Solvent-free microwave-assisted synthesis and biological evaluation of 2,2-dimethylchroman-4-one based benzofurans
-
Dongamanti Ashok
, Rayagiri Suneel Kumar
Abstract
A series of novel chroman-4-one fused benzofurans were synthesized by cyclization of the corresponding chalcones under microwave irradiation. All new compounds were characterized by IR, 1H NMR, 13C NMR and mass spectral data and were evaluated for their in vitro antimicrobial and antioxidant activities.
Introduction
Chroman-4-ones and chromones are used as scaffolds for the development of bioactive compounds. These frameworks are naturally occurring derivatives containing an oxa-pyran ring [1], [2]. The most frequently found chromone-based natural products are 2-aryl substituted chromones (flavonoids) carrying hydroxy and/or methoxy groups on the A and/or B rings [3], [4]. They are constituents of pigments in leaves and are present in a range of food sources such as olive oil, tea, fruits and red wine. The substitution pattern of the chroman-4-one and chromone scaffolds determines their different biological effects. Known effects of these types of compounds are antioxidant [5], [6], antiviral [7], antibacterial activities [8] or kinase inhibition [9], [10]. On the other hand, benzofuran derivatives possess a wide range of biological activities such as antitumor [11], [12], antibacterial [13], antifungal [14], antidepressant [15], analgesic [16] and hypoglycemic [17] activities. Hybrid compounds containing both chroman-4-ones and furan moieties, called furochromanones may exhibit better biological activity. Such compounds may be prepared using microwave assisted organic synthesis (MAOS) [18], [19], [20], [21], [22]. This technique offers simple, clean, fast and efficient synthesis of a large number of organic molecules.
Encouraged by the biological importance of chromanones and benzofurans, in this report, we describe the synthesis of (E)-7,7-dimethyl-2-pivaloyl-3-aryl-6,7-dihydro-5H-furo-[3,2-g]chromen-5-ones 6a–j under microwave irradiation in good yields. The products were evaluated for their antimicrobial and antioxidant activities.
Results and discussion
Chemistry
Synthesis of benzofurans 6a–j was accomplished in two steps as shown below. The starting materials 6-acetyl-7-hydroxy-2,2-dimethylchroman-4-one (2a) and 2,2,8,8,-tetramethyl-2,3,7,8-tetrahydropyrano[3,2-g]chromene-4,6-dione (2b) were prepared as previously described [23] (Scheme 1). The precursor chalcones 4a–j (Scheme 2) were synthesized by the Claisen-Schmidt condensation of 6-acetyl-7-hydroxy-2,2-dimethylchroman-4-one (2a) and substituted aromatic aldehydes 3a–j in the presence of potassium hydroxide in ethanol. The chalcones were subjected to cyclization with 1-bromo-3,3-dimethylbutan-2-one (5) to give the 2,2-dimethylchroman-4-one based benzofurans 6a–j in good yields. All these compounds were thoroughly characterized by spectroscopic techniques and elemental analysis.
![Scheme 1 Synthesis of 7-hydroxy-2,2-dimethylchroman-4-one (2a) and 2,2,8,8,-tetramethyl-2,3,7,8-tetrahydropyrano[3,2-g]chromene-4,6-dione (2b).](/document/doi/10.1515/hc-2016-0147/asset/graphic/j_hc-2016-0147_scheme_001.jpg)
Synthesis of 7-hydroxy-2,2-dimethylchroman-4-one (2a) and 2,2,8,8,-tetramethyl-2,3,7,8-tetrahydropyrano[3,2-g]chromene-4,6-dione (2b).
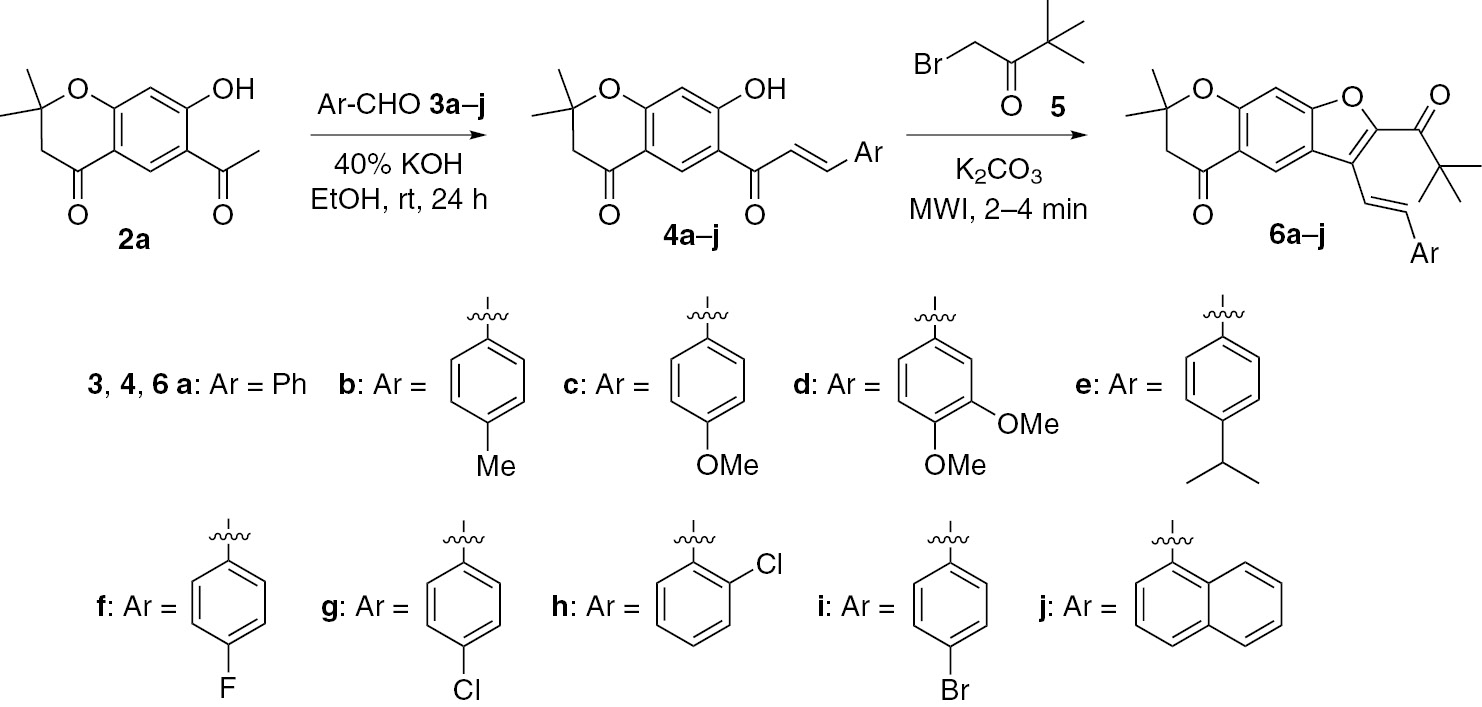
Synthetic route to 7-hydroxy-2, 2-dimethylchroman-4-one derived chalcones 4a–j and benzofurans 6a–j.
Antimicrobial activity
Compounds 6a–j were screened for their in vitro antibacterial activity against two Gram-positive bacterial strains Staphylococcus aureus (ATCC-6538), Bacillus faecalis (ATCC-6633) and two Gram-negative bacterial strains Escherichia coli (ATCC-25922) and Klebsiella pneumonia (ATCC-13883) by the disc diffusion method [24] at concentrations of 20 μg/mL and 40 μg/mL. The zones of inhibition (in mm) were compared with that for the standard drug ciproflaxin. As can be seen from Table 1, among newly synthesized benzofurans, compound 6f (4-fluorophenyl derivative) shows good activity against S. aureus, K. pneumonia and E. coli bacterial strains. The inhibitory efficiencies of compounds 6h and 6i against K. pneumonia are close to that of standard. Compounds with withdrawing groups (4-F, 4-Cl and 4-Br) at the phenyl ring show zones of inhibition that are comparable to that of the standard against all the bacterial strains.
Antimicrobial activity of benzofurans 6a–j by zone of inhibition (mm).
| Cmpd | Bacillus faecalis (ATCC-6633) | Staphylococcus aureus (ATCC-6538) | Klebsiella pneumoniae (ATCC-13883) | Escherichia coli (ATCC-25922) | Aspergillus niger | Fusarium oxysporum | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| 20 μg/mL | 40 μg/mL | 20 μg/mL | 40 μg/mL | 20 μg/mL | 40 μg/mL | 20 μg/mL | 40 μg/mL | 500 μg/mL | 500 μg/mL | |
| 6e | 11.4 | 20.9 | 11.2 | 18.6 | 19.8 | 30.5 | 21.2 | 25.6 | 11.2 | 10.9 |
| 6f | 13.2 | 26.2 | 12.5 | 20.1 | 22.4 | 35.3 | 24.6 | 30.5 | 13.2 | 12.8 |
| 6h | 12.4 | 22.4 | 11.8 | 19.8 | 20.6 | 32.2 | 22.4 | 31.5 | 12.8 | 15.4 |
| 6i | 13.0 | 24.2 | 10.4 | 18.2 | 18.6 | 28.6 | 20.7 | 28.6 | 10.5 | 14.2 |
| Std-1 | 15.2 | 33.4 | 13.2 | 20.2 | 24.5 | 35.8 | 25.5 | 33.5 | – | – |
| Std-2 | – | – | – | – | – | – | – | – | 13.4 | 18.6 |
| Std-3 | – | – | – | – | – | – | – | – | 17.2 | 23.6 |
Std-1, ciprofloxacin; Std-2, amphotericin-B; Std-3, hymexazol.
All synthesized compounds were also screened for their antifungal activity against two pathogenic fungi, Fusarium oxysporum and Aspergillus flavus by the poison plate technique [25] (Table 1). The results of the antifungal screening were compared with the activities of the standard antifungal drugs amphotericin-B and hymexazol. All compounds show moderate activity against the tested fungal strains.
Antioxidant activity
In vitro antioxidant activities of the benzofurans 6a–j were determined using the 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay [22]. The DPPH radical scavenging activity evaluation is a rapid and convenient screening technique. The results are shown in Table 2. As can be seen, the best compounds are 6d and 6c, the radical scavenging abilities of which compare favorably with that of the standard. The remaining compounds show moderate activities.
Antioxidant activity (DPPH inhibition percent) of benzofurans 6a–j.
| Cmpd | DPPH radical scavenging | Cmpd | DPPH radical scavenging |
|---|---|---|---|
| 6a | 55.58±1.85 | 6f | 70.34±1.46 |
| 6b | 60.68±1.36 | 6g | 70.22±1.45 |
| 6c | 78.22±1.25 | 6h | 67.47±1.24 |
| 6d | 80.86±1.18 | 6i | 68.67±1.24 |
| 6e | 65.58±1.84 | 6j | 56.37±1.34 |
| Std | 84.45± 2.42 |
The results are expressed as mean percent of inhibition of three independent measurements.
Conclusion
The synthesized compounds 6a–j were evaluated for their antimicrobial and antioxidant activities. Compounds 6f and 6h show promising antimicrobial activities compared with the standard. Compounds 6c and 6d show better antioxidant activities than the standard.
Experimental
All reactions were monitored by TLC on Merck Kieselgel 60 F524. Visualization was done by UV light irradiation and/or spraying with a solution of a 5% sulfuric acid in ethanol followed by heating. Column chromatography was performed on Silica Gel 60 (60–120 mesh). Melting points were determined in open capillary tubes and are uncorrected. FT-IR spectra were recorded in KBr pellets on a Perkin-Elmer spectrophotometer. 1H NMR and 13C NMR spectra were recorded on a Brucker DRX-400 spectrometer (400 and 100 MHz, respectively) in CDCl3. Mass spectra were recorded using a Jeol SX-102 mass spectrometer.
General procedure for the synthesis of benzofurans 6a–j
A mixture of substituted 2′-hydroxychalcone 4a–i (10.0 mmol), 1-bromo-3,3-dimethylbutan-2-one (15.0 mmol) and potassium carbonate (25.0 mmol) was placed in a quartz tube and inserted into a Teflon vial that was screw capped and subjected to microwave irradiation at 320 W for 2–4 min. After completion of reaction (as indicated by TLC), the mixture was poured into ice water, extracted with dichloromethane (2×30 mL), dried over Na2SO4, and purified by column chromatography eluting with n-hexane/ethyl acetate (9:1).
(E)-7,7-dimethyl-2-pivaloyl-3-styryl-6,7-dihydro-5H-furo-[3,2-g]chromen-5-one (6a)
Pale yellow solid; mp 134–136°C; reaction time 3.5 min; yield 92%; IR: 2928, 1738, 1625, 1475, 1360, 1230, 1008 cm−1; 1H NMR: δ 8.64 (s, 1H, Ar-H5), 8.04 (d, J=16.8 Hz, 1H, Hβ), 7.68–7.63 (m, 2H, Ar-H), 7.55–7.48 (m, 1H, Ar-H), 7.40 (dd, J=10.2 Hz and 4.7 Hz, 2H), 7.33–7.29 (m, 1H, Ar-H), 7.05 (s, 1H, Ar-H8), 2.82 (s, 2H, CH2), 1.51 (s, 6H, 2×CH3), 1.42 (s, 9H, 3×CH3); 13C NMR: δ 198.2, 192.0, 160.1, 158.7, 148.1, 136.9, 135.7, 128.7, 128.5, 127.1, 126.9, 122.5, 120.3, 119.8, 118.4, 100.5, 79.9, 48.9, 44.4, 26.7, 26.4; ESI-MS: 403 (M+H)+. Anal. Calcd for C26H26O4: C, 77.59; H, 6.51. Found: C, 77.54; H, 6.45.
(E)-7,7-dimethyl-3-(4-methylstyryl)-2-pivaloyl-6,7-dihydro-5H-furo-[3,2-g]chromen-5-one (6b)
White solid; mp 158–160°C; reaction time 3.0 min; yield 93%; IR: 2930, 1740, 1624, 1474, 1358, 1229, 1009 cm−1; 1H NMR: δ 8.64 (s, 1H, Ar-H5), 8.01 (d, J=16.8 Hz, 1H, Hβ), 7.53 (dd, J=20.1 Hz and 12.5 Hz, 3H), 7.20 (d, J=8.0 Hz, 2H), 7.04 (s, 1H, Ar-H8), 2.81 (s, 2H, CH2), 2.38 (s, 3H, CH3), 1.51 (s, 6H, 2×CH3), 1.42 (s, 9H, 3×CH3); 13C NMR: δ 198.6, 192.9, 160.6, 152.7, 151.5, 137.6, 134.5, 133.4, 133.2, 128.7, 128.5, 127.4, 122.1, 120.1, 119.1, 100.0, 79.9, 48.5, 44.4, 26.7, 26.4, 21.3; ESI-MS: 417 (M+H)+. Anal. Calcd for C27H28O4: C, 77.86; H, 6.78. Found: C, 77.81; H, 6.72.
(E)-3-(4-methoxylstyryl)-7,7-dimethyl-2-pivaloyl-6,7-dihydro-5H-furo-[3,2-g]chromen-5-one (6c)
Pale yellow solid; mp 166–168°C; reaction time 3.5 min; yield 89%; IR: 2929, 1741, 1625, 1473, 1356, 1230, 1010 cm−1; 1H NMR: δ 8.64 (s, 1H, Ar-H5), 7.94 (d, J=16.8 Hz, 1H, Hβ), 7.60 (d, J=8.7 Hz, 2H, Ar-H), 7.49 (d, J=16.8 Hz, 1H, Hα), 7.04 (s, 1H, Ar-H8), 6.92 (d, J=8.7 Hz, 2H, Ar-H), 3.85 (s, 3H, O-CH3), 2.81 (s, 2H, CH2), 1.51 (s, 6H, 2×CH3), 1.42 (s, 9H, 3×CH3); 13C NMR: δ 198.0, 191.9, 159.8, 157.9, 151.1, 149.7, 134.3, 133.2, 130.2, 129.8, 127.4, 122.6, 120.8, 117.8, 114.1, 100.4, 79.8, 55.8, 48.3, 44.3, 26.7, 26.4; ESI-MS: 433 (M+H)+. Anal. Calcd for C27H28O5: C, 74.98; H, 6.53. Found: C, 74.95; H, 6.50.
(E)-3-(3,4-dimethoxylstyryl)-7,7-dimethyl-2-pivaloyl-6,7-dihydro-5H-furo-[3,2-g]chromen-5-one (6d)
Yellow solid; mp 185–187°C; reaction time 3.5 min; yield 90%; IR: 2928, 1745, 1628, 1478, 1356, 1230, 1005 cm−1; 1H NMR: δ 8.64 (s, 1H, Ar-H5), 7.93 (d, J=16.8 Hz, 1H, Hβ), 7.47 (d, J=16.8 Hz, 1H, Hα), 7.24–7.15 (m, 2H, Ar-H), 7.04 (s, 1H, Ar-H8), 6.89 (d, J=8.3 Hz, 1H, Ar-H), 3.99 (s, 3H, O-CH3), 3.94 (s, 3H, O-CH3), 2.82 (s, 2H, CH2), 1.52 (s, 6H, 2×CH3), 1.42 (s, 9H, 3×CH3); 13C NMR: δ 198.2, 192.1, 160.1, 158.8, 149.7, 149.1, 147.8, 135.7, 130.1, 127.2, 122.6, 120.6, 120.4, 118.3, 117.8, 111.1, 109.3, 100.5, 79.8, 56.0, 48.9, 44.3, 26.7, 26.4; ESI-MS: 463 (M+H)+. Anal. Calcd for C28H30O6: C, 72.71; H, 6.54. Found: C, 72.69; H, 6.50.
(E)-3-(4-isopropylstyryl)-7,7-dimethyl-2-pivaloyl-6,7-dihydro-5H-furo-[3,2-g]chromen-5-one (6e)
Pale yellow solid; mp 128–130°C; reaction time 4.0 min; yield 88%; IR: 2965, 1740, 1695, 1623, 1474, 1359, 1226, 1009 cm−1; 1H NMR: δ 8.64 (s, 1H, Ar-H5), 8.01 (d, J=16.8 Hz, 1H, Hβ), 7.59 (d, J=8.2 Hz, 2H), 7.51 (d, J=16.8 Hz, 1H, Hα), 7.24 (m, 2H, Ar-H), 7.04 (s, 1H, Ar-H8), 2.93 (d, J=13.8, 6.9 Hz, 1H), 2.81 (s, 2H, CH2), 1.51 (s, 6H, 2×CH3), 1.42 (s, 9H, 3×CH3), 1.28 (d, J=6.9 Hz, 6H); 13C NMR: δ 198.1, 192.0, 160.1, 158.8, 149.6, 148.0, 135.7, 134.6, 127.1, 126.8, 122.6, 120.4, 118.9, 118.3, 100.5, 79.8, 48.9, 44.3, 34.0, 26.7, 26.4, 23.9; ESI-MS: 437 (M+H)+. Anal. Calcd for C29H32O4: C, 78.35; H, 7.26. Found: C, 78.31; H, 7.22.
(E)-3-(4-fluorostyryl)-7,7-dimethyl-2-pivaloyl-6,7-dihydro-5H-furo-[3,2-g]chromen-5-one (6f)
Pale yellow solid; mp 132–134°C; reaction time 4.5 min; yield 89%; IR: 2972, 1740, 1625, 1474, 1359, 1225, 1150, 1008 cm−1; 1H NMR: δ 8.61 (s, 1H, Ar-H5), 7.96 (d, J=16.8 Hz, 1H, Hβ), 7.66–7.59 (m, 2H, Ar-H), 7.47 (d, J=16.8 Hz, 1H, Hα), 7.12–7.02 (m, 3H, Ar-H), 2.82 (s, 2H, CH2), 1.51 (s, 6H, 2×CH3), 1.42 (s, 9H, 3×CH3); 13C NMR : δ 198.6, 192.0, 162.1, 160.1, 158.1, 150.2, 135.4, 134.3, 134.1, 130.4, 128.2, 122.2, 120.4, 120.0, 118.4, 115.4, 100.6, 79.9, 48.3, 43.5, 26.7, 26.3; ESI-MS: 421 (M+H)+. Anal. Calcd for C26H25FO4: C, 74.27; H, 5.99. Found: C, 74.23; H, 5.94.
(E)-3-(2-chlorostyryl)-7,7-dimethyl-2-pivaloyl-6,7-dihydro-5H-furo-[3,2-g]chromen-5-one (6g)
White solid; mp 133–135°C; reaction time 4.5 min; yield 89%; IR: 2971, 1741, 1688, 1623, 1470, 1231, 1003; 1H NMR : δ 8.70 (s, 1H, Ar-H5), 7.98 (d, J=16.8 Hz, 1H, Hβ), 7.90 (d, J=16.8 Hz, 1H, Hα), 7.83 (dd, J=7.7 Hz and 1.8 Hz, 1H, Ar-H), 7.42 (dd, J=7.8 Hz and 1.4 Hz, 1H, Ar-H), 7.32–7.27 (m, 1H, Ar-H), 7.24 (dd, J=7.5 Hz and 1.8 Hz, 1H, Ar-H), 7.06 (s, 1H, Ar-H8), 2.81 (s, 2H, CH2), 1.51 (s, 6H, 2×CH3), 1.42 (s, 9H, 3×CH3); 13C NMR: δ 198.8, 192.9, 159.8, 153.4, 150.2, 135.0, 133.4, 133.2, 133.0, 129.9, 129.3, 128.5, 127.8, 126.6, 122.0, 120.2, 117.8, 100.6, 79.9, 48.9, 44.3, 26.7, 26.3; ESI-MS: 437 (M+H)+. Anal. Calcd for C26H25ClO4: C, 71.47; H, 5.77. Found: C, 71.42; H, 5.71.
(E)-3-(4-chlorostyryl)-7,7-dimethyl-2-pivaloyl-6,7-dihydro-5H-furo-[3,2-g]chromen-5-one (6h)
Pale yellow solid; mp 134–136°C; reaction time 4.5 min; yield 89%; IR: 2973, 1740, 1692, 1624, 1472, 1357, 1230, 1006 cm−1; 1H NMR: δ 8.61 (s, 1H, Ar-H5), 8.02 (d, J=16.8 Hz, 1H, Hβ), 7.58 (d, J=8.5 Hz, 2H, Ar-H), 7.45 (d, J=16.8 Hz, 1H), 7.39–7.32 (m, 2H, Ar-H), 7.06 (s, 1H, Ar-H8), 2.82 (s, 2H, CH2), 1.51 (s, 6H, 2×CH3), 1.42 (s, 9H, 3×CH3); 13C NMR: δ 198.3, 192.0, 160.2, 158.7, 148.2, 135.5, 134.2, 134.1, 128.9, 128.2, 126.6, 122.4, 120.4, 120.1, 118.4, 100.6, 79.9, 48.9, 44.4, 26.7, 26.3; ESI-MS: 437 (M+H)+. Anal. Calcd for C26H25ClO4: C, 71.47; H, 5.77. Found: C, 71.42; H, 5.71.
(E)-3-(4-bromostyryl)-7,7-dimethyl-2-pivaloyl-6,7-dihydro-5H-furo-[3,2-g]chromen-5-one (6i)
Pale yellow solid; mp 124–126°C; reaction time 5.0 min; yield 85%; IR: 2972, 1740, 1689, 1623, 1472, 1230, 1004 cm−1; 1H NMR: δ 8.60 (s, 1H, Ar-H5), 8.03 (d, J=16.8 Hz, 1H, Hβ), 7.51 (s, 4H, Ar-H), 7.44 (d, J=16.8 Hz, 1H, Hα), 7.06 (s, 1H, Ar-H8), 2.82 (s, 2H, CH2), 1.51 (s, 6H, 2×CH3), 1.42 (s, 9H, 3×CH3); 13C NMR: δ 198.4, 192.9, 160.2, 158.6, 149.0, 136.5, 134.2, 133.1, 133.0, 129.9, 128.6, 126.6, 122.4, 120.4, 120.0, 117.4, 99.8, 79.9, 48.3, 44.5, 27.7, 26.3; ESI-MS: 481 (M+H)+. Anal. Calcd for C26H25BrO4: C, 64.87; H, 5.23. Found: C, 64.82; H, 5.18.
(E)-7,7-dimethyl-3-(2-(napthalen-1-yl)vinyl)-2-pivaloyl-6,7-dihydro-5H-furo-[3,2-g]chromen-5-one (6j)
Pale yellow solid; mp 194–196°C; reaction time 4.0 min; yield 80%; IR: 2974, 1742, 1691, 1623, 1475, 1228, 1009 cm−1; 1H NMR: δ 8.75 (s, 1H, Ar-H5), 8.28 (dd, J=18.1 Hz and 12.5 Hz, 2H), 8.06 (d, J=16.5 Hz, 1H, Hβ), 7.95–7.81 (m, 3H, Ar-H), 7.66–7.61 (m, 1H, Ar-H), 7.52–7.54 (m, 2H, Ar-H), 7.09 (s, 1H, Ar-H8), 2.83 (s, 2H, CH2), 1.53 (s, 6H, 2×CH3), 1.43 (s, 9H, 3×CH3); 13C NMR: δ 198.1, 191.9, 160.2, 158.8, 148.3, 134.5, 133.7, 132.8, 131.2, 128.9, 128.6, 127.1, 126.5, 125.9, 125.7, 124.4, 123.6, 122.4, 122.4, 120.4, 118.5, 100.6, 79.9, 48.9, 44.3, 26.8, 26.4; ESI-MS: 453 (M+H)+. Anal. Calcd for C30H28O4: C, 79.62; H, 6.24. Found: C, 79.58; H, 6.20.
Biological assays
Antibacterial activity
Compounds 5a–j were screened for their in vitro antibacterial activity against the bacterial cultures S. aureus, B. subtilis (Gram-positive) and E. coli and Klebsiella (Gram-negative) by the disc diffusion method at concentrations of 20 μg/mL and 40 μg/mL. The cultures were grown in nutrient agar media and sub-cultured for log phasic cultures in a liquid nutrient broth medium for zone of inhibition and further sub-cultured onto media in Petri plates. The broth cultures were diluted with a sterilized saline to bring the final size of inoculum approximately to 105–106 CFU/mL. The compounds were dissolved in acetone, DMSO and diethyl ether for biological assays. Diethyl ether was the preferred solvent. The bacterial cultures were placed on the media and incubated at 37°C for 24 h–48 h along with the diluted compounds introduced through discs dipped and placed over the nutrient media. The zones of bacterial growth inhibitions were measured using the diameter of the zone. All the results were expressed as zone of inhibition (ZOI) in mm. The results were compared with the activity of the standard antibiotic ciproflaxin (20 μg/mL and 40 μg/mL). For the disc diffusion method, the diluted test compounds were introduced onto the disc and once the disc was found completely saturated it was immediately transferred on to surface of the medium with bacterial inoculums spread on the plate evenly. The Petri dishes were incubated at 37°C for 24 h. Bioactivity was determined by measuring diameter of the inhibition zone (DIZ) in mm.
Antifungal activity
The antifungal activity of synthesized compounds was tested against two pathogenic fungi, F. oxysporum and A. flavus, by the poison plate technique. Test compounds were dissolved in diethyl ether (10 mL) before mixing with potato dextrose agar medium (PDA, 90 mL). The final concentration of compounds in the medium was maintained at 500 μg/mL. The fungi were incubated in PDA at 25±1°C for 48–72 h to get long mycelium for antifungal assay. The mycelia disks of approximately 0.45 cm in diameter were cut from the PDA medium with a sterilized inoculation needle and inoculated in the center of a PDA plate. The inoculated plates were incubated at 27±1°C for 3 days. Diethyl ether in sterilized distilled water was taken as control, while hymexazole was used as positive control for the treatment. The growth of the fungal colonies was measured on the third day and the data were statistically analyzed. The in vitro inhibition effects of the test compounds on the fungi were calculated by the given formula CV=(A–B)/A, where A represents the diameter of fungi growth on untreated PDA, B represents the diameter of the fungi growth on treated PDA, and CV represents the rate of inhibition.
DPPH radical scavenging
DPPH radical scavenging activity was measured by the method of Cotelle [26] after standardization with some modifications. A mixture containing 0.2 mL of DPPH (100 μm in methanol) and 2.0 mL of the test solution containing the compound (50, 100, 200 μg/mL) was incubated at 37°C for 30 min. Absorbance of the mixture was measured at 517 nm using a Beckman model DU-40 spectrophotometer. The percentage inhibition of DPPH radical was calculated by comparing the result of the test with that of the control (not treated with extract) using the following equation:
Acknowledgments
The authors thank the Head of Department of Chemistry, Osmania University and JNTU, Hyderabad, India and the Managing Director, Richmond Vivek Laboratories, Hyderabad, India, for providing laboratory facilities to carry out the research work. We also thank the Director of Central Facilities for Research and Development (CFRD), Osmania University for providing spectral analysis facilities.
References
[1] Saengchantara, S. T.; Wallace, T. W. Chromanols, chromanones, and chromones. Nat. Prod. Rep.1986, 3, 465–475.10.1039/np9860300465Suche in Google Scholar
[2] Cottiglia, F.; Dhanapal, B.; Sticher, O.; Heilmann, J. New chromanone acids with antibacterial activity from Calophyllum brasiliense. J. Nat. Prod.2004, 67, 537–541.10.1021/np030438nSuche in Google Scholar
[3] Edwards, A. M.; Howell, J. B. L. The chromones: history, chemistry and clinical development. A tribute to the work of Dr R. E. C. Altounyan. Clin. Exp. Allergy2000, 30, 756–774.10.1046/j.1365-2222.2000.00879.xSuche in Google Scholar
[4] Sharma, S. K.; Kumar, S.; Chand, K.; Kathuria, A.; Gupta, A.; Jain, R. An update on natural occurrence and biological activity of chromones. Curr. Med. Chem.2011, 18, 3825–3852.10.2174/092986711803414359Suche in Google Scholar
[5] Heim, K. E.; Tagliaferro, A. R.; Bobilya, D. J. Flavonoid antioxidants: chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem.2002, 13, 572–584.10.1016/S0955-2863(02)00208-5Suche in Google Scholar
[6] Lee, H.; Lee, K.; Jung, J. K.; Cho, J.; Theodorakis, E. A. Synthesis and evaluation of 6-hydroxy-7-methoxy-4chromanone-and chroman-2-carboxamides as antioxidants. Bioorg. Med. Chem. Lett.2005, 15, 2745–2748.10.1016/j.bmcl.2005.03.118Suche in Google Scholar PubMed
[7] Yu, D. L.; Suzuki, M.; Xie, L.; Morris-Natschke, S. L.; Lee, K. H. Recent progress in the development of coumarin derivatives as potent anti-HIV agents. Med. Res. Rev.2003, 23, 322–34510.1002/med.10034Suche in Google Scholar PubMed
[8] Chandler, I. M.; Mcintyre, C. R.; Simpson, T. J. Structural revision and synthesis of Ll-D253-α and related chromanone fungal metabolites. J. Chem. Soc. Perkin Trans. 11992, (18), 2271–2284.10.1039/p19920002271Suche in Google Scholar
[9] Nguyen, T. B.; Lozach, O.; Surpateanu, G.; Wang, Q.; Retailleau, P.; Iorga, B. I.; Meijer, L.; Gueritte, F. Synthesis, biological evaluation, and molecular modelling of natural and unnatural flavonoidal alkaloids, inhibitors of kinases. J. Med. Chem.2012, 55, 2811–2819.10.1021/jm201727wSuche in Google Scholar PubMed
[10] Dyrager, C.; Mollers, L. N.; Kjall, L. K.; Alao, J. P.; Diner, P.; Wallner, F. K.; Sunnerhagen, P.; Grotli, M. Design, synthesis, and biological evaluation of chromone-based p38 MAP kinase inhibitors. J. Med. Chem.2011, 54, 7427–7431.10.1021/jm200818jSuche in Google Scholar PubMed
[11] Hayakawa, I.; Shioya, R.; Agatsuma, T.; Furukawa, H.; Naruto S.; Sugano, Y. 4-Hydroxy-3-methyl-6-phenylbenzofuran-2-carboxylic acid ethyl ester derivatives as potent anti-tumor agents. Bioorg. Med. Chem. Lett.2004, 14, 455–458.10.1016/j.bmcl.2003.10.039Suche in Google Scholar
[12] Galal, S. A.; Abd El All; Abdullah, M. M.; Amira S.; EL- Diwani H. I. Synthesis of potent antitumor and antiviral benzofuran derivatives. Bioorg. Med. Chem. Lett.2009, 19, 2420–2428.10.1016/j.bmcl.2009.03.069Suche in Google Scholar
[13] Kirilmis, C.; Ahmedzade, M.; Süleyman, S.; Koca, M.; Kizirgil, A. Synthesis and antimicrobial activity of some novel derivatives of benzofuran: Part 2. The synthesis and antimicrobial activity of some novel 1-(1-benzofuran-2-yl)-2-mesitylethanone derivatives. Eur. J. Med. Chem.2008, 43, 300–308.10.1016/j.ejmech.2007.03.023Suche in Google Scholar
[14] Aslam, S. N.; Stevenson, P. C.; Phythian, S. J.; Veitch N. C.; Hall, D. R. Synthesis of cicerfuran, an antifungal benzofuran, and some related analogs. Tetrahedron2006, 62, 4214–4226.10.1016/j.tet.2006.02.015Suche in Google Scholar
[15] Malik, W. U.; Mahesh V. K.; Raishighani, M. Synthesis of some benzofuranopyrazoles and benzofuranopyrimidines, two new heterocyclic systems. Ind. J. Chem.1971, 9, 655–657.Suche in Google Scholar
[16] Fry, D. J.; Ficken E. G.; Burrows. R. W. Cyanine dyes for photographic emulsions. British Patent 1,68,495, May 14, 1969.Suche in Google Scholar
[17] Brady, B. A.; Kennedy, J. A.; Sullivan, W. I. Configuration of aurones. Tetrahedron1973, 29, 359–362.10.1016/S0040-4020(01)93302-2Suche in Google Scholar
[18] Ashok, D.; Mohan Gandhi, D.; Vikas Kumar, A.; Srinivas, G. Microwave-assisted synthesis and antimicrobial evaluation of novel pyrazolines. Chem. Heterocycl. Compd. 2015, 51, 872–882.10.1007/s10593-015-1790-6Suche in Google Scholar
[19] Ashok, D.; Ganesh, A.; Vijaya Lakshmi, B.; Ravi, S. One pot multicomponent microwave and ultrasound assisted synthesis and antimicrobial activity of 2-(2-ethoxy-5-substituted indol-3-ylidene)-1-arylethanones. Russ. J. Gen. Chem.2015, 85, 2141–2142.10.1134/S1070363215090194Suche in Google Scholar
[20] Ashok, D.; Vijaya Lakshmi, B.; Ravi, S.; Ganesh, A.; Shaik, A. Microwave-assisted synthesis of 10-aryl-4-methyl-2-oxo-8-phenyl-2,8-dihydropyrano[2,3-f]chromene-9-carbaldehydes by Suzuki coupling and their antimicrobial activity. Chem. Heterocycl. Compd. 2015, 51, 462–466.10.1007/s10593-015-1719-0Suche in Google Scholar
[21] Ashok, D.; Srinivas, G.; Vikas Kumar, A.; Mohan Gandhi, D.; Srinivas Reddy, M. Facile ionic liquid-mediated, microwave assisted green synthesis, and antioxidant studies of novel Indolin-2-one annulated spirochromanone conjugates. Russ. J. Gen. Chem.2015, 85, 708–712.10.1134/S1070363215030305Suche in Google Scholar
[22] Ashok, D.; Vijaya Lakshmi, B.; Ravi, S.; Ganesh, A. Microwave-assisted synthesis of some new coumarin-pyrazoline hybrids and their antimicrobial activity. J. Serb. Chem. Soc.2014, 80, 305–313.10.2298/JSC140021101ASuche in Google Scholar
[23] Ashok, D.; Suneel Kumar, R.; Mohan Gandhi, D.; Jayashree, A. Synthesis of novel 2,4,6-trisubstituted pyrimidine derivatives and their in vitro antimicrobial activity. Russ. J. Gen. Chem.2016, 86, 1396–1404.10.1134/S1070363216060268Suche in Google Scholar
[24] Song, B. A.; Zhang, H. P.; Wang, H.; Yang, S.; Jin, L. H.; Hu, D. Y.; Pang, L. L.; Xue, W. Synthesis and antiviral activity of novel chiral cyanoacrylate derivatives. J. Agric. Food Chem. 2005, 53, 7886–7891.10.1021/jf051050wSuche in Google Scholar
[25] Song, S. Q.; Zhou, L. G.; Li, D.; Tang, D.; Li, J. Q.; Jiang, W. B. Antifungal activity of five plants from Xinjiang. Nat. Prod. Res. Dev.2004, 16, 157–159.Suche in Google Scholar
[26] Cotelle, N.; Bemier, J. L.; Catteau, J. P.; Pommery, J.; Wallet, J. C.; Gaydou, E. M. Antioxidant properties of hydroxy-flavones. Free Radic. Biol. Med.1996, 20, 35–43.10.1016/0891-5849(95)02014-4Suche in Google Scholar
©2016 Walter de Gruyter GmbH, Berlin/Boston
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Artikel in diesem Heft
- Frontmatter
- Research Articles
- Pyrimidinethione as a building block in heterocyclic synthesis: synthesis of pyrano[2,3-d]pyrimidine, chromeno[2,3-d]pyrimidine, pyrido[3′,2′:5,6]pyrano[2,3-b]pyridine, and pyrimido[5′,4′:5,6]pyrano[2,3-d]pyrimidine derivatives
- C1-Substituted N-tert-butoxycarbonyl-5-syn-tert-butyldimethylsilyloxymethyl-2-azabicyclo[2.1.1]hexanes as conformationally constrained β-amino acid precursors
- Synthesis and characterization of 1,3,4-thiadiazole-2,5-dithio crown ethers
- Oxidative reaction of 2-aminopyridine-3-sulfonyl chlorides with tertiary amines
- New 8-substituted BODIPY-based chromophores: synthesis, optical and electrochemical properties
- Heterocyclization of 5,6-disubstituted 3-alkenyl-2-thioxothieno[2,3-d]pyrimidin-4-one with p-alkoxyphenyltellurium trichloride
- Synthesis and biological evaluation of 4-(2′,4′-difluorobiphenyl-4-yl)-6-arylpyrimidin- 2-amine derivatives
- Synthesis and antimicrobial properties of cycloheptyl substituted benzimidazolium salts and their silver(I) carbene complexes
- Solvent-free microwave-assisted synthesis and biological evaluation of 2,2-dimethylchroman-4-one based benzofurans
Artikel in diesem Heft
- Frontmatter
- Research Articles
- Pyrimidinethione as a building block in heterocyclic synthesis: synthesis of pyrano[2,3-d]pyrimidine, chromeno[2,3-d]pyrimidine, pyrido[3′,2′:5,6]pyrano[2,3-b]pyridine, and pyrimido[5′,4′:5,6]pyrano[2,3-d]pyrimidine derivatives
- C1-Substituted N-tert-butoxycarbonyl-5-syn-tert-butyldimethylsilyloxymethyl-2-azabicyclo[2.1.1]hexanes as conformationally constrained β-amino acid precursors
- Synthesis and characterization of 1,3,4-thiadiazole-2,5-dithio crown ethers
- Oxidative reaction of 2-aminopyridine-3-sulfonyl chlorides with tertiary amines
- New 8-substituted BODIPY-based chromophores: synthesis, optical and electrochemical properties
- Heterocyclization of 5,6-disubstituted 3-alkenyl-2-thioxothieno[2,3-d]pyrimidin-4-one with p-alkoxyphenyltellurium trichloride
- Synthesis and biological evaluation of 4-(2′,4′-difluorobiphenyl-4-yl)-6-arylpyrimidin- 2-amine derivatives
- Synthesis and antimicrobial properties of cycloheptyl substituted benzimidazolium salts and their silver(I) carbene complexes
- Solvent-free microwave-assisted synthesis and biological evaluation of 2,2-dimethylchroman-4-one based benzofurans

