Three-component one-pot synthesis of dihydrochromeno[4,3-b]pyrazolo[4,3-e]pyridines
-
Seyed Esmail Sadat-Ebrahimi
Abstract
A series of dihydrochromeno[4,3-b]pyrazolo[4,3-e]pyridines has been synthesized using a simple and efficient one-pot, three-component procedure. The products were obtained in good yields in the presence of heterogeneous nanoporous acid catalyst (SBA-Pr-SO3H).
Introduction
Pyrazoles and fused pyrazoles are well-recognized central units in medicine and therapeutics [1]. Among the various fused systems, pyrazolopyridines show promising biological activities as antibacterial [2], antitumor [3], antiviral [4] and anti-inflammatory [5] agents, and antagonists of corticotropin-releasing factor 1 (CRF1) [6], chemokine receptor type 1 (CCR1) [7] and dopamine D3 receptors antagonists [8]. They are also known to be cholesterol forming [9], acetyl-CoA carboxylase (ACC) [10], HIV reverse transcriptase [11], phosphodiesterase 3/4 (PDE3/4) cyclin-dependent 1 (CDK1) [12], and B-Raf kinase inhibitors [13]. Several methods have been devised for the synthesis of substituted pyrazolopyridines [14], [15], [16], [17], [18], [19], [20]. Among them multi-component reactions (MCRs) [21], [22], [23], [24], [25] are of increasing importance because of their convergent, atom-economical and productive nature.
MCRs [26], [27] constitute an important pathway for one-pot construction of polycyclic compounds. This valuable feature has made the MCR chemistry a powerful procedure. Heterogeneous catalysts are often used to resolve growing concerns about environmental issues. In this context, Santa Barbara Amorphous (SBA-15) [28], in which various functional groups could be covalently bound to its mesostructured silica surface, has gained considerable attention. Sulfonic acid functionalized SBA-15 (SBA-Pr-SO3H) [29] with high acid strength could be regarded as a replacement for conventional homogenous acidic catalysts suffering from various drawbacks, mainly their toxicity and hazards to humans. Considering environmental and industrial aspects, sulfonic acid functionalized nanoporous silica (SBA-Pr-SO3H) has emerged as an important catalyst in an efficient construction of complex frameworks. In continuation of our interest toward developing new pathways for the synthesis of heterocyclic compounds [30], [31], [32], herein we report the catalytic synthesis of novel compounds incorporating coumarin and pyrazolopyridine moieties.
Results and discussion
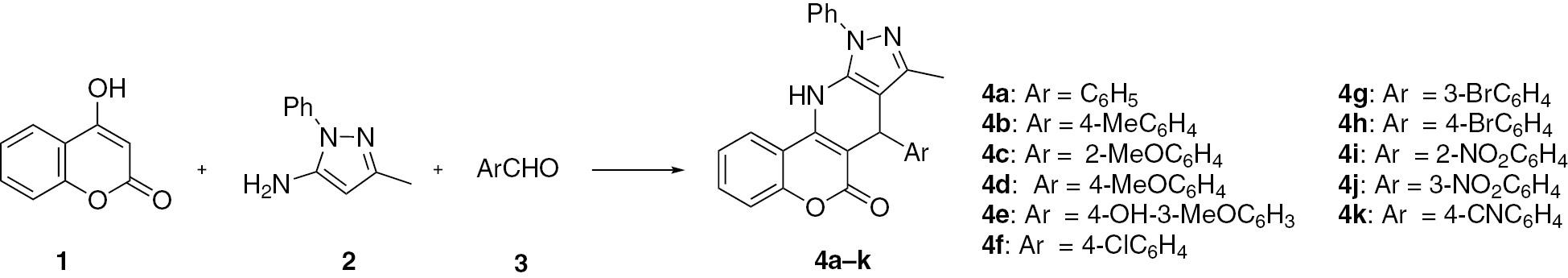
The reaction of 4-hydroxycoumarin (1), 3-methyl-1-phenyl-1H-pyrazol-5-amine (2) and benzaldehyde (3a) was selected as a model reaction to establish the appropriate conditions (Scheme 1). The reaction was investigated by utilizing 5 mol% SBA-Pr-SO3H, prepared according to the literature [33], in different solvents at reflux temperature (including ethanol, water, ethanol/water and methanol). The highest yield of 83% of product 4a was obtained for the reaction conducted in ethanol under reflux for 2 h. No improvement in the isolated yield was observed with increasing amounts of the catalyst, up to 20 mol% and no product 4a was observed in the absence of the catalyst. Heating the mixture under reflux in acetic acid furnished product 4a in a comparable yield, but after a long reaction time (18 h). Additional dihydrochromeno[4,3-b]pyrazolo [4,3-e]pyridines 4b–k were obtained in good yields under similar conditions, as shown in Scheme 1. No significant substituent effects were observed for electron-donating groups Me and OMe or electron-withdrawing groups including Cl, Br, NO2 and CN.
Conclusion
Pyrazolo[4,3-e]pyridines 4a–k were synthesized in good yields using our straightforward, one-pot and multi-component procedure.
Experimental
All chemicals were purchased from Merck and Fluka and used without further purification. Melting points were measured with a Kofler hot stage apparatus and are uncorrected. 1H NMR (500 MHz) and 13C NMR (125 MHz) spectra were recorded on a Bruker FT-500 spectrometer in DMSO-d6, using tetramethylsilane as an internal standard. IR spectra were recorded on a Shimadzu 470 spectrophotometer in KBr disks. Mass spectra were obtained using an Agilent Technology mass spectrometer operating at an ionization potential of 70 eV. Elemental analysis was performed using an Elemental Analysen system, GmbH VarioELCHNS. The synthesis of SBA-15 and SBA-Pr-SO3H was performed as described in the literature [33].
General synthesis of compounds 4a–k
A mixture of 4-hydroxycoumarin (1 mmol), an aldehyde (1 mmol), 3-methyl-1-phenyl-1H-pyrazol-5-amine (1 mmol) and 5 mol% SBA-SO3H in ethanol (5 mL) was stirred and heated under reflux. Upon completion, which was indicated by TLC analysis, the mixture was filtered off and the filtrate was cooled to room temperature. The precipitate of 4a–k was collected and purified by silica gel chromatography eluting with petroleum ether/ethyl acetate (8:2). The catalyst was washed with water and acetone. After drying under a reduced pressure, the catalyst could be reused several times without the loss of activity.
8-Methyl-7,10-diphenyl-10,11-dihydrochromeno[4,3-b]pyrazolo[4,3-e]pyridin-6(7H)-one (4a)
The product was obtained within 2 h in 83% yield as a pale yellow solid; mp 221–222°C; IR: 3400, 1698, 1631, 1535, 1455, 1024, 748, 701 cm-1; 1H NMR: δ 1.93 (s, 3H), 5.19 (s, 1H), 7.15 (t, 1H, J = 7.3 Hz), 7.25–7.30 (m, 4H), 7.35 (d, 1H, J = 8.0 Hz), 7.38–7.43 (m, 2H), 7.57 (t, 2H, J = 7.5 Hz), 7.62 (t, 1H, J = 7.3 Hz), 7.68 (d, 2H, J = 7.0 Hz), 8.06 (d, 1H, J = 8.0 Hz), 9.95 (s, NH); 13C NMR: δ 11.9, 37.3, 101.4, 103.9, 113.9, 116.4, 122.5, 123.6, 123.7, 126.2, 126.6, 127.8, 128.0, 129.3, 131.7, 136.0, 138.7, 143.8, 145.8, 146.0, 152.0, 160.4; MS: m/z 405 ([M]+, 26), 328 (100), 284 (60), 77 (41%). Anal. Calcd for C26H19N3O2: C, 77.02; H, 4.72; N, 10.36. Found: C, 76.94; H, 4.59; N, 10.48.
8-Methyl-10-phenyl-7-(p-tolyl)-10,11-dihydrochromeno[4,3-b]pyrazolo[4,3-e]pyridin-6(7H)-one (4b)
The product was obtained within 1.5 h in 80% yield as a pale yellow solid; mp 154–156°C; IR: 3348, 1690, 1627, 1551, 1440, 1011, 819 cm-1; 1H NMR: δ 1.93 (s, 3H), 3.69 (s, 3H), 5.13 (s, 1H), 6.82 (d, 2H, J = 8.5 Hz), 7.18 (d, 2H, J = 8.5 Hz), 7.35 (d, 1H, J = 8.0 Hz), 7.38–7.42 (m, 2H), 7.57 (t, 2H, J = 8.0 Hz), 7.62 (t, 1H, J = 7.5 Hz), 7.67 (d, 2H, J = 7.5 Hz), 8.04 (d, 1H, J = 8.0 Hz), 9.94 (s, NH); 13C NMR: δ 11.9, 20.9, 36.4, 101.4, 104.6, 113.7, 114.0, 116.4, 122.8, 123.1, 123.7, 126.2, 128.8, 129.6, 131.3, 136.0, 138.3, 138.7, 143.5, 145.6, 151.3, 157.6, 160.0. Anal. Calcd for C27H21N3O2: C, 77.31; H, 5.05; N, 10.02. Found: C, 77.21; H, 4.96; N, 10.06.
7-(2-Methoxyphenyl)-8-methyl-10-phenyl-10,11-dihydrochromeno[4,3-b]pyrazolo[4,3-e]pyridin-6(7H)-one (4c)
The product was obtained within 2.5 h in 81% yield as a pale yellow solid; mp 202°C; IR: 3236, 1681, 1631, 1536, 1458, 1027, 755, 698 cm-1; 1H NMR: δ 1.93 (s, 3H), 3.78 (s, 3H), 5.50 (s, 1H), 6.82 (t, 1H, J = 7.5 Hz), 6.94 (d, 1H, J = 8.0 Hz), 7.10–7.15 (m, 2H), 7.33 (d, 1H, J = 8.5 Hz), 7.36–7.41 (m, 2H), 7.56 (t, 2H, J = 7.5 Hz), 7.61 (t, 1H, J = 7.5 Hz), 7.65 (d, 2H, J = 8.5 Hz), 8.04 (d, 1H, J = 8.5 Hz), 9.83 (s, NH); 13C NMR: δ 11.7, 31.4, 55.5, 95.4, 101.1, 103.7, 111.3, 114.0, 116.3, 120.4, 122.3, 123.4, 123.5, 126.4, 127.4, 129.2, 129.3, 131.5, 134.2, 136.2, 138.8, 144.6, 145.6, 152.0, 159.8. Anal. Calcd for C27H21N3O3: C, 74.47; H, 4.86; N, 9.65. Found: C, 74.50; H, 4.92; N, 9.71.
7-(4-Methoxyphenyl)-8-methyl-10-phenyl-10,11-dihydrochromeno [4,3-b]pyrazolo[4,3-e]pyridin-6(7H)-one (4d)
The product was obtained within 2 h in 79% yield as a pale yellow solid; mp 196–198°C; IR: 3244, 1711, 1628, 1539, 1457, 1027, 841, 758, 689 cm-1; 1H NMR: δ 1.94 (s, 3H), 3.69 (s, 3H), 5.14 (s, 1H), 6.82 (d, 2H, J = 7.5 Hz), 7.19 (d, 2H, J = 7.5 Hz), 7.34 (d, 1H, J = 8.5 Hz), 7.41 (t, 2H, J = 7.5 Hz), 7.55–7.62 (m, 3H), 7.68 (d, 2H, J = 8.5 Hz), 8.05 (d, 1H, J = 7.5 Hz), 9.89 (s, NH); 13C NMR: δ 11.9, 36.4, 54.9, 101.7, 104.1, 113.4, 113.9, 116.4, 122.5, 123.5, 123.6, 126.5, 128.8, 129.3, 131.6, 135.9, 138.3, 138.7, 143.5, 145.8, 151.9, 157.6, 160.4; MS: m/z 435 ([M]+, 25), 328 (100), 107 (51), 251 (41), 77 (32%). Anal. Calcd for C27H21N3O3: C, 74.47; H, 4.86; N, 9.65. Found: C, 74.56; H, 4.92; N, 9.54.
7-(4-Hydroxy-3-methoxyphenyl)-8-methyl-10-phenyl-10,11-dihydrochromeno[4,3-b]pyrazolo[4,3-e]pyridin-6(7H)-one (4e)
The product was obtained within 2 h in 82% yield as a pale yellow solid; mp 230–232°C; IR: 3351, 1683, 1630, 1541, 1382, 741 cm-1; 1H NMR: δ 1.99 (s, 3H), 3.71 (s, 3H), 5.10 (s, 1H), 6.58 (d, 1H, J = 8.0 Hz), 6.65 (d, 1H, J = 7.3 Hz), 6.91 (s, 1H), 7.35 (d, 1H, J = 8.0 Hz), 7.38–7.42 (m, 2H), 7.57 (t, 2H, J = 7.5 Hz), 7.61 (t, 1H, J = 7.5 Hz), 7.68 (d, 2H, J = 8.0 Hz), 8.04 (d, 1H, J = 8.0 Hz), 8.72 (s, 1H), 9.91 (s, NH); 13C NMR: δ 12.0, 36.7, 55.7, 101.8, 104.3, 112.5, 114.0, 115.3, 116.4, 120.1, 122.4, 123.6, 126.5, 129.3, 131.6, 135.9, 137.4, 138.8, 143.5, 144.9, 145.8, 146.9, 151.9, 160.5. Anal. Calcd for C27H21N3O4: C, 71.83; H, 4.69; N, 9.31. Found: C, 71.74; H, 4.58; N, 9.40.
7-(4-Chlorophenyl)-8-methyl-10-phenyl-10,11-dihydrochromeno[4,3-b]pyrazolo[4,3-e]pyridin-6(7H)-one (4f)
The product was obtained within 3 h in 75% yield as a pale yellow solid; mp 236–237°C; IR: 3189, 1665, 1623, 1530, 1457, 1047, 837, 753 cm-1; 1H NMR: δ 1.93 (s, 3H), 5.22 (s, 1H), 7.32–7.43 (m, 7H), 7.58 (t, 2H, J = 7.5 Hz), 7.63 (t, 1H, J = 7.8 Hz), 7.68 (d, 2H, J = 8.5 Hz), 8.06 (d, 1H, J = 8.0 Hz), 10.02 (s, NH); 13C NMR: δ 11.9, 36.8, 100.9, 103.3, 113.8, 116.4, 122.6, 123.6, 123.7, 126.7, 128.0, 129.3, 129.7, 130.7, 131.8, 136.0, 138.6, 144.0, 144.9, 145.8, 152.0, 160.4. Anal. Calcd for C26H18ClN3O2: C, 70.99; H, 4.12; N, 9.55. Found: C, 70.88; H, 4.21; N, 9.49.
7-(3-Bromophenyl)-8-methyl-10-phenyl-10,11-dihydrochromeno[4,3-b]pyrazolo[4,3-e]pyridin-6(7H)-one (4g)
The product was obtained within 3.5 h in 72% yield as a pale yellow solid; mp 166–168°C; IR: 3230, 1677, 1628, 1534, 1459, 1045, 756, 687 cm-1; 1H NMR: δ 1.94 (s, 3H), 5.23 (s, 1H), 7.25 (t, 1H, J = 7.5 Hz), 7.30 (d, 1H, J = 7.3 Hz), 7.34–7.44 (m, 4H), 7.46 (s, 1H), 7.58 (d, 2H, J = 7.8 Hz), 7.64 (t, 1H, J = 7.5 Hz), 7.69 (d, 2H, J = 8.0 Hz), 8.06 (d, 1H, J = 7.8 Hz), 10.02 (s, NH); 13C NMR: δ 11.9, 37.2, 100.7, 103.2, 113.8, 116.3, 116.5, 121.4, 122.6, 123.1 (2C), 126.7, 127.1, 129.2, 130.3, 131.9, 132.6, 1360, 138.6, 144.1, 145.8, 148.6, 152.0, 160.4. Anal. Calcd for C26H18BrN3O2: C, 64.47; H, 3.75; N, 8.68. Found: C, 64.39; H, 3.82; N, 8.58.
7-(4-Bromophenyl)-8-methyl-10-phenyl-10,11-dihydrochromeno[4,3-b]pyrazolo[4,3-e]pyridin-6(7H)-one (4h)
The product was obtained within 3 h in 78% yield as a pale yellow solid; mp 177–179°C; IR: 3230, 1677, 1628, 1534, 1459, 1045, 756, 687 cm-1; 1H NMR: δ 1.93 (s, 3H), 5.20 (s, 1H), 7.25 (d, 2H, J = 8.5 Hz), 7.36 (d, 1H, J = 8.0 Hz), 7.38–7.43 (m, 2H), 7.45 (d, 2H, J = 8.5 Hz,), 7.57 (t, 2H, J = 7.5 Hz), 7.63 (t, 1H, J = 7.5 Hz), 7.67 (d, 2H, J = 8.5 Hz), 8.05 (d, 1H, J = 8.0 Hz), 10.01 (s, NH); 13C NMR: δ 11.9, 36.9, 100.8, 103.3, 113.8, 116.5, 119.2, 122.6, 123.6, 123.7, 126.7, 129.3, 130.1, 130.9, 131.9, 136.0, 138.6, 144.0, 145.4, 145.8, 152.0, 160.4. Anal. Calcd for C26H18BrN3O2: C, 64.47; H, 3.75; N, 8.68. Found: C, 64.58; H, 3.69; N, 8.58.
8-Methyl-7-(2-nitrophenyl)-10-phenyl-10,11-dihydrochromeno[4,3-b]pyrazolo[4,3-e]pyridin-6(7H)-one (4i)
The product was obtained within 3.5 h in 71% yield as a pale yellow solid; mp 189–191°C; IR: 3339, 1652, 1640, 1559, 1370, 731, 682 cm-1; 1H NMR: δ 1.96 (s, 3H), 5.93 (s, 1H), 7.31 (d, J = 8.0 Hz, 1H), 7.38–7.44 (m, 4H), 7.56–7.62 (m, 4H), 7.71 (d, 2H, J = 8.0 Hz), 7.83 (d, 1H, J = 8.5 Hz), 8.05 (d, 1H, J = 8.0 Hz), 10.09 (s, NH); 13C NMR: δ 12.0, 31.9, 101.0, 102.0, 113.6, 116.5, 120.6, 122.8, 123.1, 124.5, 126.7, 127.4, 129.3, 130.2, 131.6, 133.1, 134.3, 137.0, 139.7, 143.8, 146.0, 148.8, 152.0, 160.5; MS: m/z 450 ([M]+, 19), 328 (100), 284 (42), 122 (23), 77 (54%). Anal. Calcd for C26H18N4O4: C, 69.33; H, 4.03; N, 12.44. Found: C, 69.42; H, 4.11; N, 12.37.
8-Methyl-7-(3-nitrophenyl)-10-phenyl-10,11-dihydrochromeno[4,3-b]pyrazolo[4,3-e]pyridin-6(7H)-one (4j)
The product was obtained within 3 h in 74% yield as a pale yellow solid; mp 244–246°C; IR: 3361, 1701, 1632, 1531, 1455, 1043, 755 cm-1; 1H NMR: δ 1.93 (s, 3H), 5.44 (s, 1H), 7.36 (d, 1H, J = 8.5 Hz), 7.41–7.45 (m, 2H), 7.58–7.62 (m, 3H), 7.65 (t, 1H, J = 7.5 Hz), 7.71 (d, 2H, J = 8.0 Hz), 7.80 (d, 1H, J = 7.5 Hz), 8.05 (d, 1H, J = 7.7 Hz), 8.10 (d, 1H, J = 8.0 Hz), 8.14 (s, 1H), 10.12 (s, NH); 13C NMR: δ 11.9, 37.2, 100.3, 102.9, 113.8, 116.5, 121.4, 122.3, 122.7, 123.7, 123.8, 126.8, 129.4, 129.6, 132.0, 134.7, 136.2, 138.6, 144.4, 145.9, 147.7, 148.0, 152.1, 160.3. Anal. Calcd for C26H18N4O4: C, 69.33; H, 4.03; N, 12.44. Found: C, 69.20; H, 3.97; N, 12.32.
4-(8-Methyl-6-oxo-10-phenyl-6,7,10,11-tetrahydrochromeno[4,3-b]pyrazolo[4,3-e]pyridin-7-yl)benzonitrile (4k)
The product was obtained within 3 h in 76% yield as a pale yellow solid; mp 159–161°C; IR: 3303, 2227, 1694, 1631, 1537, 1456, 1041, 843, 763, 688 cm-1; 1H NMR: δ 1.91 (s, 3H), 5.33 (s, 1H), 7.37 (d, 1H, J = 8.0 Hz), 7.40–7.44 (m, 2H), 7.50 (d, 2H, J = 8.5 Hz), 7.58 (t, 2H, J = 7.5 Hz), 7.63–7.69 (m, 3H), 7.75 (d, 2H, J = 8.5 Hz), 8.07 (d, 1H, J = 8.0 Hz), 10.07 (s, NH); 13C NMR: δ 11.9, 37.6, 100.2, 102.7, 109.0, 113.7, 116.5, 118.7, 122.7, 123.7, 124.2, 126.7, 128.9, 129.3, 132.0, 132.1, 136.0, 138.5, 144.4, 145.8, 151.2, 152.0, 160.4; MS: m/z 430 ([M]+, 26), 328 (100), 284 (29), 102 (62), 77 (39%). Anal. Calcd for C27H18N4O2: C, 75.34; H, 4.21; N, 13.02. Found: C, 75.28; H, 4.30; N, 12.95.
Acknowledgments
This study was supported by the research council of Tehran University of Medical Sciences (TUMS) and Iran National Science Foundation (INSF).
References
[1] Li, M.; Zhao, B. -X. Progress of the synthesis of condensed pyrazole derivatives (from 2010 to mid-2013). Eur. J. Med. Chem. 2014, 85, 311–340.10.1016/j.ejmech.2014.07.102Search in Google Scholar PubMed
[2] Guo, X.; Liu, M. L.; Guo, H. Y.; Wang, Y. C.; Wang, J. X. Synthesis and In Vitro Antibacterial Activity of 7-(3-Amino-6,7-dihydro-2-methyl-2H-pyrazolo[4,3-c]pyridin-5(4H)-yl)fluoroquinolone Derivatives. Molecules2011, 16, 2626–2635.10.3390/molecules16032626Search in Google Scholar PubMed PubMed Central
[3] El-borai, M. A.; Rizk, H. F.; Abd-Aal, M. F.; El-Deeb, I. Y. Synthesis of pyrazolo[3,4-b]pyridines under microwave irradiation in multi-component reactions and their antitumor and antimicrobial activities – Part 1. Eur. J. Med. Chem. 2011, 48, 92–96.10.1016/j.ejmech.2011.11.038Search in Google Scholar PubMed
[4] Crenshaw, R.; Luke, G. M.; Siminoff, P. Interferon inducing activities of derivatives of 1,3-dimethyl-4-(3-dimethylaminopropylamino)-1H-pyrazolo[3,4-b]quinoline and related compounds. J. Med. Chem. 1976, 19, 262–275.10.1021/jm00224a013Search in Google Scholar PubMed
[5] Krapcho, J.; Turk, C. F. Bicyclic pyrazolines, potential central nervous system depressants and antiinflammatory agents. J. Med. Chem. 1979, 22, 207–210.10.1021/jm00188a018Search in Google Scholar PubMed
[6] Takahashi, Y.; Hibi, S.; Hoshino, Y.; Kikuchi, K.; Shin, K.; Murata-Tai, K. M.; Fujisawa, M.; Ino, Shibata, H.; Yonaga, M. Synthesis and structure–activity relationships of pyrazolo[1,5-a]pyridine derivatives: potent and orally active antagonists of corticotropin-releasing Factor 1 receptor. J. Med. Chem. 2012, 55, 5255–5269.10.1021/jm300259rSearch in Google Scholar PubMed
[7] Zhang, P.; Pennell, A. M. K.; Wright, J. J. K.; Chen, W.; Leleti, M. R.; Li, Y.; Li, L.; Xu, Y. PCT Int. Appl, WO2007002293, Chemocentryx, USA.Search in Google Scholar
[8] Bettinetti, L.; Schlotter, K.; Hübner, H.; Gmeiner, P. Interactive SAR studies: rational discovery of super-potent and highly selective dopamine D3 receptor antagonists and partial agonists. J. Med. Chem. 2002, 45, 4594–4597.10.1021/jm025558rSearch in Google Scholar PubMed
[9] Fujikawa, Y.; Suzuki, M.; Iwasaki, H.; Sakashita, M.; Kitahara, M. European Patent Application EP, 1989, 339–358.Search in Google Scholar
[10] Huard, K.; Bagley, S. W.; Menhaji-Klotz, E.; Preville, C.; Southers, J. A.; Smith, A. C.; Edmonds, D. J.; Lucas, J. C.; Dunn, M. F.; Allanson, N. M.; et al. Synthesis of Spiropiperidine lactam acetyl-CoA carboxylase inhibitors. J. Org. Chem.2012, 77, 10050–10057.10.1021/jo3014808Search in Google Scholar PubMed
[11] Saggar, S. A.; Sisko, J. T.; Tucker, T. J.; Thomas, R. M.; Su, D. S.; Anthony, N. J. US 2007021442, 2007.Search in Google Scholar
[12] Lukasik, P. M.; Elabar, S.; Lam, F.; Shao, H.; Liu, X.; Abbas, A. Y.; Wang, S. Synthesis and biological evaluation of imidazo[4,5-b]pyridine and 4-heteroaryl-pyrimidine derivatives as anti-cancer agents. Eur. J. Med. Chem. 2012, 57, 311–322.10.1016/j.ejmech.2012.09.034Search in Google Scholar PubMed
[13] Wang, M.; Gao, M.; Miller, K. D.; Zheng, Q. H. Synthesis of 2,6-difluoro-N-(3-[11C]methoxy-1H-pyrazolo[3,4-b]pyridine-5-yl)-3-(propylsulfonamidio)benzamide as a new potential PET agent for imaging of B-RafV600Ein cancers. Bioorg. Med. Chem. Lett. 2013, 23, 1017–1021.10.1016/j.bmcl.2012.12.027Search in Google Scholar PubMed
[14] Diaz-Ortiz, A.; De la Hoz, A. A.; Langa, F. Microwave irradiation in solvent-free conditions: an eco-friendly methodology to prepare indazoles, pyrazolopyridines and bipyrazoles by cycloaddition reactions. Green Chem. 2000, 2, 165–172.10.1039/b003752oSearch in Google Scholar
[15] Krygowski, T. M.; Anulewicz, R. R.; Cyranski, M. K.; Puchala, A.; Rasala, D. InCl3 and In(OTf)3 catalyzed reactions: synthesis of 3-acetyl indoles, bis-indolylmethane and indolylquinoline derivatives. Tetrahedron1998, 54, 1229–1232.Search in Google Scholar
[16] Sanghvi, Y. S.; Larson, S. B.; Willis, R. C.; Robins, R. K.; Revankar, G. R. Synthesis and biological evaluation of certain C-4 substituted pyrazolo[3,4-b]pyridine nucleosides. J. Med. Chem. 1989, 32, 945–951.10.1021/jm00125a004Search in Google Scholar PubMed
[17] Lynch, B. M.; Khan, M. A.; Teo, H. C.; Pedrotti, F. Pyrazolo[3,4-b]pyridines: Syntheses, reactions, and nuclear magnetic resonance spectra. Can. J. Chem. 1988, 66, 420–428.10.1139/v88-074Search in Google Scholar
[18] Nascimento-Júnior, N. M.; Mendes, T. C. F.; Leal, D. M.; Corrêa, C. M. N.; Sudo, R. T.; Sudo, G. Z.; Barreiro, E. J.; Fraga, C. A. M. Microwave-assisted synthesis and structure–activity relationships of neuroactive pyrazolo[3,4-b]pyrrolo[3,4-d]pyridine derivatives. Bioorg. Med. Chem. Lett. 2010, 20, 74–77.10.1016/j.bmcl.2009.11.038Search in Google Scholar PubMed
[19] Chen, J.; Liu, W.; Ma, J.; Xu, H.; Wu, J.; Tang, X.; Fan, Z.; Wang, P. Synthesis and Properties of Fluorescence Dyes: Tetracyclic Pyrazolo[3,4-b]Pyridine-Based Coumarin Chromophores with Intramolecular Charge Transfer Character. J. Org. Chem. 2012, 77, 3475–3482.10.1021/jo3002722Search in Google Scholar PubMed
[20] Ghahremanzadeh, R.; Ahadi, S.; Bazgir, A. A one-pot, four-component synthesis of α-carboline derivatives. Tetrahedron Lett. 2009, 50, 7379–7381.10.1016/j.tetlet.2009.10.077Search in Google Scholar
[21] Gunasekaran, P.; Indumathi, S.; Perumal, S. L-Proline-catalyzed three-component domino reactions in the regioselective synthesis of novel densely functionalized pyrazolo[3,4-b]pyridines. RSC Adv. 2013, 3, 8318–8325.10.1039/c3ra00136aSearch in Google Scholar
[22] Pal, S.; Khan, M. N.; Karamthulla, S.; Choudhury, L. H. Molecular iodine catalyzed one-pot multicomponent reactions for the synthesis of dihydrochromeno[4,3-b]pyrazolo[4,3-e]pyridin-6(7H)-ones. RSC Adv. 2013, 3, 15705–15711.10.1039/c3ra41569dSearch in Google Scholar
[23] Ghosh, A.; Khan, A. T. Synthesis of dihydrochromeno[4,3-b]pyrazolo[4,3-e]pyridin-6(7H)-ones involving one-pot three-component tandem Knoevenagel–Michael reaction catalyzed by n-tetrabutylammonium tribromide (TBATB). Tetrahedron Lett. 2014, 55, 2006–2009.10.1016/j.tetlet.2014.02.014Search in Google Scholar
[24] El-Emary, T. I.; El-Mohsen, S. A. A. Multi-Component One-Pot Synthesis and Antimicrobial Activities of 3-Methyl-1,4-diphenyl-7-thioxo-4,6,8,9-tetrahydro-pyrazolo[5,4-b]pyrimidino[5,4-e]pyridine-5-one and Related Derivatives. Molecules, 2012, 17, 14464–14483.10.3390/molecules171214464Search in Google Scholar PubMed PubMed Central
[25] Gunasekaran, P.; Prasanna, P.; Perumal, S. l-Proline-catalyzed three-component domino reactions for the synthesis of highly functionalized pyrazolo[3,4-b]pyridines. Tetrahedron Lett. 2014, 55, 329–332.10.1016/j.tetlet.2013.11.016Search in Google Scholar
[26] Zhu, J.; Bienayme, H. Multicomponent Reactions; Wiley-VCH: Weinheim, Germany, 2005.10.1002/3527605118Search in Google Scholar
[27] Huang, Y.; Yazbak, A.; Domling, A. Multicomponent Reactions. In Green Techniques for Organic Synthesis and Medicinal Chemistry, Zhang, W. and Cue, B. W. Eds. John Wiley and Sons, Ltd, Chichester, UK, 2012.10.1002/9780470711828.ch18Search in Google Scholar
[28] Margolese, D.; Melero, J. A.; Christiansen, S. C.; Chmelka, B. F.; Stucky, G. D. Direct Syntheses of ordered SBA-15 mesoporous silica containing sulfonic acid groups. Chem. Mater. 2000, 12, 2448–2459.10.1021/cm0010304Search in Google Scholar
[29] Ziarani, G. M.; Lashgari, L.; Badiei, A. Sulfonic acid-functionalized mesoporous silica (SBA-Pr-SO3H) as solid acid catalyst in organic reactions. J. Mol. Catal. A: Chemical2015, 397, 166–191.10.1016/j.molcata.2014.10.009Search in Google Scholar
[30] Noushini, S.; Mahdavi, M.; Firoozpour, L.; Moghimi, S.; Shafiee, A.; Foroumadi, A. Efficient multi-component synthesis of 1,4-benzodiazepine-3,5-diones. Tetrahedron2015, 71, 6272–6275.10.1016/j.tet.2015.06.060Search in Google Scholar
[31] Mahdavi, M.; Najafi, R.; Saeedi, M.; Alipour, E.; Shafiee, A.; Foroumadi, A. Synthesis of isoindolo[2,1-a]quinazoline-5,11-dione derivatives via the reductive one-pot reaction of N-substituted 2-nitrobenzamides and 2-formylbenzoic acids. Helv. Chim. Acta2013, 96, 419–423.10.1002/chin.201332181Search in Google Scholar
[32] Rasouli, M. A.; Mahdavi, M.; Ranjbar, P. R.; Saeedi, M.; Shafiee, A.; Foroumadi, A. A green one-pot synthesis of N-alkyl-2-(2-oxoazepan-1-yl)-2-arylacetamide derivatives via an Ugi four-center, three-component reaction in water. Tetrahedron Lett. 2012, 53, 7088–7092.10.1016/j.tetlet.2012.10.075Search in Google Scholar
[33] Mohammadi Ziarani, G.; Badiei, A. R.; Khaniania, Y.; Haddadpour, M. One pot synthesis of polyhydroquinolines catalyzed by sulfonic acid functionalized SBA-15 as a new nanoporous acid catalyst under solvent free conditions. Iran. J. Chem. Chem. Eng. (IJCCE), 2010, 29, 1–10.Search in Google Scholar
©2016 Walter de Gruyter GmbH, Berlin/Boston
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Articles in the same Issue
- Frontmatter
- Review
- Constituents from Chloranthaceae plants and their biological activities
- Preliminary Communications
- Design, synthesis and cytotoxicity evaluation of novel (E)-3-(3-aryl-1-phenyl-1H-pyrazol-4-yl)-1-(pyridin-3-yl)prop-2-en-1-ones as anticancer agents
- Synthesis of first selenodecalines: 2-aryl-4-phenyl octahydroselenochromenes
- Reasearch Articles
- Microwave-assisted and conventional synthesis of novel antimicrobial 1,2,4-triazole derivatives containing nalidixic acid skeleton
- One-pot synthesis of new triazole-sucrose derivatives via click chemistry and evaluation of their antitubercular activity
- One-pot synthesis of 2-hydrazonyl-4-phenylthiazoles via [PDBMDIm]Br-catalyzed reaction under solvent-free conditions
- Three-component one-pot synthesis of dihydrochromeno[4,3-b]pyrazolo[4,3-e]pyridines
Articles in the same Issue
- Frontmatter
- Review
- Constituents from Chloranthaceae plants and their biological activities
- Preliminary Communications
- Design, synthesis and cytotoxicity evaluation of novel (E)-3-(3-aryl-1-phenyl-1H-pyrazol-4-yl)-1-(pyridin-3-yl)prop-2-en-1-ones as anticancer agents
- Synthesis of first selenodecalines: 2-aryl-4-phenyl octahydroselenochromenes
- Reasearch Articles
- Microwave-assisted and conventional synthesis of novel antimicrobial 1,2,4-triazole derivatives containing nalidixic acid skeleton
- One-pot synthesis of new triazole-sucrose derivatives via click chemistry and evaluation of their antitubercular activity
- One-pot synthesis of 2-hydrazonyl-4-phenylthiazoles via [PDBMDIm]Br-catalyzed reaction under solvent-free conditions
- Three-component one-pot synthesis of dihydrochromeno[4,3-b]pyrazolo[4,3-e]pyridines

