Abstract
The reaction of 2-amino-4-aryl-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitriles with excess aliphatic carboxylic acids in the presence of phosphoryl chloride (POCl3) afforded new 2-alkyl-5-aryl-8,8-dimethyl-8,9-dihydro-3H-chromeno[2,3-d]pyrimidine-4,6(5H,7H)-diones in high yields. The suggested mechanism involves a tandem intramolecular Pinner/Dimroth rearrangement. The synthesized compounds were characterized by infrared (IR), proton nuclear magnetic resonance (1H NMR), carbon-13 nuclear magnetic resonance (13C NMR) and elemental analysis.
Introduction
Certain chromenes possess important biological properties such as anticancer [1], [2], antimicrobial [3], [4], antiproliferative [5], anticonvulsant [6], antimalarial [7], antibacterial [8], anti-influenza [9] and anti-rhinovirus [10], [11] activities. Other compounds with a chromene moiety are potential inhibitors of aldose reductase [12], tumor necrosis fact (TNF)-α [13], PTP1B [14], DPP-IV [14], α-glucosidase [14], [15], h-MAO-B [16], PI3Kβ [17], PI3Kδ [17] and Src kinase [18]. On the other hand, the pyrimidine moiety is found in a range of compounds exhibiting a broad spectrum of biological activities such as antitumor [19], antiproliferative [20], antileishmanial [21], antibacterial [22], antifungal [23] and antioxidant [24] properties.
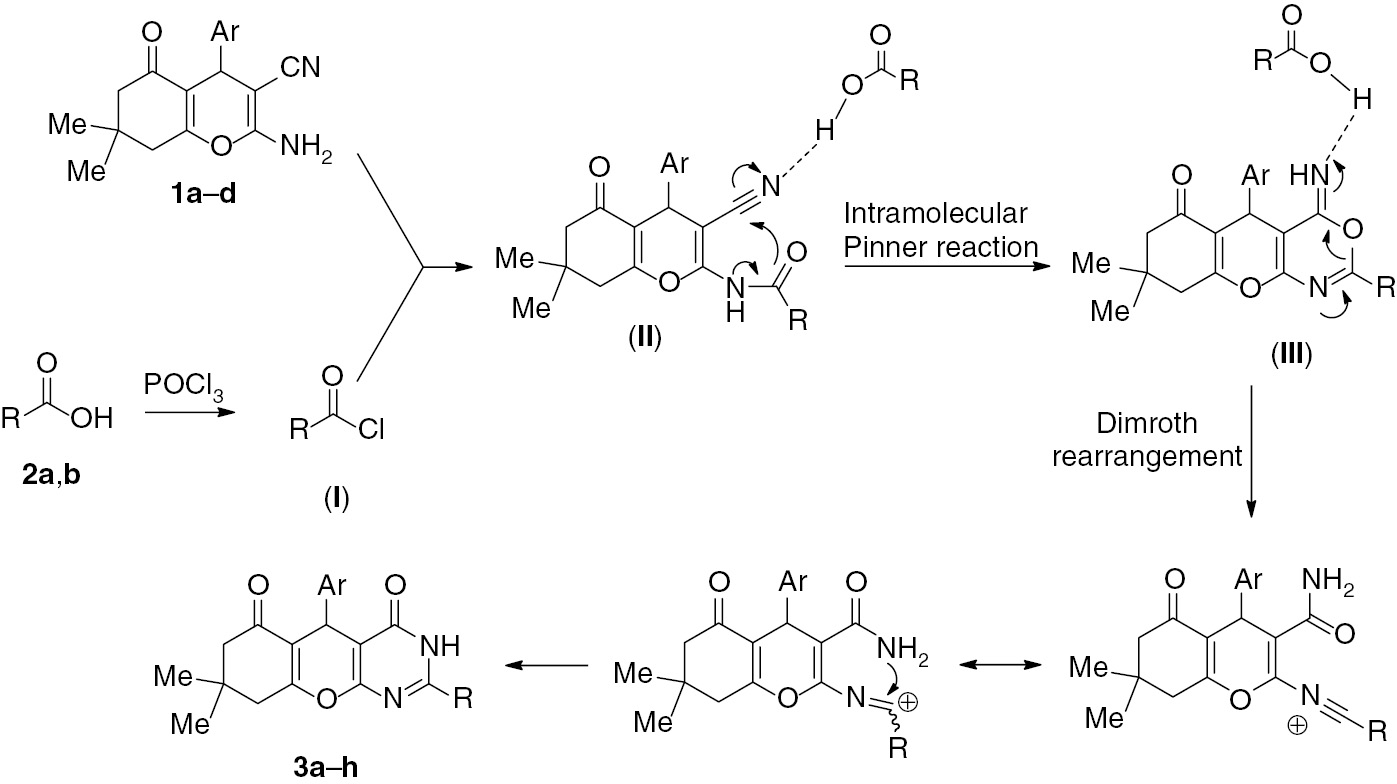
Chromeno[2,3-d]pyrimidines have received much attention because of their interesting biological properties such as anticancer [25], antimicrobial [26], [27], antitubercular [27], antibacterial [28], antiproliferative [29] and antioxidant [30] activities. In conjunction with our interest in the synthesis of heterocyclic compounds [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], in this paper we report the synthesis of new chromeno[2,3-d]pyrimidine-4,6(5H,7H)-diones 3a–h by the reaction of substituted 5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitriles 1a–d with excess aliphatic carboxylic acids 2a, b in the presence of phosphoryl chloride (POCl3) (Scheme 1).

Results and discussion
The starting materials 1a–d were prepared according to methods cited in the literature [41], [42], [43], [44], [45], [46]. In a model reaction, 2-amino-7,7-dimethyl-5-oxo-4-phenyl-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile (1a) was allowed to react with excess acetic acid (2a) under reflux without any solvent and catalyst. After a prolonged reaction time, only a low yield of the product was observed and a large amount of the starting material was recovered. Next, the reaction was investigated in excess acetic acid (2a) in the presence of POCl3 as the chlorinating agent. After stirring at room temperature for 300 min the mixture showed only little conversion. However, after reflux for 150 min, no starting material was observed on monitoring of the reaction by thin-layer chromatography (TLC), and a single spot for the formation of a product was seen. The product was identified as 2,8,8-trimethyl-5-phenyl-8,9-dihydro-3H-chromeno[2,3-d]pyrimidine-4,6(5H,7H)-dione (3a). Decreasing the reaction temperature to 100°C decreased the yield of the product from 90% to 78% under otherwise identical conditions. For comparison, synthesis of the model compound 3a was also carried out using thionyl chloride (SOCl2) under reflux conditions. Under these conditions, the product 3a was obtained in 82% yield after 180 min. Consequently, all subsequent reactions for the synthesis of compounds 3b–h were carried out in the presence of POCl3 at reflux temperature.
The structures of the newly synthesized compounds 3a–h were confirmed by analysis of spectral data and elemental analysis. For example, the proton nuclear magnetic resonance (1H NMR) spectrum of compound 3a in dimethyl sulfoxide-d6 (DMSO-d6) contains a broad singlet at δ 12.55 for the NH group and a singlet at δ 2.27 for new methyl group. These new signals along with other signals including two singlets at δ 0.97 and 1.07 for diastereotopic methyl groups and a singlet at δ 4.70 for aliphatic methine group are fully consistent with the formation of compound 3a. The infrared (IR) spectrum is devoid of the CN absorption band at 2199 cm−1 of the precursor but instead shows the NH absorption band at 3434 cm−1, which indicates the involvement of the nitrile group in the cyclization process.
On the basis of a related transformation [40], [41], a plausible mechanism for the formation of compounds 3a–hvia the tandem intramolecular Pinner/Dimroth rearrangement is presented in Scheme 2. First, chlorination of carboxylic acid 2a or 2b with POCl3 affords acyl chloride I which undergoes a reaction with the starting material 1a–d to give the intermediate product II. This compound undergoes an intramolecular Pinner reaction followed by the Dimroth rearrangement to give the final product 3a–hvia the oxazine intermediate III. Unfortunately, attempts to isolate the proposed intermediate products failed even with careful monitoring of the reaction.
Conclusion
Synthesis of new 2-alkyl-5-aryl-8,8-dimethyl-8,9-dihydro-3H-chromeno[2,3-d]pyrimidine-4,6(5H,7H)-dione derivatives 3a–h by the reaction of 2-amino-4-aryl-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitriles 1a–d with excess aliphatic carboxylic acid 2a or 2b in the presence of POCl3 is reported. An intramolecular Pinner/Dimroth rearrangement was suggested for the formation of the products.
Experimental
All chemicals were purchased from Merck and Aldrich and used without additional purification. IR spectra were obtained as KBr pellets using a Tensor 27 Bruker spectrophotometer. The 1H NMR (300 MHz) and carbon-13 nuclear magnetic resonance (13C NMR) (75 MHz) spectra were recorded on a Bruker 300 FT spectrometer in DMSO-d6 using tetramethylsilane (TMS) as the internal standard. Elemental analyses were performed on a Thermo Finnigan Flash EA microanalyzer. Melting points were recorded on a Stuart SMP3 melting point apparatus and are not corrected. The starting materials 1a–d were prepared according to the methods cited in the literature [41], [42], [43], [44], [45], [46].
General procedure for synthesis of compounds 3a–h
A mixture of 2-amino-4-aryl-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4H- chromene-3-carbonitrile 1a–d (1 mmol) and excess acetic acid (2a, 2 mL) or propionic acid (2b, 2 mL) in POCl3 (1 mL) was heated under reflux for 60–150 min. Upon completion, as monitored using TLC plates, the mixture was poured into cold water (40 mL). The crude product 3a–h was collected, washed with water and crystallized from ethyl acetate.
2,8,8-Trimethyl-5-phenyl-8,9-dihydro-3H-chromeno[2,3-d]pyrimidine-4,6(5H,7H)-dione (3a)
White powder; yield 90%; mp 318–320°C; IR: ν 3434 (NH), 1676 cm−1 (C=O); 1H NMR: δ 0.97 (s, 3H, CH3), 1.07 (s, 3H, CH3), 2.13 (d, 1H, J=16.1 Hz, one proton of diastereotopic protons in CH2), 2.22–2.35 (m, 4H, a doublet for one proton of diastereotopic protons in CH2 overlapped with a singlet for CH3), 2.62 (s, 2H, CH2), 4.70 (s, 1H, CH), 7.10–7.30 (m, 5H, HAr), 12.55 (s br., 1H, NH); 13C NMR: δ 21.4, 27.2, 29.0, 32.4, 32.9, 50.5, 102.2, 114.2, 126.8, 128.4, 128.5, 144.3, 159.1, 160.5, 162.5, 164.2, 196.3. Anal. Calcd for C20H20N2O3: C, 71.41; H, 5.99; N, 8.33. Found: C, 71.16; H, 6.17; N, 8.54.
2-Ethyl-8,8-dimethyl-5-phenyl-8,9-dihydro-3H-chromeno[2,3-d]pyrimidine-4,6(5H,7H)-dione (3b)
Creamy powder; yield 85%; mp 315–317°C; IR: ν 3432 (NH), 1674 cm−1 (C=O); 1H NMR: δ 0.98 (s, 3H, CH3), 1.07 (s, 3H, CH3), 1.17 (t, 3H, J=7.5 Hz, CH3), 2.22 (ABq, 2H, Δν=49.4 Hz, JAB=16.1 Hz, CH2), 2.45–2.60 (m, 2H, CH2 overlapped with solvent), 2.63 (s, 2H, CH2), 4.70 (s, 1H, CH), 7.10–7.30 (m, 5H, HAr), 12.51 (s br., 1H, NH); 13C NMR: δ 11.3, 27.2, 27.6, 29.0, 32.4, 33.0, 50.5, 102.4, 114.1, 126.8, 128.4, 128.6, 144.3, 160.6, 162.6, 163.0, 164.3, 196.4. Anal. Calcd for C21H22N2O3: C, 71.98; H, 6.33; N, 7.99. Found: C, 72.27; H, 6.14; N, 8.23.
2,8,8-Trimethyl-5-(3-nitrophenyl)-8,9-dihydro-3H-chromeno[2,3-d]pyrimidine-4,6(5H,7H)-dione (3c)
Creamy powder; yield 82%; mp 320–322°C; IR: ν 3437 (NH), 1669 cm−1 (C=O); 1H NMR: δ 0.98 (s, 3H, CH3), 1.08 (s, 3H, CH3), 2.15 (d, 1H, J=16.1 Hz, one proton of diastereotopic protons in CH2), 2.28 (s, 3H, CH3), 2.32 (d, 1H, J=16.1 Hz, one proton of diastereotopic protons in CH2), 2.66 (s, 2H, CH2), 4.82 (s, 1H, CH), 7.54–7.61 (m, 1H, HAr), 7.69–7.73 (m, 1H, HAr), 8.02–8.07 (m, 2H, HAr), 12.58 (br s, 1H, NH); 13C NMR: δ 21.5, 27.1, 28.9, 32.4, 33.4, 50.4, 101.1, 113.1, 122.1, 123.1, 130.0, 135.4, 146.3, 147.9, 159.8, 160.5, 162.5, 164.9, 196.5. Anal. Calcd for C20H19N3O5: C, 62.99; H, 5.02; N, 11.02. Found: C, 63.30; H, 5.24; N, 10.78.
2-Ethyl-8,8-dimethyl-5-(3-nitrophenyl)-8,9-dihydro-3H-chromeno[2,3-d]pyrimidine-4,6(5H,7H)-dione (3d)
Creamy powder; yield 80%; mp 301–303°C; IR: ν 3426 (NH), 1673 cm−1 (C=O); 1H NMR: δ 0.99 (s, 3H, CH3), 1.08 (s, 3H, CH3), 1.17 (t, 3H, J=7.4 Hz, CH3), 2.24 (ABq, 2H, Δν=49.4 Hz, JAB=16.0 Hz, CH2), 2.45–2.62 (m, 2H, CH2 overlapped with solvent), 2.67 (s, 2H, CH2), 4.82 (s, 1H, CH), 7.57 (t, 1H, J=7.7 Hz, HAr), 7.71 (t, 1H, J=7.6 Hz, HAr), 8.01–8.10 (m, 2H, HAr), 12.49 (br., 1H, NH); 13C NMR: δ 11.2, 19.0, 27.2, 27.8, 28.9, 32.4, 33.5, 50.4, 101.2, 113.1, 122.0, 123.2, 130.0, 135.4, 146.4, 147.9, 160.6, 162.8, 163.8, 165.0, 196.5. Anal. Calcd for C21H21N3O5: C, 63.79; H, 5.35; N, 10.63. Found: C, 63.50; H, 5.54; N, 10.85.
5-(4-Chlorophenyl)-2,8,8-trimethyl-8,9-dihydro-3H-chromeno[2,3-d]pyrimidine-4,6(5H,7H)-dione (3e)
White powder; yield 87%; mp 316–318°C; IR: ν 3423 (NH), 1663 cm−1 (C=O); 1H NMR: δ 0.97 (s, 3H, CH3), 1.07 (s, 3H, CH3), 1.90–2.40 (m, 5H, CH2 overlapped with CH3), 2.61 (s, 2H, CH2), 4.68 (s, 1H, CH), 7.15–7.45 (m, 4H, HAr), 12.60 (s br., 1H, NH); 13C NMR: δ 21.4, 27.2, 28.9, 31.2, 32.4, 32.7, 50.4, 101.7, 113.7, 128.4, 130.4, 131.4, 143.2, 159.3, 160.4, 162.5, 164.4, 196.4. Anal. Calcd for C20H19ClN2O3: C, 64.78; H, 5.16; N, 7.55. Found: C, 64.59; H, 5.01; N, 7.73.
5-(4-Chlorophenyl)-2-ethyl-8,8-dimethyl-8,9-dihydro-3H-chromeno[2,3-d]pyrimidine-4,6(5H,7H)-dione (3f)
Yellow powder; yield 86%; mp 260–262°C; IR: ν 3441 (NH), 1673 cm−1 (C=O); 1H NMR: δ 0.98 (s, 3H, CH3), 1.07 (s, 3H, CH3), 1.17 (t, 3H, J=7.5 Hz, CH3), 2.22 (ABq, 2H, Δν=47.5 Hz, JAB=16.1 Hz, CH2), 2.50–2.60 (m, 2H, CH2 overlapped with solvent), 2.63 (s, 2H, CH2), 4.68 (s, 1H, CH), 7.24 (d, 2H, J=8.6 Hz, HAr), 7.31 (d, 2H, J=8.6 Hz, HAr), 12.57 (br., 1H, NH); 13C NMR: δ 11.2, 27.3, 27.7, 28.9, 32.4, 32.8, 50.5, 101.9, 113.7, 128.4, 130.5, 131.4, 143.2, 160.6, 162.5, 163.3, 164.4, 196.4. Anal. Calcd for C21H21ClN2O3: C, 65.54; H, 5.50; N, 7.28. Found: C, 65.77; H, 5.31; N, 7.49.
2,8,8-Trimethyl-5-(4-methylphenyl)-8,9-dihydro-3H-chromeno[2,3-d]pyrimidine-4,6(5H,7H)-dione (3g)
Yellow powder; yield 88%; mp 262–265°C; IR: ν 3446 (NH), 1672 cm−1 (C=O); 1H NMR: δ 0.97 (s, 3H, CH3), 1.07 (s, 3H, CH3), 2.12 (d, 1H, J=16.1 Hz, one proton of diastereotopic protons in CH2), 2.22 (s, 3H, CH3), 2.24–2.34 (m, 4H, a doublet for one proton of diastereotopic protons in CH2 overlapped with a singlet for CH3), 2.60 (s, 2H, CH2), 4.65 (s, 1H, CH), 7.03 (d, 2H, J=8.0 Hz, HAr), 7.10 (d, 2H, J=8.0 Hz, HAr), 12.54 (s br., 1H, NH); 13C NMR: δ 14.6, 21.0, 21.4, 27.1, 29.0, 32.4, 32.5, 50.5, 102.3, 114.3, 128.4, 129.0, 135.9, 141.4, 158.9, 160.4, 162.5, 164.0, 196.3. Anal. Calcd for C21H22N2O3: C, 71.98; H, 6.33; N, 7.99. Found: C, 71.70; H, 6.17; N, 8.19.
2-Ethyl-8,8-dimethyl-5-(4-methylphenyl)-8,9-dihydro-3H-chromeno[2,3-d]pyrimidine-4,6(5H,7H)-dione (3h)
White powder; yield 87%; mp 318–320°C; IR: ν 3433 (NH), 1672 cm−1 (C=O); 1H NMR: δ 0.97 (s, 3H, CH3), 1.07 (s, 3H, CH3), 1.16 (t, 3H, J=7.5 Hz, CH3), 2.12 (d, 1H, J=16.1 Hz, one proton of diastereotopic protons in CH2), 2.22 (s, 3H, CH3), 2.29 (d, 1H, J=16.1 Hz, one proton of diastereotopic protons in CH2), 2.50–2.60 (m, 2H, CH2 overlapped with solvent), 2.62 (s, 2H, CH2), 4.66 (s, 1H, CH), 7.03 (d, 2H, J=7.9 Hz, HAr), 7.10 (d, 2H, J=7.9 Hz, HAr), 12.51 (s br., 1H, NH); 13C NMR: δ 11.3, 21.0, 27.2, 27.6, 29.0, 32.4, 32.6, 50.5, 102.5, 114.2, 128.4, 129.0, 135.9, 141.4, 160.5, 162.5, 162.9, 164.1, 196.3. Anal. Calcd for C22H24N2O3: C, 72.50; H, 6.64; N, 7.69. Found: C, 72.73; H, 6.46; N, 7.88.
Acknowledgments
This work was supported by the Islamic Azad University, Mashhad Branch, Iran.
References
[1] Narsimha Reddy, P.; Thirupathi Reddy, Y.; Kanakalingeswara Rao, M.; Rajitha, B. Synthesis and anticancer activity of novel benzimidazole chromenes, thiadiazolyl chromenes under microwave irradiation conditions. Heterocycl. Commun.2003, 9, 647–652.10.1515/HC.2003.9.6.647Search in Google Scholar
[2] Parthiban, A.; Kumaravel, M.; Muthukumaran, J.; Rukkumani, R.; Krishna, R.; Surya Prakash Rao, H. Design, synthesis, in vitro and in silico anti-cancer activity of 4H-chromenes with C4-active methane groups. Med. Chem. Res.2015, 24, 1226–1240.10.1007/s00044-014-1190-ySearch in Google Scholar
[3] Suresh Babu, K.; China Raju, B.; Praveen, B.; Hara Kishore, K.; Suryanarayana Murty, U.; Madhusudana Rao, J. Microwave assisted synthesis and antimicrobial activity of 2,2-dimethyl chromenes. Heterocycl. Commun.2003, 9, 519–526.10.1515/HC.2003.9.5.519Search in Google Scholar
[4] Bingi, C.; Narender Reddy, E.; Chennapuram, M.; Poornachandra, Y.; Kumar, C. G.; Jagadeesh Babu, N.; Atmakur, K. One-pot catalyst free synthesis of novel kojic acid tagged 2-aryl/alkyl substituted-4H-chromenes and evaluation of their antimicrobial and anti-biofilm activities. Bioorg. Med. Chem. Lett.2015, 25, 1915–1919.10.1016/j.bmcl.2015.03.034Search in Google Scholar PubMed
[5] Zhang, D.; Ma, Y.; Liu, Y.; Liu, Z. -P. Synthesis of sulfonylhydrazone- and acylhydrazone-substituted 8-ethoxy-3-nitro-2H-chromenes as potent antiproliferative and apoptosis inducing agents. Arch. Pharm. (Weinheim, Ger.)2014, 347, 576–588.10.1002/ardp.201400082Search in Google Scholar PubMed
[6] Bhat, M. A.; Siddiqui, N.; Khan, S. A. Synthesis of novel 3-(4-acetyl-5H/methyl-5-substituted phenyl-4,5-dihydro- 1,3,4-oxadiazol-2-yl)-2H-chromen-2-ones as potential anticonvulsant agents. Acta Pol. Pharm. Drug Res.2008, 65, 235–239.Search in Google Scholar
[7] Parthiban, A.; Muthukumaran, J.; Manhas, A.; Srivastava, K.; Krishna, R.; Rao, H. S. P. Synthesis, in vitro and in silico antimalarial activity of 7-chloroquinoline and 4H-chromene conjugates. Bioorg. Med. Chem. Lett.2015, 25, 4657–4663.10.1016/j.bmcl.2015.08.030Search in Google Scholar PubMed
[8] Tanna, J. A.; Chaudhary, R. G.; Gandhare, N. V.; Rai, A. R.; Yerpude, S.; Juneja, H. D. Copper nanoparticles catalysed an efficient one-pot multicomponents synthesis of chromenes derivatives and its antibacterial activity. J. Exp. Nanosci.2016, 11, 884–900.10.1080/17458080.2016.1177216Search in Google Scholar
[9] Patrusheva, O. S.; Zarubaev, V. V.; Shtro, A. A.; Orshanskaya, Y. R.; Boldyrev, S. A.; Ilyina, I. V.; Kurbakova, S. Y.; Korchagina, D. V.; Volcho, K. P.; Salakhutdinov, N. F. Anti-influenza activity of monoterpene-derived substituted hexahydro-2H-chromenes. Bioorg. Med. Chem.2016, 24, 5158–5161.10.1016/j.bmc.2016.08.037Search in Google Scholar PubMed
[10] Conti, C.; Proietti Monaco, L.; Desideri, N. Synthesis and anti-rhinovirus activity of novel 3-[2-(pyridinyl)vinyl] substituted-2H-chromenes and -4H-chromen-4-ones. Bioorg. Med. Chem.2014, 22, 1201–1207.10.1016/j.bmc.2013.11.054Search in Google Scholar PubMed
[11] Conti, C.; Proietti Monaco, L.; Desideri, N. 3-Phenylalkyl-2H-chromenes and -chromans as novel rhinovirus infection inhibitors. Bioorg. Med. Chem.2017, 25, 2074–2083.10.1016/j.bmc.2017.02.012Search in Google Scholar PubMed
[12] Gopinath, G.; Sankeshi, V.; Perugu, S.; Alaparthi, M. D.; Bandaru, S.; Pasala, V.K.; Chittineni, P. R.; Krupadanam, G. L. D.; Sagurthi, S. R. Design and synthesis of chiral 2H-chromene-N-imidazolo-amino acid conjugates as aldose reductase inhibitors. Eur. J. Med. Chem.2016, 124, 750–762.10.1016/j.ejmech.2016.08.070Search in Google Scholar PubMed
[13] Cheng, J. F.; Ishikawa, A.; Ono, Y.; Arrhenius, T.; Nadzan, A. Novel chromene derivatives as TNF-α inhibitors. Bioorg. Med. Chem. Lett.2003, 13, 3647–3650.10.1016/j.bmcl.2003.08.025Search in Google Scholar PubMed
[14] Zhao, B. T.; Le, D. D.; Nguyen, P. H.; Ali, M. Y.; Choi, J. -S.; Min, B. S.; Shin, H. M.; Rhee, H. I.; Woo, M. H. PTP1B, α-glucosidase, and DPP-IV inhibitory effects for chromene derivatives from the leaves of Smilax China L. Chem.-Biol. Interact.2016, 253, 27–37.10.1016/j.cbi.2016.04.012Search in Google Scholar PubMed
[15] Perumal, O.; Peddakotla, S. V. K.; Suresh, L.; Chandramouli, G. V. P.; Pydisetty, Y. α-Glucosidase inhibitory activity, molecular docking, QSAR and ADMET properties of novel 2-amino-phenyldiazenyl-4H-chromene derivatives. J. Biomol. Struct. Dyn.2017, 35, 2620–2630.10.1080/07391102.2016.1227278Search in Google Scholar PubMed
[16] Gomes, L. R.; Low, J. N.; Cagide, F.; Chavarria, D.; Borges, F. New insights in the discovery of novel h-MAO-B inhibitors: structural characterization of a series of N-phenyl-4-oxo-4H-chromene-3-carboxamide derivatives. Acta Crystallogr., Sect. E: Crystallogr. Commun.2015, 71, 547–554.10.1107/S2056989015007859Search in Google Scholar PubMed PubMed Central
[17] Barlaam, B.; Cosulich, S.; Degorce, S.; Fitzek, M.; Green, S.; Hancox, U.; Lambert-Van Der Brempt, C.; Lohmann, J. -J.; Maudet, M.; Morgentin, R.; et al. Discovery of a series of 8-(2,3-dihydro-1,4-benzoxazin-4-ylmethyl)-2-morpholino-4-oxo-chromene-6-carboxamides as PI3Kβ/δ inhibitors for the treatment of PTEN-deficient tumours. Bioorg. Med. Chem. Lett.2016, 26, 2318–2323.10.1016/j.bmcl.2016.03.034Search in Google Scholar PubMed
[18] Fallah-Tafti, A.; Tiwari, R.; Shirazi, A. N.; Akbarzadeh, T.; Mandal, D.; Shafiee, A.; Parang, K.; Foroumadi, A. 4-Aryl-4H-chromene-3-carbonitrile derivatives: evaluation of Src kinase inhibitory and anticancer activities. Med. Chem.2011, 7, 466–472.10.2174/157340611796799258Search in Google Scholar PubMed
[19] Guo, Y.; Wang, S. -Q.; Ding, Z. -Q.; Zhou, J.; Ruan, B. -F. Synthesis, characterization and antitumor activity of novel ferrocene bisamide derivatives containing pyrimidine-moiety. J. Organomet. Chem. 2017, 851, 150–159.10.1016/j.jorganchem.2017.09.032Search in Google Scholar
[20] Bhuma, N.; Burade, S. S.; Bagade, A. V.; Kumbhar, N. M.; Kodam, K. M.; Dhavale, D. D. Synthesis and anti-proliferative activity of 3′-deoxy-3′-fluoro-3′-C-hydroxymethyl-pyrimidine and purine nucleosides. Tetrahedron2017, 73, 6157–6163.10.1016/j.tet.2017.09.006Search in Google Scholar
[21] Agarwal, A.; Ramesh; Ashutosh; Goyal, N.; Chauhan, P. M. S.; Gupta, S. Dihydropyrido[2,3-d]pyrimidines as a new class of antileishmanial agents. Bioorg. Med. Chem.2005, 13, 6678–6684.10.1016/j.bmc.2005.07.043Search in Google Scholar PubMed
[22] Jhala, Y. S.; Gahlot, U. S.; Dulawat, S. S.; Verma, B. L. A facile one pot microwave-assisted solid phase synthesis of 2-amino-4, 6-diaryl pyrimidines and their antibacterial activity. Heterocycl. Commun.2006, 12, 253–258.10.1515/HC.2006.12.3-4.253Search in Google Scholar
[23] Chen, Q.; Zhu, X.; Jiang, L.; Yang, L. M.; Fu, G. Synthesis, antifungal activity and CoMFA analysis of novel 1,2,4-triazolo[1,5-a]pyrimidine derivatives. Eur. J. Med. Chem.2008, 43, 595–603.10.1016/j.ejmech.2007.04.021Search in Google Scholar PubMed
[24] Kabeer, S. A.; Reddy, G. R.; Sreelakshmi, P.; Manidhar, D. M.; Reddy, C. S. TiO2-SiO2 Catalyzed eco-friendly synthesis and antioxidant activity of benzopyrano[2,3-d]pyrimidine derivatives. J. Heterocycl. Chem. 2017, 54, 2598–2604.10.1002/jhet.2856Search in Google Scholar
[25] Halawa, A. H.; Elaasser, M. M.; El Kerdawy, A. M.; Abd El-Hady, A. M. A. I.; Emam, H. A.; El-Agrody, A. M. Anticancer activities, molecular docking and structure–activity relationship of novel synthesized 4H-chromene, and 5H-chromeno[2,3-d]pyrimidine candidates. Med. Chem. Res.2017, 26, 2624–2638.10.1007/s00044-017-1961-3Search in Google Scholar
[26] Patel, A. J.; Patel, M. P. Ultrasound promoted L-proline catalyzed facile synthesis and antimicrobial evaluation of 4H-chromeno[2,3-d] pyrimidine derivatives incorporated with quinoline moiety. Indian Drugs2017, 54, 16–23.10.53879/id.54.09.11091Search in Google Scholar
[27] Kamdar, N. R.; Haveliwala, D. D.; Mistry, P. T.; Patel, S. K. Synthesis and evaluation of in vitro antitubercular activity and antimicrobial activity of some novel 4H-chromeno[2,3-d] pyrimidine via 2-amino-4-phenyl-4H-chromene-3-carbonitriles. Med. Chem. Res.2011, 20, 854–864.10.1007/s00044-010-9399-xSearch in Google Scholar
[28] Ameli, S.; Pordel, M.; Davoodnia, A.; Jajarmi, M. Synthesis and antibacterial activity of some new benzo[5,6]chromeno[2,3-d]pyrimidines. Russ. J. Bioorg. Chem.2017, 43, 429–434.10.1134/S1068162017040100Search in Google Scholar
[29] El-Agrody, A. M.; Halawa, A. H.; Fouda, A. M.; Al-Dies, A. -A. M. The anti-proliferative activity of novel 4H-benzo[h]chromenes, 7H-benzo[h]-chromeno[2,3-d]pyrimidines and the structure–activity relationships of the 2-, 3-positions and fused rings at the 2, 3-positions. J. Saudi Chem. Soc.2017, 21, 82–90.10.1016/j.jscs.2016.03.002Search in Google Scholar
[30] Yalagala, K.; Jonnalagadda, S. B.; Maddila, S.; Rana, S.; Maddila, S. N. Novel chromeno[2,3-d]pyrimidines-design, synthesis and antioxidant activity. Lett. Drug Des. Discovery2017, 14, 763–772.10.2174/1570180814666161123143637Search in Google Scholar
[31] Davoodnia, A.; Bakavoli, M.; Pooryaghoobi, N.; Roshani, M. A convenient approach for the synthesis of new substituted isoxazolo[5,4-d] pyrimidin-4(5H)-ones. Heterocycl. Commun.2007, 13, 323–325.10.1515/HC.2007.13.5.323Search in Google Scholar
[32] Davoodnia, A.; Rahimizadeh, M.; Atapour-Mashhad, H.; Tavakoli-Hoseini, N. Investigation into the reaction of 2-amino-4,5-dimethylthiophene-3-carboxamide with iso(and isothio)cyanates under microwave irradiation. Heteroat. Chem.2009, 20, 346–349.10.1002/hc.20557Search in Google Scholar
[33] Davoodnia, A.; Behmadi, H.; Zare-Bidaki, A.; Bakavoli, M.; Tavakoli-Hoseini, N. A facile one-pot synthesis of new thieno[2,3-d]pyrimidine-2,4(1H,3H)-dione derivatives. Chin. Chem. Lett.2007, 18, 1163–1165.10.1016/j.cclet.2007.07.024Search in Google Scholar
[34] Davoodnia, A.; Bakavoli, M.; Bashash, M.; Roshani, M.; Zhiani, R. Synthesis of new 5-arylpyrido[3′,2′:4,5]thieno[2,3-e][1,2,3,4]tetrazolo[1,5-c]pyrimidine derivatives. Turk. J. Chem. 2007, 31, 599–603.Search in Google Scholar
[35] Davoodnia, A.; Roshani, M.; Saleh Nadim, E.; Bakavoli, M.; Tavakoli Hoseini, N. Microwave-assisted synthesis of new pyrimido[4′,5′:4,5]thiazolo[3,2-a] benzimidazol-4(3H)-one derivatives in solvent-free condition. Chin. Chem. Lett.2007, 18, 1327–1330.10.1016/j.cclet.2007.09.004Search in Google Scholar
[36] Davoodnia, A.; Bakavoli, M.; Mohseni, S.; Tavakoli-Hoseini, N. Synthesis of pyrido[3′,2′:4,5]thieno[2,3-e][1,2,4]triazolo[4,3-a]pyrimidin-5(4H)-one derivatives. Monatsh. Chem.2008, 139, 963–965.10.1007/s00706-007-0844-6Search in Google Scholar
[37] Nakhaei, A.; Davoodnia, A.; Yadegarian, S. Nano isopolyoxomolybdate catalyzed biginelli reaction for one-pot synthesis of 3,4-dihydropyrimidin-2(1H)-ones and 3,4-dihydropyrimidin-2(1H)-thiones under solvent-free conditions. Russ. J. Gen. Chem. 2016, 86, 2870–2876.10.1134/S1070363216120537Search in Google Scholar
[38] Vazirimehr, S.; Davoodnia, A.; Nakhaei-Moghaddam, M.; Tavakoli-Hoseini, N. Ultrasonic synthesis, characterization, and antibacterial evaluation of novel heterocycles containing hexahydroquinoline and pyrrole moieties. Heterocycl. Commun. 2017, 23, 65–70.10.1515/hc-2016-0164Search in Google Scholar
[39] Ameli, S.; Davoodnia, A.; Pordel, M.; Behmadi, H. Synthesis of new imino containing tetrahydrochromeno[2,3-d]pyrimidines. J. Heterocycl. Chem. 2017, 54, 1437–1441.10.1002/jhet.2729Search in Google Scholar
[40] Fattahi, M.; Davoodnia, A.; Pordel, M. Efficient one-pot synthesis of hsome new pyrimido[5′,4′:5,6]pyrido[2,3-d]pyrimidines catalyzed by magnetically recyclable Fe3O4 nanoparticles. Russ. J. Gen. Chem. 2017, 87, 863–867.10.1134/S1070363217040326Search in Google Scholar
[41] Ameli, S.; Davoodnia, A.; Pordel, M. Rapid one-pot aspartic acid-promoted synthesis of tetrahydrobenzo[b]pyrans in water. Org. Prep. Proced. Int.2016, 48, 328–336.10.1080/00304948.2016.1194127Search in Google Scholar
[42] Moghaddas, M.; Davoodnia, A. Atom-economy click synthesis of tetrahydrobenzo[b]pyrans using carbon-based solid acid as a novel, highly efficient and reusable heterogeneous catalyst. Res. Chem Intermed.2015, 41, 4373–4386.10.1007/s11164-014-1536-6Search in Google Scholar
[43] Maleki, B.; Ashrafi, S. S. Nano α-Al2O3 supported ammonium dihydrogen phosphate (NH4H2PO4/Al2O3): preparation, characterization and its application as a novel and heterogeneous catalyst for the one-pot synthesis of tetrahydrobenzo[b]pyran and pyrano[2,3-c]pyrazole derivatives. RSC Adv.2014, 4, 42873–42891.10.1002/chin.201512201Search in Google Scholar
[44] Maleki, B.; Eshghi, H.; Barghamadi, M.; Nasiri, N.; Khojastehnezhad, A.; Ashrafi, S. S.; Pourshiani, O. Silica-coated magnetic NiFe2O4 nanoparticles-supported H3PW12O40; synthesis, preparation, and application as an efficient, magnetic, green catalyst for one-pot synthesis of tetrahydrobenzo[b]pyran and pyrano[2,3-c]pyrazole derivatives. Res. Chem. Intermed.2016, 42, 3071–3093.10.1007/s11164-015-2198-8Search in Google Scholar
[45] Maleki, B.; Baghayeri, M.; Ayazi Jannat Abadi, S.; Tayebee, R.; Khojastehnezhad, A. Ultrasound promoted facile one pot synthesis of highly substituted pyran derivatives catalyzed by silica-coated magnetic NiFe2O4 nanoparticle-supported H14[NaP5W30O110] under mild conditions. RSC Adv.2016, 6, 96644–96661.10.1039/C6RA20895ASearch in Google Scholar
[46] Maleki, B.; Nasiri, N.; Tayebee, R.; Khojastehnezhad, A.; Akhlaghi, H. A. Green synthesis of tetrahydrobenzo[b]pyrans, pyrano[2,3-c]pyrazoles and spiro[indoline-3,4′-pyrano[2,3-c]pyrazoles catalyzed by nano-structured diphosphate in water. RSC Adv.2016, 6, 79128–79134.10.1039/C6RA15800ESearch in Google Scholar
©2018 Walter de Gruyter GmbH, Berlin/Boston
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Articles in the same Issue
- Frontmatter
- Review
- Research progress in quinazoline derivatives as multi-target tyrosine kinase inhibitors
- Research Articles
- A bio-inspired approach to proline-derived 2,4-disubstituted oxazoles
- Solvent-free preparation of 3-aryl-2-[(aryl)(arylamino)]methyl-4H-furo[3,2-c]chromen-4-one derivatives using ZnO-ZnAl2O4 nanocomposite as a heterogeneous catalyst
- Synthesis of 4-arylethyl-6-arylpyrimidine-2-thiols through aza-Michael addition/nucleophilic addition/aromatization tandem reactions
- 3-Carboxy-1-sulfopyridin-1-ium chloride ([CPySO3H]+Cl−): an efficient catalyst for one-pot synthesis of hexahydroquinoline-3-carboxamides
- Synthesis of new 3H-chromeno[2,3-d]pyrimidine-4,6(5H,7H)-diones via the tandem intramolecular Pinner/Dimroth rearrangement
- A new synthesis of pyrrolo[3,2-d]pyrimidine derivatives by a one-pot, three-component reaction in the presence of L-proline as an organocatalyst
- Diversity-oriented synthesis of amide derivatives of tricyclic thieno[2,3-d]pyrimidin-4(3H)-ones and evaluation of their influence on melanin synthesis in murine B16 cells
- Synthesis, crystal structure, molecular docking studies and bio-evaluation of some N4-benzyl-substituted isatin- 3-thiosemicarbazones as urease and glycation inhibitors
- Ultrasound-assisted synthesis and antimicrobial activity of tetrazole-based pyrazole and pyrimidine derivatives
Articles in the same Issue
- Frontmatter
- Review
- Research progress in quinazoline derivatives as multi-target tyrosine kinase inhibitors
- Research Articles
- A bio-inspired approach to proline-derived 2,4-disubstituted oxazoles
- Solvent-free preparation of 3-aryl-2-[(aryl)(arylamino)]methyl-4H-furo[3,2-c]chromen-4-one derivatives using ZnO-ZnAl2O4 nanocomposite as a heterogeneous catalyst
- Synthesis of 4-arylethyl-6-arylpyrimidine-2-thiols through aza-Michael addition/nucleophilic addition/aromatization tandem reactions
- 3-Carboxy-1-sulfopyridin-1-ium chloride ([CPySO3H]+Cl−): an efficient catalyst for one-pot synthesis of hexahydroquinoline-3-carboxamides
- Synthesis of new 3H-chromeno[2,3-d]pyrimidine-4,6(5H,7H)-diones via the tandem intramolecular Pinner/Dimroth rearrangement
- A new synthesis of pyrrolo[3,2-d]pyrimidine derivatives by a one-pot, three-component reaction in the presence of L-proline as an organocatalyst
- Diversity-oriented synthesis of amide derivatives of tricyclic thieno[2,3-d]pyrimidin-4(3H)-ones and evaluation of their influence on melanin synthesis in murine B16 cells
- Synthesis, crystal structure, molecular docking studies and bio-evaluation of some N4-benzyl-substituted isatin- 3-thiosemicarbazones as urease and glycation inhibitors
- Ultrasound-assisted synthesis and antimicrobial activity of tetrazole-based pyrazole and pyrimidine derivatives

