transient receptor potential vanilloid 1 receptor antagonists for the treatment of pain: structure-activity relationships for ureas with quinoline, isoquinoline, quinazoline, phthalazine, quinoxaline, and cinnoline moieties. J. Med. Chem.2005, 48, 744–752.10.1021/jm0492958Suche in Google Scholar PubMed
[17] Perumal, R. V.; Mahesh, R. Synthesis and biological evaluation of a novel structural type of serotonin 5-HT3 receptor antagonists. Bioorg. Med. Chem. Lett. 2006, 16, 2769–2772.10.1002/chin.200633174Suche in Google Scholar
[18] Tandon, V. K.; Yadav, D. B.; Maurya, H. K., Chaturvedi, A. K.; Shukla, P. K. Design, synthesis, and biological evaluation of 1,2,3-trisubstituted-1,4-dihydrobenzo[g]quinoxaline-5,10-diones and related compounds as antifungal and antibacterial agents. J. Med. Chem.2006, 14, 6120–6126.10.1016/j.bmc.2006.04.029Suche in Google Scholar PubMed
[19] Carta, A.; Loriga, M.; Paglietti, G.; Mattana, A.; Fiori, P. L.; Mollicotti, P.; Echi, L.; Zanetti, S. Synthesis, anti-mycobacterial, anti-trichomonas and anti-candida in vitro activities of 2-substituted-6,7-difluoro-3-methylquinoxaline-1,4-dioxides. J. Med. Chem. 2004, 39, 195–203.10.1016/j.ejmech.2003.11.008Suche in Google Scholar PubMed
[20] Mashevskaya, I.; Makhmudov, R. R.; Aleksandrova, G. A.; Golovnira, O. V.; Duvalov, A. V.; Maslivets, A. N. Synthesis and study of the antibacterial and analgesic activity of 3-acyl-1,2,4,5-tetrahydro-[1,2-a]quinoxaline-1,2,4-triones. Pharm. Chem. J.2001, 35, 196–198.10.1023/A:1010475811489Suche in Google Scholar
[21] Vyas, D. A.; Chauhan, N. A.; Parikh, A. R. Synthesis and antimicrobial activity of quinoxaline based thiazolidinones and azetidinones. Indian J. Chem. 2007, 46B, 1699–1702.Suche in Google Scholar
[22] Burguete, A.; Pontiki, E.; Litina, D. H.; Villar, R.; Vicente, E.; Solano, B.; Ancizu, S.; Aldana, I.; Monge, A. Synthesis and anti-inflammatory/antioxidant activities of some new ring substituted 3-phenyl-1-(1,4-di-N-oxide quinoxalin-2-yl)-2-propen-1-one derivatives and of their 4,5-dihydro-(1H)-pyrazole analogues. Bioorg. Med. Chem. Lett. 2007, 17, 6439–6443.10.1016/j.bmcl.2007.10.002Suche in Google Scholar PubMed
[23] Wagle, S.; Adhikari, A. V.; Kumari, N. S. Synthesis of some new 2-(3-methyl-7-substituted-2-oxoquinoxalinyl)-5-(aryl)-1,3,4-oxadiazoles as potential nonsteroidal anti-inflammatory and analgesic agents. Indian J. Chem.2008, 47B, 439–448.10.1002/chin.200830164Suche in Google Scholar
[24] Kim, Y. B.; Kim, Y. H.; Park, J. Y.; Kim, S. K. Synthesis and biological activity of new quinoxaline antibiotics of echinomycin analogues. Bioorg. Med. Chem. Lett. 2004, 14, 541–544.10.1016/j.bmcl.2003.09.086Suche in Google Scholar PubMed
[25] Palanaki, M. S. S.; Dneprovskaia, E.; Doukas, J.; Fine, R. M.; Hood, J.; Kang, X.; Lohse, D.; Martin, M.; Noronha, G.; Soll, R. M.; et al. Discovery of 3,3′-(2,4-diaminopteridine-6,7-diyl)diphenol as an isozyme-selective inhibitor of PI3K for the treatment of ischemia reperfusion injury associated with myocardial infarction. J. Med. Chem.2007, 50, 4279–4294.10.1021/jm051056cSuche in Google Scholar PubMed
[26] Bandyopadhayay, D.; Cruz, J.; Morales, D. L.; Arman, H.; Cuate, E.; Lee, Y. S.; Banik, B. K.; Kim, D. An expeditious green route toward 2-aryl-4-phenyl-1H-imidazoles. J. Med. Chem.2013, 5, 1377.10.1186/s13588-014-0009-7Suche in Google Scholar PubMed PubMed Central
[27] Sako, M. Methods of Molecular Transformations. In Science of Synthesis. Yamamoto, Y., Ed. Houben-Weyl Thieme: Stuttgart, New York, 2003; Vol. 16, pp 1269–1290.Suche in Google Scholar
[28] Brown, D. J. A Series of Monographs; Quinoxalines: Supplement II. In The Chemistry of Heterocyclic Compounds. Taylor, E. C., Wipf, P., Eds. John Wiley & Sons: New Jersey, 2004; Vol. 61.Suche in Google Scholar
[29] Porter, A. E. A. Quinoxalines. In Comprehensive Heterocyclic Chemistry A. Katritsky, R., Rees, C. W., Eds. Oxford: Pergamum, 1984; p 157.10.1016/B978-008096519-2.00036-9Suche in Google Scholar
[30] Khalafy, J.; Poursattar Marjani, A.; Haghipour, M. Regioselective synthesis of 3-arylpyrido[2,3-b]pyrazines by reaction of arylglyoxals with 2,3-diaminopyridine. Curr. Chem. Lett.2013, 2, 21–26.10.5267/j.ccl.2012.12.003Suche in Google Scholar
©2018 Walter de Gruyter GmbH, Berlin/Boston
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Artikel in diesem Heft
- Frontmatter
- Research Articles
- Synthesis of benzofuro[3,2-b]furo[2,3-d]pyridin-4(5H)-ones, derivatives of a novel heterocyclic system
- Reactions of 3H-furan-2-ones and 2H-chromen-2-ones with pyrazole-3(5)-diazonium salts
- Synthesis of 1,4-oxathian-2-ones by triton B-catalyzed one-pot reaction of epoxides with ethyl mercaptoacetate
- The regioselective catalyst-free synthesis of bis-quinoxalines and bis-pyrido[2,3-b]pyrazines by double condensation of 1,4-phenylene-bis-glyoxal with 1,2-diamines
- [1,3]Thiazolo[3,2-b][1,2,4]triazol-7-ium salts: synthesis, properties and structural studies
- Crystal structure and molecular docking studies of 1,2,4,5-tetraaryl substituted imidazoles
- Design, synthesis and cytotoxicity evaluation of indibulin analogs
- Synthesis of dibenzothiazepine analogues by one-pot S-arylation and intramolecular cyclization of diaryl sulfides and evaluation of antibacterial properties
- Synthesis and preliminary anti-inflammatory evaluation of xanthone derivatives
- Synthesis and antimicrobial evaluation of 3-(4-arylthieno[2,3-d]pyrimidin-2-yl)- 2H-chromen-2-ones
- Corrigendum
- Corrigendum to: Diversity-oriented synthesis of amide derivatives of tricyclic thieno[2,3-d]pyrimidin-4(3H)-ones and evaluation of their influence on melanin synthesis in murine B16 cells
Artikel in diesem Heft
- Frontmatter
- Research Articles
- Synthesis of benzofuro[3,2-b]furo[2,3-d]pyridin-4(5H)-ones, derivatives of a novel heterocyclic system
- Reactions of 3H-furan-2-ones and 2H-chromen-2-ones with pyrazole-3(5)-diazonium salts
- Synthesis of 1,4-oxathian-2-ones by triton B-catalyzed one-pot reaction of epoxides with ethyl mercaptoacetate
- The regioselective catalyst-free synthesis of bis-quinoxalines and bis-pyrido[2,3-b]pyrazines by double condensation of 1,4-phenylene-bis-glyoxal with 1,2-diamines
- [1,3]Thiazolo[3,2-b][1,2,4]triazol-7-ium salts: synthesis, properties and structural studies
- Crystal structure and molecular docking studies of 1,2,4,5-tetraaryl substituted imidazoles
- Design, synthesis and cytotoxicity evaluation of indibulin analogs
- Synthesis of dibenzothiazepine analogues by one-pot S-arylation and intramolecular cyclization of diaryl sulfides and evaluation of antibacterial properties
- Synthesis and preliminary anti-inflammatory evaluation of xanthone derivatives
- Synthesis and antimicrobial evaluation of 3-(4-arylthieno[2,3-d]pyrimidin-2-yl)- 2H-chromen-2-ones
- Corrigendum
- Corrigendum to: Diversity-oriented synthesis of amide derivatives of tricyclic thieno[2,3-d]pyrimidin-4(3H)-ones and evaluation of their influence on melanin synthesis in murine B16 cells

