Abstract
The reaction of 2-allyl(propargyl)thioquinolin-3-carbaldehyde with halogens (Br or I) results in formation of 1-halogenomethyl(halogenomethylidene)-4-formyl-1,2-dihydrothiazolo[3,2-a]-quinolinium trihalogenide. In the case of the propargylic derivative the process is stereoselective.
Introduction
Derivatives of fused quinolines possess diverse biological properties such as antibacterial, antifungal [1], [2], [3], [4], [5], [6], anti-inflammatory [7], antitubercular [8] and anticonvulsant [9] activity. Considering these observations, it was envisaged to synthesize new quinoline derivatives containing a fused thiazole ring. In recent years, heteroannulation processes based on electrophilic halocyclization have produced various heterocycles including furans [10], [11], [12], [13], pyrroles [10], [14], selenophenes [15], pyrazoles [16], piperazines [17], imidazothiazoles [18], imidazotriazines [19], thiazolo(oxazolo)thienopyrimidines [20], [21], [22], [23], thiazolopyrazolopyrimidines [24], [25], [26], [27] and thiazolotriazoles [28]. Halogenoheterocyclization of unsaturated methallyl thioethers of quinoline has been described [28], [29], [30], [31], [32], [33], [34], [35], [36]. In continuation of these studies we now present halogenoheterocyclization of 2-allylthio and 2-propargylthio substituted quinolin-3-carbaldehydes 2 and 6.
Results and discussion
Compound 2 was obtained by alkylation of 3-formylquinolin-2-thione (1) with allyl bromide in DMF in the presence of KOH [37]. Bromine and iodine were used as electrophilic agents for halogenoheterocyclization. Halogenation was carried out in chloroform with a two-fold excess of halogen to give the respective 1-halogenomethyl-2,3-dihydrothiazolo[3,2-a]quinolinium trihalogenides 3 and 4. The monobromide 5 was obtained after treatment of the tribromide 3 with acetone (Scheme 1).
Compounds 3 and 4 were extensively characterized by elemental analysis, 1H NMR, 13C NMR, COSY, NOESY, and by heteronuclear correlation methods HMQC and HMBC.
1H NMR spectrum of compound 3 is fully consistent with the proposed structure and the proton assignments were obtained by analysis of two-dimensional spectra COSY and NOESY. Analysis of the COSY spectrum cross peaks gave the scheme of correlations shown in Figure 1. These assignments are fully consistent with the analysis of the NOESY spectrum of 3 (Figure 2).
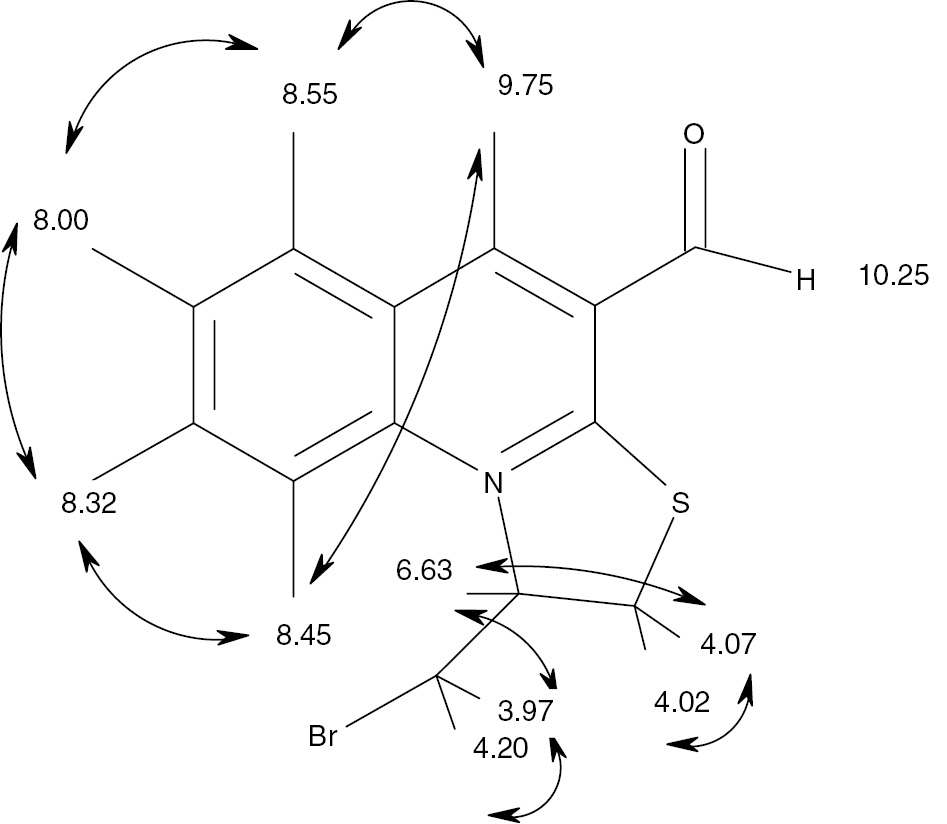
Correlations in the COSY spectrum of compound 3.
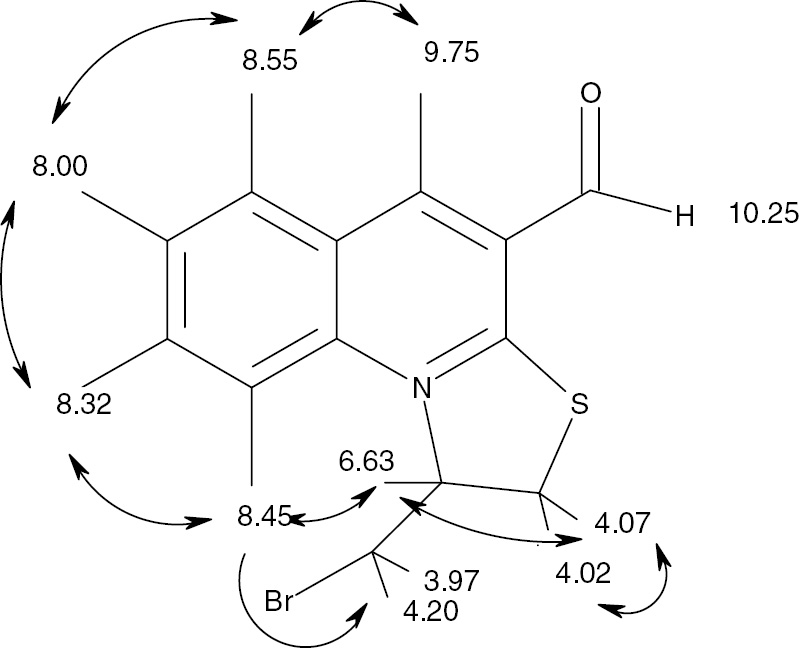
Correlations in the NOESY spectrum of compound 3.
The heteronuclear correlations in the HMQC and HMBC spectra were measured for full assimilation of signals in spectral data of compound 3. Table 1 provides a complete list of observed correlations and these correlations are shown graphically in Figure 3.
Heteronuclear 1H-13C correlations for compound 3.
| Signal in 1H NMR spectrum, δ | The cross-peaks in 13C NMR spectrum | |
|---|---|---|
| HMQC | HMBCa | |
| 10.25 | 190.00 | 165.40; 126.64; 152.91 s |
| 9.75 | 152.91 | 165.40; 138.63; 133.24; 126.64 |
| 8.55 | 133.24 | 152.91; 139.13; 126.57; 119.49s; |
| 8.45 | 119.49 | 152.91s; 139.13; 133.24s; 129.93; 126.57; |
| 8.32 | 139.13 | 138.63; 133.24; 129.93s; 126.57s; 119.49s |
| 7.99 | 129.93 | 139.13; 133.24s; 126.57; 119.49 |
| 6.63 | 66.93 | 165.40 |
| 4.20 | 34.90 | 66.93; 32.82 |
| 4.07 | 32.82 | 66.93; 34.90; |
| 4.02 | 32.82 | 66.93; 34.90 |
| 3.97 | 34.90 | 66.93; 32.82 |
aCorrelation of low intensity.
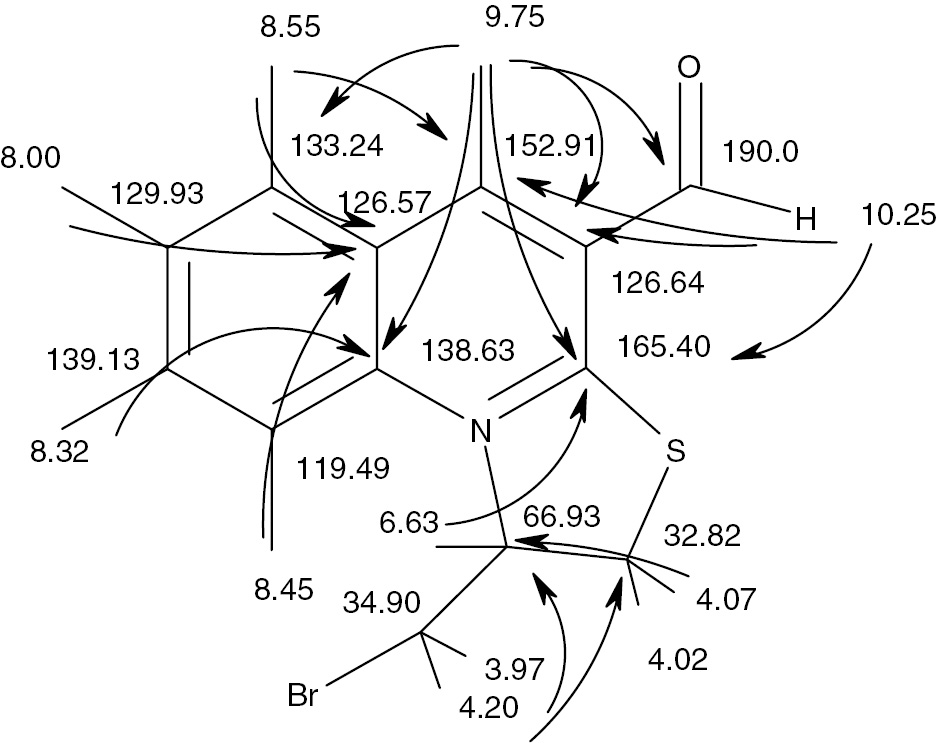
Heteronuclear correlations of compound 3. Similar results were obtained for the iodo derivative 4.
In principle, halogenocyclization involving a propargylic substituent as unsaturated nucleophilic moiety in a quinoline system may result in the formation of two geometrical isomers. In our previous studies [31] we have found that the process of halogenocyclization of a similar propargyl thioether is stereoselective, however, the geometric configuration of the resulting product has not been established. In this work, halogenoheterocyclization of 7-methyl-2-propargylthioquinolin-3-carbaldehyde 6 [31] (Scheme 2), was carried out. 1H NMR and 13C NMR spectral data were used to establish structure of the synthesized compounds 7, 8. The location of the halogen atom at the exocyclic double bond and the predominant conformation in solution of the aldehyde group are the main structural features which required additional investigation. This matter was addressed by using homonuclear overhauser effect (NOE) and the results for compound 7 are shown in Figure 4.
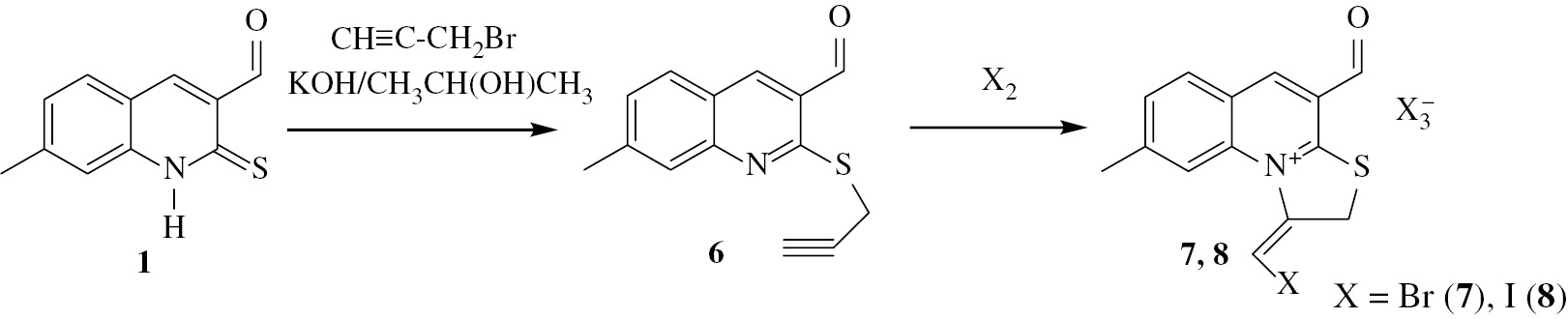
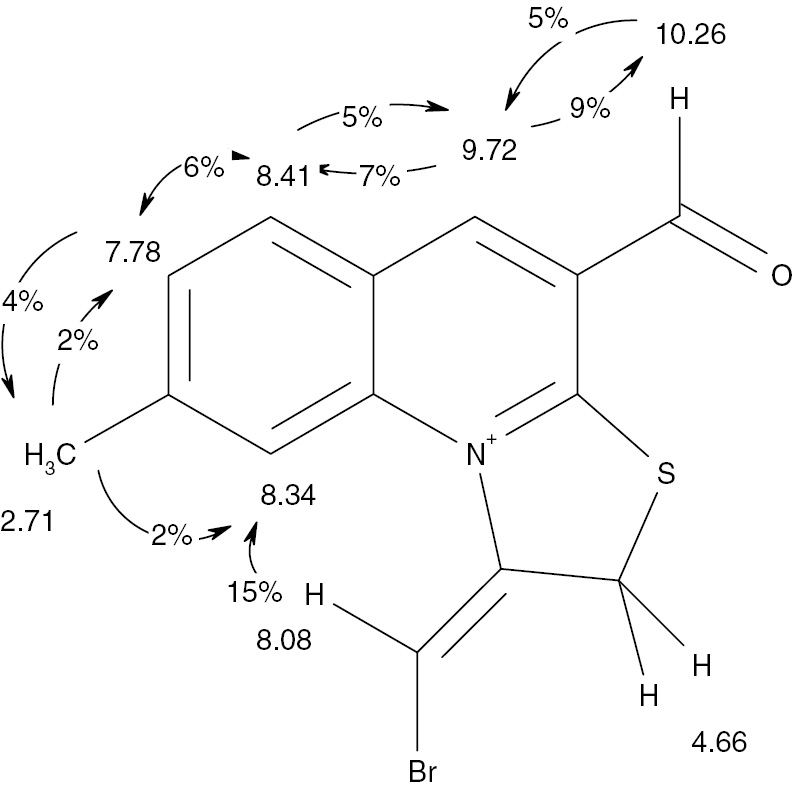
Correlations of NOE for compound 7.
The large NOE value for protons with chemical shifts at 8.34 ppm and 8.08 ppm indicates that the bromine atom in the olefin moiety has the E configuration relative to the thiazolium moiety. The large NOE value between the signals of the aldehyde proton and the aromatic proton with chemical shift of 9.72 ppm shows that the aldehyde group has s-syn orientation relative to the pyridinium moiety. Similar values of the chemical shifts for products 7 and 8 indicate identical stereochemical features in the products of bromination and iodination.
Conclusions
Heterocyclization of 2-allyl(propargyl)thioquinolin-3-carbaldehyde by reaction with halogens (Br and I) was investigated in detail.
Experimental
1H NMR (400 MHz) and 13C NMR (100 MHz) spectra were recorded in (CD3)2SO on a Varian Mercury-400 instrument. 2D-NOESY and COSY experiments were carried out for the compounds 3, 4, 7, 8 in (CD3)2SO on the same instrument. Melting points were determined on a Stuart SMP30 instrument. Elemental analyses were performed on an Elementar Vario MICRO cube analyzer. All reagents were obtained from commercial suppliers and used without any further purification. Anhydrous solvents were prepared according to standard methods. Compounds 1 [38], 2 [37] and 6 [31] were synthesized as previously described. The Rf values were obtained using silica gel plates.
1-Bromomethyl-4-formyl-1,2-dihydro[1,8]thiazolo[3,2-a]quinolinium tribromide (3)
A solution of bromine (7.2 mmol) in chloroform (7 mL) was added to a solution of allyl thioether 2 (3.6 mmol) in chloroform (15 mL) under constant stirring. After 5 h, the precipitated yellow solid was filtered and washed with chloroform; yield 71%; mp 132–133°C; Rf 0.81 (ethanol/hexane/diethyl ether, 1:2:3); 1H NMR: δ 3.97 (d, J=10.4 Hz, 1H), 4.02 (t, J=7.6 Hz, 1H), 4.07 (d, J=6.4 Hz, 1H), 4.20 (t, J=10.4 Hz, 1H), 6.63 (m, 1H), 8.00 (t, J=8.0 Hz, 1H), 8.32 (t, J=8.0 Hz, 1H), 8.45 (d, J=8.0 Hz, 1H), 8.55 (d, J=8.0 Hz, 1H), 9.75 (s, 1H), 10.25 (s, 1H); 13C NMR: δ 32.8, 34.9, 66.9, 119.5, 126.6, 126.6, 129.9, 133.2, 138.6, 139.1, 152.9, 165.4, 190.0. Anal. Calcd for C13H11Br4NOS: N, 2.55; Br, 58.23. Found: N, 2.48; Br 57.13.
1-Bromomethyl-4-formyl-1,2-dihydro[1,3]thiazolo[3,2-a]quinolinium bromide (5)
A solution of tribromide 3 (0.001 mol) in acetone (10 mL) was slowly concentrated to give a crystalline residue of 5; mp 245–247°C. Anal. Calcd for C13H11Br2NOS: N, 3.60; Br, 41.07. Found: N, 3.49; Br, 40.28.
1-Iodomethyl-4-formyl-1,2-dihydro[1,3]thiazolo[3,2-a]quinolinium triiodide (4)
A solution of allyl thioether 2 (1.8 mmol) in chloroform (15 mL) was stirred and slowly treated with a solution of iodine (3.6 mmol) in chloroform (20 mL). The mixture was stirred for 5 h and left for a day. The resultant precipitate of 4 was filtered off and washed with chloroform; yield 83%; mp 127–128°C; Rf 0.7 (ethanol/hexane/diethyl ether, 1: 2: 3); 1H NMR: δ 3.66 (d, J=10.5 Hz, 1H), 3.75 (t, J=8.5 Hz, 1H), 3.92 (d, J=12.3 Hz, 1H), 4.15 (t, J=10.5 Hz, 1H), 6.43 (m, 1H), 7.99 (t, J=6.9 Hz, 1H), 8.33 (t, J=6.9 Hz, 1H), 8.38 (d, J=8.0 Hz, 1H), 8.56 (d, J=8.0 Hz, 1H), 9.73 (s, 1H), 10.26 (s, 1H). Anal. Calcd for C13H11I4NOS: N, 1.90; I, 68.93. Found: N, 1.85; I, 67.48.
1-Bromomethylidene-7-methyl-4-formyl-1,2-dihydro[1,3]thiazolo[3,2-a]quinolinium tribromide (7)
To a solution of propargylic thioether 6 (0.62 mmol) in chloroform (15 mL) was added under constant stirring a solution of bromine (1.2 mmol) in chloroform (7 mL). After 5 h, the precipitated yellow solid of 7 was filtered and washed with chloroform; yield 73%; mp 178–179°C; Rf 0.80 (ethanol/hexane/diethyl ether, 1: 2: 3); 1H NMR: δ 2.71 (s, 3H), 4.66 (s, 2H), 7.78 (d, J=8.0 Hz, 1H), 8.08 (s, 1H), 8.34 (s, 1H), 8.41 (d, J=8.0 Hz, 1H), 9.72 (s, 1H), 10.26 (s, 1H); 13C NMR: δ 23.4, 36.4, 111.8, 119.8, 125.4, 125.5, 131.8, 133.0, 138.7, 140.1, 151.5, 152.2, 165.5, 189.5. Anal. Calcd for C14H11Br4NOS: N, 2.50; Br, 56.98. Found: N, 2.41; Br, 56.25.
1-Iodomethylidene-7-methyl-4-formyl-1,2-dihydro[1,3]thiazolo[3,2-a]quinolinium triiodide (8)
To a solution of propargylic thioether 6 (0.33 mmol) in chloroform (15 mL) was added under constant stirring a solution of iodine (0.66 mmol) in chloroform (15 mL). The mixture was stirred for 5 h and left for a day. The precipitate was filtered off and washed with chloroform; yield 81%; mp 210–211°C; Rf 0.72 (ethanol/hexane/diethyl ether, 1: 2: 3); 1H NMR: δ 2.73 (s, 3H), 4.64 (s, 2H), 7.80 (d, J=8.0 Hz, 1H), 8.18 (s, 1H), 8.32 (s, 1H), 8.40 (d, J=8.0 Hz, 1H), 9.64 (s, 1H), 10.28 (s, 1H); 13C NMR: δ 23.6, 36.1, 112.0, 119.6, 125.4, 125.5, 131.6, 133.0, 138.7, 140.1, 151.5, 152.2, 165.5, 189.6. Anal. Calcd for C14H11I4NOS: N, 1.87; I, 67.78. Found: N, 1.85; I, 66.48.
Acknowledgments
The authors are grateful to Alexander Turov (Taras Shevchenko National University of Kyiv) for assistance in conducting spectral studies.
References
[1] Chu, D.; Fernandes, P.; Pernet, A. Synthesis and biological activity of benzothiazolo[3,2-a]quinolone antibacterial agents. J. Med. Chem.1986, 29, 1531–1534.10.1021/jm00158a037Search in Google Scholar PubMed
[2] Chu, D.; Fernandes, P.; MaleczkaJr, R.; Nordeen, C.; Pernet, A. Synthesis and structure-activity relationship of 1-aryl-6,8-difluoroquinolone antibacterial agents. J. Med. Chem.1987, 30, 504–509.10.1021/jm00386a011Search in Google Scholar PubMed
[3] Chu, D. T. W.; Fernandes, P. B.; Claiborne, A.; Pihuleac, E.; Nordeen, C.; Maleczka Jr, R.; Pernet, A. Synthesis and structure-activity relationships of novel arylfluoroquinolone antibacterial agents. J. Med. Chem.1985, 28, 1558–1564.10.1021/jm00149a003Search in Google Scholar PubMed
[4] Cooper, C.; Klock, P.; Chu, D.; Fernandes, P. The synthesis and antibacterial activities of quinolones containing five- and six-membered heterocyclic substituents at the 7-position. J. Med. Chem.1990, 33, 1246–1252.10.1021/jm00166a025Search in Google Scholar PubMed
[5] Mitscher, L.; Sharma, P.; Chu, D.; Shen, L.; Pernet, A. Chiral DNA gyrase inhibitors. 1. Synthesis and antimicrobial activity of the enantiomers of 6-fluoro-7-(1-piperazinyl)-1-(2-trans-phenylcyclopropyl)-1,4-dihydro-4-oxoquinoline-3-carboxylic acid. J. Med. Chem.1986, 29, 2044–2047.10.1021/jm00160a042Search in Google Scholar PubMed
[6] Cooper, C.; Klock, P.; Chu, D.; Hardy, D.; Swanson, R.; Plattner, J. Preparation and in vitro and in vivo evaluation of quinolones with selective activity against Gram-positive organisms. J. Med. Chem.1992, 35, 1392–1398.10.1021/jm00086a007Search in Google Scholar PubMed
[7] Ukrainets, I. V.; Mospanova, E. V.; Davidenko, A. A.; Tkach, A. A.; Gorokhova, O. V. 4-Hydroxy-2-quinolones. 179. Synthesis, structure, and anti-inflammatory activity of 4-hydroxy-1-methyl-2-oxo-1,2-dihydroquinolin-3-ylacetic acid and its derivatives. Chem. Heterocycl. Comp.2010, 46, 947–956.10.1007/s10593-010-0607-xSearch in Google Scholar
[8] Ukrainets, I. V.; Taran, S. G.; Gorokhova, O. V.; Taran, E. A.; Jaradat, N. A.; Petukhova, I. Yu. 4-Hydroxy-2-quinolones. 40. Synthesis and biological properties of anilides of 1H-2-oxo-4-hydroxyquinoline-3-carboxylic acid. Chem. Heterocycl. Comp.2000, 36, 166–169.10.1007/BF02283545Search in Google Scholar
[9] Ukrainets, I. V.; Gorokhova, O. V.; Taran, S. G.; Bezuglyi, P. A.; Turov, A. V. 2-Car-bethoxymethyl-4H-3,1-benzoxazin-4-one 6. Synthesis of some new 3-acylamino-4-oxaquinazolin-2-yl-acetic acid benzylamides as possible anticonvulsants. Chem. Heterocycl. Comp.1994, 30, 208–212.10.1007/BF01165014Search in Google Scholar
[10] Godoi, B.; Schumacher, R. F.; Zeni, G. Synthesis of heterocycles via electrophilic cyclization of alkynes containing heteroatom. Chem Rev. 2011, 111, 2937–2980.10.1021/cr100214dSearch in Google Scholar PubMed
[11] Windmon, N.; Dragojlovic, V. Phase-vanishing halolactonization of neat substrates. Beilstein J. Org. Chem. 2008, 4, 29–32.10.3762/bjoc.4.29Search in Google Scholar PubMed PubMed Central
[12] Raffa, G.; Balme, G.; Monterio, N. Direct access to fully substituted 3-formyl-4-iodofurans through iodocyclization of α-alkynyl β-alkoxy enones. Eur. J. Org. Chem.2013, 9, 105–110.10.1002/ejoc.201201203Search in Google Scholar
[13] He, Y.; Pu, Y.; Shao, B.; J. Yan. Novel catalytic bromolactonization of alkenoic acids using iodobenzene and oxone. J. Heterocycl. Chem.2011, 48, 695–698.10.1002/jhet.617Search in Google Scholar
[14] Spina, R.; Colacino, E.; Gabriele, B.; Salerno, G.; Martinez, J.; Lamaty, F. Preparation of enantioenriched iodinated pyrrolinones by iodocyclization of a-amino-ynones. Org. Biomol. Chem.2012, 10, 9085–9089.10.1039/c2ob26427gSearch in Google Scholar PubMed
[15] Gai, R. M.; Schumacher, R. F.; Back, D. F.; Zeni, G. Regioselective formation of tetrahydroselenophenes via 5-exo-dig-cyclization of 1-butylseleno-4-alkynes. Org. Lett. 2012, 14, 6072–6075.10.1021/ol302919bSearch in Google Scholar PubMed
[16] Zora, M.; Kivrak, A.; Yazici, C. Synthesis of pyrazoles via electrophilic cyclization. J. Org. Chem.2011, 76, 6726–6742.10.1021/jo201119eSearch in Google Scholar PubMed
[17] Gulevskaya, A. V.; Lazarevich, R. Yu.; Pozharskii, A. F. Electrophilic cyclizations of 2,3-dialkynylquinoxalines and 1,2-dialkynylbenzenes: a comparative study. Tetrahedron2013, 69, 910–917.10.1016/j.tet.2012.10.098Search in Google Scholar
[18] Jasiński, M.; Mlostoń, G.; Heimgartner, H. Synthesis of 2,3-dihydroimidazo[2,1-b]thiazole derivatives via cyclization of N-allylimidazoline-2-thiones. J. Heterocycl. Chem. 2010, 47, 1287–1293.10.1002/jhet.469Search in Google Scholar
[19] Krasnova, L. B.; Hein, J. E.; Fokin, V. V. Synthesis of 7-Aza-5-deazapurine Analogues via Copper(I)-Catalyzed Hydroamination of Alkynes and 1-Iodoalkynes. J. Org. Chem. 2010, 75, 8662–8665.10.1021/jo1016376Search in Google Scholar PubMed PubMed Central
[20] Slivka, Mar. V.; Krivovjaz, A. A.; Slivka, M. V.; Lendel, V. G. Stereoselective synthesis of (E)-halogenomethylidene[1,3]thiazolo[3,2-a]thieno[3,2-e]pyrimidinium and analogous [1,3]oxazolo[3,2-a]thieno[3,2-e]pyrimidinium halogenides from 3-N-substituted 2-propargylthio(oxy-)thieno-[2,3-d]pyrimidin-4-ones. Heterocycl. Commun. 2013, 19, 189–193.10.1515/hc-2013-0036Search in Google Scholar
[21] Nesterenko, A. M.; Vas’kevich, R. I.; Zborovskii, Yu. L.; Staninets, V. I. Reactions of 3-allyl-4-oxothieno [2,3-d] pyrimidin-2-yl disulfides with iodine. Russ. Chem. Bull.2005, 54, 2582–2585.10.1007/s11172-006-0159-5Search in Google Scholar
[22] Khripak, S. M.; Plesha, M. V.; Slivka, M. V.; Yakubets, V. I.; Krivovyaz, A. A. Syntesis and reactivity of 1-bromomethyl-5-oxo-4-phenyl-1,2,4,5,6,7,8,9-octahydrobenzo [4,5]thieno [3, 2-e][1, 3]oxazolo [3,2-a]-pyrimidin-11-ium bromides. Russ. J. Org. Chem. 2004, 40, 1705–1706.10.1007/s11178-005-0086-1Search in Google Scholar
[23] Wippich, P.; Gutschow, M.; Leistner, S. Regioselective preparation of 1-(bromomethyl)-5H-thiazolo[3,2-a]quinazolin-5-ones and analogous 5H-thieno[3,2-e]thiazolo[3,2-a]pyrimidin-5-ones from fused 2-(alkenylthio)pyrimidin-4-ones. Synthesis2000, 5, 714–718.10.1055/s-2000-6390Search in Google Scholar
[24] Onisko, M. Yu.; Svalyavin, O. V.; Lendel, V. G. Synthesis and halogenation of allylthioethers of pyrazolo[3,4-d]pyrimidine. Chem. Heterocycl. Compd. 2007, 4, 602–605.10.1002/chin.200752157Search in Google Scholar
[25] Onysko, M. Yu.; Svalyavin, O. V.; Turov, A. V.; Lendel, V. G. Synthesis and halogenation of propargyl pyrazolo-[3,4-d]pyrimidine thioether. Chem. Heterocycl. Compd.2008, 7, 1085–1089.10.1007/s10593-008-0123-4Search in Google Scholar
[26] Onysko, M. Yu.; Svalyavin, O. V.; Lendel, V. G. Synthesis of alkyl thioethers of pyrazolo[3,4-d]pyrimidine. Chem. Heterocycl. Compd.2009, 7, 1044–1046.10.1007/s10593-009-0352-1Search in Google Scholar
[27] Svalyavin, O. V.; Onysko, M. Yu.; Turov, A. V.; Vlasenko, Uy. G.; Lendel, V. G. Peculiar electrophilic heterocyclization of 5-allyl-6-thioxopyrazolo[3,4-d]pyrimidin-4-one. Chem. Heterocycl. Comp.2013, 3, 526–531.10.1002/chin.201349179Search in Google Scholar
[28] Slivka, M.; Korol, N.; Rusyn, I.; Lendel, V. Synthesis of [1,3]thiazolo[3,2-b][1,2,4]triazol-7-ium and [1,2,4]triazolo[5,1-b][1,3]thiazin-4-ium salts via regioselective electrophilic cyclization of 3-S-alkenylthio-4H-1,2,4-triazoles. Heterocycl. Commun. 2015, 21, 397–401.10.1515/hc-2015-0158Search in Google Scholar
[29] Kim, D.; Vershinina, E. Synthesis and properties of thiazolo- and oxazolo-[3,2-a]quinolinium systems and their hydrogenated derivatives (review). Chem. Heterocycl. Comp.2014,50, 911–915.10.1007/s10593-014-1546-8Search in Google Scholar
[30] Onysko, M. Yu.; Lendel, V. G. Haloheterocyclization of 2-allyl(propargyl)oxyquinoline-3-carbaldehydes. Chem. Heterocycl. Comp.2007, 43, 1020–1023.10.1007/s10593-007-0159-xSearch in Google Scholar
[31] Onysko, M. Yu.; Lendel, V. G. Haloheterocyclization of 2-methallyl(propargyl)-thioquinoline-3-carbaldehydes. Chem. Heterocycl. Comp.2009, 45, 853–857.10.1007/s10593-009-0349-9Search in Google Scholar
[32] Kim, D.; Vershinina, E. Synthesis of the thiazolo[3,2-a]quinolinium system. Chem. Heterocycl. Comp.2010, 46, 773–774.10.1007/s10593-010-0585-zSearch in Google Scholar
[33] Kim, D. Synthesis and halocyclization of 2-alkenylthioquinolines. Chem. Heterocycl. Comp.2008, 44, 1355–1358.10.1007/s10593-009-0195-9Search in Google Scholar
[34] Bartashevich, E.; Yushina, E.; Vershinina, E.; Slepukhin, P.; Kim, D. Complex structure tri- and polyiodides of iodocyclization products of 2-allylthioquinoline. J. Struct. Chem.2014, 55, 112–124.10.1134/S0022476614010181Search in Google Scholar
[35] Litvinov, V. P.; Sharanin, Yu. A.; Apenöva, E.; Shestopalov, A. M.; Mortikov, V. Yu.; Nesterov, V. N.; Shklover, V. E.; Struchkov, Yu. T. Condensed pyridines. 6. Synthesis and strucutre of adamantyl-, cyclopropyl- and alkyl-substituted 3-halomethyl-2,3-dihydro-8-cyanothiazolo[3,2-a]pyridinium salts and their oxazolo and selenazolo derivatives. Chem. Heterocycl. Comp. 1987, 23, 574–582.10.1007/BF00476391Search in Google Scholar
[36] Vershinina, E.; Kim, D.; Slepuhin, P. Tandem reactions in the iodination of 2-(2-bromoallyl)thioquinoline. Chem. Heterocycl. Comp.2011, 46, 1415–1417.10.1007/s10593-011-0684-5Search in Google Scholar
[37] Prabhuswamy, B.; Ambekar Sarwottam, Y. Synthesis of 3a,4-dihydro-8-substituted-3H-isoxazolo [c-4,3]thiapyrano[5,6-3,2]quinolines. Synth. Commun. 1999, 20, 3477–3485.10.1080/00397919908085980Search in Google Scholar
[38] Meth-Cohn, O.; Narine, B.; Tarnowski, B.; Hayes, R.; Keyzad, A.; Rhouati, S.; Robinson, A. A versatile new synthesis of quinolines and related fused pyridines. Part 9. Synthetic application of the 2-chloroquinoline-3-carbaldehydes. J. Chem. Soc. Perkin Trans.1981, 1, 2509–2517.10.1039/p19810002509Search in Google Scholar
©2016 Walter de Gruyter GmbH, Berlin/Boston
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Articles in the same Issue
- Frontmatter
- Preliminary Communications
- Efficient synthesis of 4-amino-2,6-dichloropyridine and its derivatives
- Reactions of 3-arylmethylene-3H-furan(pyrrol)-2-ones with azomethine ylide: synthesis of substituted azaspirononenes
- Research Articles
- Microwave-assisted one-pot synthesis and antimicrobial evaluation of 2-(1-phenyl-3-(2-thienyl)-1H-pyrazol-4-yl)chroman-4-one derivatives
- Structural characterization of copper complexes with chiral 1,2,4-triazine-oxazoline ligands
- Synthesis of metallophthalocyanines with four oxy-2,2-diphenylacetic acid substituents and their structural and electronic properties
- Polymeric (anion-π)n interactions in crystals of 2-(2,4,6-trioxo-[1,3,5]triazinan-1-yl)ethylammonium iodide
- 5-(N-Ethylcarbazol-3-yl)thiophene-2-carbaldehyde (ECTC): a novel fluorescent sensor for ferric ion
- Novel 5,6,7,8-tetrahydroimidazo[2′,1′:2,3]thiazolo[5,4-c]pyridine derivatives
- Halogenoheterocyclization of 2-(allylthio)quinolin-3-carbaldehyde and 2-(propargylthio)quinolin-3-carbaldehyde
- Convenient synthesis of the functionalized 1′,3′-dihydrospiro[cyclopentane-1,2′-inden]-2-enes via a three-component reaction
- Synthesis of spiro[pyrazole-4,8′-pyrazolo [3,4-f]quinolin]-5(1H)-ones by the reaction of aldehydes with 1H-indazol-6-amine and 1H-pyrazol-5(4H)-one
Articles in the same Issue
- Frontmatter
- Preliminary Communications
- Efficient synthesis of 4-amino-2,6-dichloropyridine and its derivatives
- Reactions of 3-arylmethylene-3H-furan(pyrrol)-2-ones with azomethine ylide: synthesis of substituted azaspirononenes
- Research Articles
- Microwave-assisted one-pot synthesis and antimicrobial evaluation of 2-(1-phenyl-3-(2-thienyl)-1H-pyrazol-4-yl)chroman-4-one derivatives
- Structural characterization of copper complexes with chiral 1,2,4-triazine-oxazoline ligands
- Synthesis of metallophthalocyanines with four oxy-2,2-diphenylacetic acid substituents and their structural and electronic properties
- Polymeric (anion-π)n interactions in crystals of 2-(2,4,6-trioxo-[1,3,5]triazinan-1-yl)ethylammonium iodide
- 5-(N-Ethylcarbazol-3-yl)thiophene-2-carbaldehyde (ECTC): a novel fluorescent sensor for ferric ion
- Novel 5,6,7,8-tetrahydroimidazo[2′,1′:2,3]thiazolo[5,4-c]pyridine derivatives
- Halogenoheterocyclization of 2-(allylthio)quinolin-3-carbaldehyde and 2-(propargylthio)quinolin-3-carbaldehyde
- Convenient synthesis of the functionalized 1′,3′-dihydrospiro[cyclopentane-1,2′-inden]-2-enes via a three-component reaction
- Synthesis of spiro[pyrazole-4,8′-pyrazolo [3,4-f]quinolin]-5(1H)-ones by the reaction of aldehydes with 1H-indazol-6-amine and 1H-pyrazol-5(4H)-one

