Abstract
A new series of chalcones 5a–l were synthesized and evaluated for in vitro antiproliferative activity against human colon cancer cell lines. The synthesis of the key intermediate compounds 3a–d was achieved by tetrakis(triphenylphosphine) palladium(II) mediated Suzuki cross coupling reaction. Chalcone 5a shows superior anticancer activity with IC50 value of 21.0 μg/mL compared to the IC50 value of the reference drug doxorubicin at 21.65 μg/mL.
Synthetic and natural aryl/heteroaryl chalcones exhibit wide and diverse pharmacological activities including antioxidant, anti-HIV, antibacterial, antileishmanial, antiplatelet, anti-infective, anti-inflammatory, antimalarial, antifungal, anticancer, and antiangiogenic properties and are widely used in traditional medicine practices [1]. Their anticancer activity is due to the involvement with multi-drug resistance (MDR) channels [2], p53 degradation [3], JAK/STAT signaling pathway [4], angiogenesis [5], and VEGFR-2 kinase inhibition [6]. Selected naturally occurring and synthetic chalcones with numerous biological activities are shown in Figure 1 [7]. Most of the clinically useful anticancer drugs have genotoxic effects due to interaction with the amino groups of nucleic acids but aryl/heteroaryl chalcones have not been found to show such undesired side effects [8]. Recent development of anticancer agents involves structural modification of chalcones with heteroaryl substituents [9]. In particular, chalcones of thiophenes exhibit various activities [10, 11]. In this work we designed and synthesized a series of 3-arylthiophene chalcone derivatives 5a–l which were not explored earlier and investigated their cytotoxicity against human colon cancer cell line HCT-15.
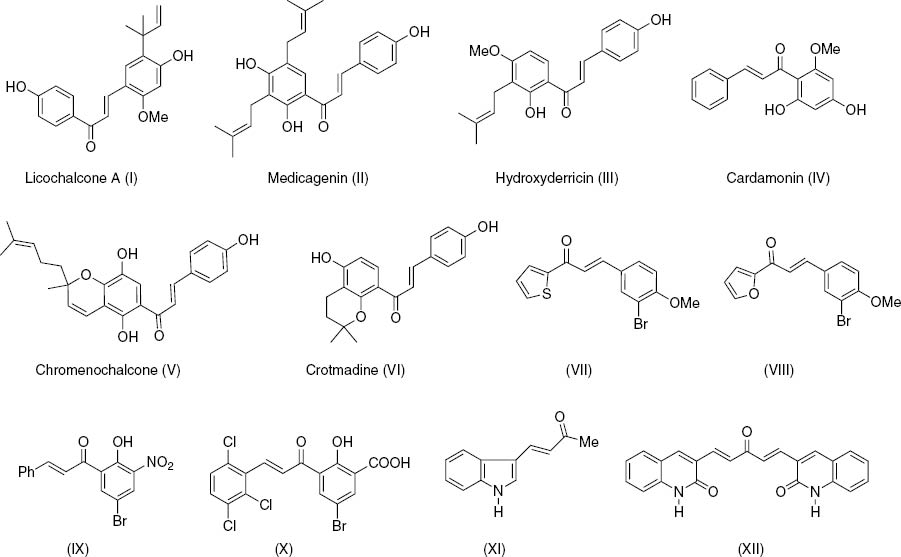
Naturally occurring (I–VI) and synthetic aryl/heteroaryl chalcones (VII–XII).
As depicted in Scheme 1, new 3-arylthiophene-2-carboxaldehydes 3a–d were synthesized by carrying out palladium catalyzed Suzuki cross coupling reaction [12–15] of 3-bromothiophene-2-carboxaldehyde (1) and arylboronic acids 2a–d. In literature, most of the results reported for this reaction have been obtained with 2-bromothiophene [16]. In this work, 3-arylthiophene carboxaldehyde derivatives 3a–d were synthesized. The starting material, 3-bromothiophene-2-carboxaldehyde (1), was synthesized from 3-bromothiophene and N-formylpiperidine in the presence of LDA. The structure of the compound obtained was confirmed by comparing the analytical data with those reported [17]. Compound 1 was allowed to react with commercially available 2,3-disubstituted arylboronic acids 2a–d in the presence of a palladium catalyst and NaHCO3 under nitrogen atmosphere to give the corresponding 3-arylthiophene-2-carboxaldehydes 3a–d in good yields. We initially directed our efforts towards the synthesis of a new series of 3-[3-(3-methoxyphenyl)thiophen-2-yl]-1-phenylprop-2-en-1-one derivatives 5a–l (Scheme 2) from 3-(3-methoxyphenyl)thiophene-2-carboxaldehyde (3a). The aldehyde 3a was allowed to react with commercially available substituted aryl methyl ketones 4a–l in alcoholic sodium hydroxide to give the corresponding chalcones 5a–l with E-geometry of olefin in good yields. The E-configuration was confirmed by analysis of the 1H NMR spectral data. Briefly, two doublets at around δ 7.00 and 7.85 for the adjacent olefinic protons show a coupling of 14.0–14.5 Hz, which is fully consistent with the E-geometry of the olefin fragment in chalcone compounds.
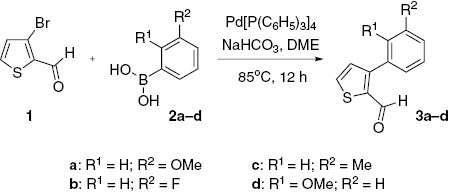
Synthesis of compounds 3a–d.
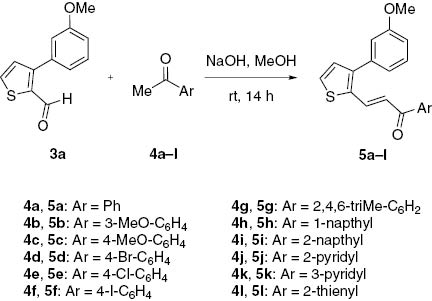
Synthesis of chalcones 5a–l.
The 3-arylthiophene chalcones 5a–l were evaluated in vitro for anti-proliferative activity against human colon cancer cell lines (HCT-15) using an MTT (3,4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay and doxorubicin (DRI) as a reference anticancer drug. Many chalcones show superior anticancer activity when compared to the reference compound. Compounds 5a, 5d, 5g, and 5j exhibit the most potent growing inhibitory activity with IC50 values in the range from 21.0 to 23.8 μg/mL which is comparable to the activity of 21.65 μg/mL of doxorubicin. The percentage of cell death along with IC50 (half maximal inhibitory concentration) values were measured at various concentrations (Table 1). In the series tested, compound 5a shows the best antitumor activity with the IC50 value of 21.0 μg/mL, which is better than the IC50 value of 21.65 μg/mL of the reference drug. Compound 5g with the IC50 value of 22.8 μg/mL is also highly active. The results of the present study provide a rationale for the further development of this class of compounds as novel cancer chemotherapeutic agents.
In vitro anticancer activity of compounds 5a-l.
| Compound | % of cell death at various concentrations (μg/mL) of compound | IC50 (μg/mL) | ||||
|---|---|---|---|---|---|---|
| 2.0 | 5.0 | 10.0 | 15.0 | 25.0 | ||
| 5a | 6.61 | 8.09 | 12.53 | 21.88 | 70.73 | 21.0 |
| 5b | 0.76 | 2.54 | 6.10 | 16.79 | 45.29 | – |
| 5c | 7.88 | 10.43 | 15.77 | 25.95 | 41.41 | – |
| 5d | 0.25 | 0.50 | 15.26 | 24.42 | 52.73 | 23.0 |
| 5e | 3.56 | 6.87 | 9.16 | 30.27 | 43.76 | – |
| 5f | 13.48 | 17.81 | 20.86 | 23.91 | 44.02 | – |
| 5g | 8.09 | 15.80 | 34.99 | 43.24 | 51.12 | 22.8 |
| 5h | 7.09 | 9.89 | 14.78 | 24.25 | 41.73 | – |
| 5i | 0.92 | 2.15 | 6.14 | 15.24 | 44.25 | – |
| 5j | 0.32 | 0.60 | 16.72 | 25.74 | 53.41 | 23.8 |
| 5k | 21.65 | 27.71 | 46.76 | 48.06 | 49.79 | 25.0 |
| 5l | 7.09 | 9.89 | 14.48 | 24.25 | 41.73 | – |
| DRIa | 6.68 | 8.42 | 11.98 | 20.75 | 70.10 | 21.65 |
aDioxorubicin.
Experimental
All chemicals and solvents were commercially available and used without purification. Melting points were determined by open glass capillary method on a Cintex melting point apparatus and are uncorrected. IR spectra were recorded on a Perkin Elmer spectrometer in KBr pellets. 1H NMR (300 MHz or 400 MHz) spectra and 13C NMR spectra (100 MHz) were recorded on Varian spectrometers. Elemental analyses were performed on a LECO-932 analyzer. Mass spectra were recorded on a LC-MSD-Trap-SL instrument in the electrospray ionization (ESI) mode. All reactions were monitored by TLC on pre-coated silica gel plates (60F 254; Merck). Column chromatography was performed on 100–200 mesh silica gel (SRL, India).
Biological assay
HCT-15 (human colon cancer cell line) was procured from NCCS (Pune, India). The cell line was grown in the RPMI-1640 with 2 mm L-glutamine, supplemented with 10% FBS, penicillin (100 IU/mL) and streptomycin (100 μg/mL) at 37°C in a 95% humidified CO2 incubator with 5% CO2 atmosphere. The cell line was passaged twice weekly for maintaining sub-confluent state.
MTT assay was used as cell viability assay [18]. The principle of the assay lies in the fact that MTT is reduced to formazan crystals by mitochondrial dehydrogenase of the viable cells. The treated and untreated cells were washed with PBS (phosphate buffer saline) and after addition of MTT (100 μg/mL) the mixture was incubated at 37°C for 5 h. The remaining MTT was then removed and the formazan crystals were dissolved in DMSO. The absorbance at 540 nm represents the viable cells. The absorbance was taken using multi-scan spectrum from Thermo Scientific. The absorbance from the untreated cells was defined as 100% viable cells. The percentage of viable cells was plotted (Y axis) against concentration (X axis). The IC50 values were then interpolated from the graph.
Synthesis of 3-bromothiophene-2-carboxaldehyde (1)
A solution of 3-bromothiophene (10 g, 61.35 mmol) in THF (100 mL) was stirred and treated dropwise with a solution of LDA in hexanes (62 mmol) at 0°C and the mixture was stirred for an additional 30 min before the addition of N-formylpiperidine (6.9 g, 61.35 mmol). The mixture was stirred further for 3 h, after which time the TLC analysis indicated that all starting material had been consumed. The mixture was quenched with 20% aqueous ammonium chloride and extracted with diethyl ether. The extract was dried with Na2SO4 and concentrated. The residue was purified by column chromatography eluting with 5% ethyl acetate-hexanes. The yield was 9 g (78%) [17].
General procedure for the synthesis of 3-(3-methoxyphenyl)thiophene-2-carboxaldehydes 3a–d
A solution of 3-bromothiophene-2-carboxaldehyde (1, 9.0 g, 47.12 mmol) and 3-methoxy-phenylboronic acid (9.3 g, 61.25 mmol) in 1,2-dimethoxyethane (90 mL) was treated with NaHCO3 (26.0 g, 188.5 mmol), water (90 mL), Pd(PPh3)4 (14 g, 1.41 mmol), and the mixture was heated at 80°C for 12 h under a nitrogen atmosphere. The mixture was then filtered on a celite bed and extracted with ethyl acetate. The extract was washed with water and brine, dried with Na2SO4 and concentrated. The residue was purified by silica gel column chromatography eluting with a mixture of hexanes and ethyl acetate (5:1) to give 3a–d as an off-white solid.
3-(3-Methoxyphenyl)thiophene-2-carboxaldehyde (3a)
Yield 93%; mp 95°C; IR: ν 1658, 1490, 1414, 1361, 1263, 1023, 895, 756 cm-1; 1H NMR (DMSO-d6): δ 9.80 (s, 1H, -CHO), 7.83 (d, 1H, =CHS), 7.52 (d, 1H, Ar-H), 7.45 (s, 1H, Ar-H), 7.32 (d, 1H, Ar-H), 7.09 (d, 1H, Ar-H), 6.93 (d, 1H, Ar-H), 3.35 (s, 3H); 13C NMR (DMSO-d6): δ 184.6, 162.0, 160.4, 160.1, 149.7, 145.4, 132.9, 131.7, 131.0, 128.0, 114.3, 28.0; MS: m/z 219.0 (M+1). Anal. Calcd for C12H10O2S: C, 66.03; H, 4.62. Found: C, 66.13; H, 4.59.
3-(3-Fluorophenyl)thiophene-2-carboxaldehyde (3b)
Yield 65%; mp 90°C; IR: ν: 1652, 1480, 1410, 1325, 1270, 1020 cm-1; 1H NMR (CDCl3): δ 9.87 (1H, s, -CHO), 7.75 (d, 1H, Ar-H), 7.46 (d, 1H, Ar-H), 7.17 (dd, 1H, Ar-H), 7.12 (d, 1H, Ar-H), 7.10 (d, 1H, Ar-H), 6.89 (d, 1H, Ar-H); 13C NMR (CDCl3): δ 183.7, 164.0, 149.6, 138.9, 135.9, 134.4, 131.4, 130.7, 125.4, 116.5, 115.8; MS: m/z 207.2 (M+1). Anal. Calcd for C11H7FOS: C, 64.06; H, 3.42. Found: C, 63.98; H, 3.49.
3-m-Tolylthiophene-2-carboxaldehyde (3c)
Yield 66%; mp 92°C; IR: ν 1658, 1458, 1413, 1320, 1260, 1030 cm-1; 1H NMR (CDCl3): δ 9.86 (1H, s, CHO), 7.68 (d, 1H, Ar-H), 7.34 (d, 3H, Ar-H), 7.25 (dd, 1H, Ar-H), 7.23 (d, 1H, Ar-H), 7.19 (d, 1H, Ar-H), 7.18 (d, 1H, Ar-H), 2.40 (CH3, 3H, s); 13C NMR (CDCl3): δ 184.4, 184.2, 151.7, 138.6, 138.4, 138.4, 134.5, 133.9, 129.6, 128.7, 126.8, 21.4; MS: (m/z) 203.2 (M+1). Anal. Calcd for C12H10OS: C, 71.25; H, 4.98. Found: C, 71.15; H, 5.01.
3-(2-Methoxyphenyl)thiophene-2-carboxaldehyde (3d)
Yield 93%; mp 90°C; IR: ν 1658, 1490, 1414, 1361, 1263, 1023, 895, 756 cm-1; 1H NMR (DMSO-d6): δ 9.81 (s, 1H, -CHO), 7.82 (d, 1H, =CH-S), 7.52 (d, 1H, Ar-H), 7.40 (s, 1H, Ar-H), 7.32 (d, 1H, Ar-H), 7.12 (d, 1H, Ar-H), 6.90 (d, 1H, Ar-H), 3.32 (s, 3H); 13C NMR (DMSO-d6): δ 184.6, 162.1, 160.4, 160.10, 149.7, 145.4, 133.0, 131.7, 131.1, 128.0, 114.3, 28.2; MS: m/z 219.0 (M+1). Anal. Calcd for C12H10O2S: C, 66.03; H, 4.62. Found: C, 66.13; H, 4.59.
General procedure for the synthesis of chalcones 5a–l
A solution of acetophenone 4a–l (389 mg, 2.29 mmol) in methanolic solution of NaOH (2 M) was treated with 3-(3-methoxyphenyl)thiophene-2-carboxaldehyde (3a, 500 mg, 2.29 mmol) and the mixture was stirred at ambient temperature for 14 h. After addition of water (10 mL) the resultant yellow precipitate was filtered and air dried to give analytically pure compound 5a–l.
3-(3-(3-Methoxyphenyl)thiophen-2-yl)-1-phenylprop-2-en-1-one (5a)
Yield 93%; mp 98°C; IR: ν 1658 (C=O), 1597, 1565 (C=C), 1468, 1261 cm-1; 1H NMR (DMSO-d6): δ 9.23 (s, 1H, Ar-H), 8.81 (d, 1H), 8.41 (d, 1H), 7.89 (d, 1H), 7.88 (d, 2H), 7.57 (m, 2H), 7.47 (d, 1H, J = 14.5 Hz, CH of olefin), 7.32 (d, 1H, J = 14.5 Hz, CH of olefin), 7.03 (3H, m, Ar-H), 3.80 (s, 3H, OCH3); 13C NMR (DMSO-d6): δ 187.9, 159.4, 153.2, 149.4, 146.9, 136.4, 136.0, 135.8, 134.1, 132.7, 130.6, 129.9, 129.8, 123.8, 121.6, 120.7, 114.1, 113.8, 55.2; MS: m/z 322.21 (M+1). Anal. Calcd for C20H16O2S: C, 74.97; H, 5.03. Found: C, 74.89; H, 5.08.
1-(3-Methoxyphenyl)-3-(3-(3-methoxyphenyl)thiophen-2-yl)prop-2-en-1-one (5b)
Yield 90%; mp 80°C; IR: ν 1652 (C=O), 1565 (C=C), 1462, 1265 cm-1; 1H NMR: (DMSO-d6): δ 7.84 (s, 1H), 7.64 (d, 1H), 7.47 (m, 4H), 7.40 (d, 1H), 7.30 (d, 1H, J = 14.5 Hz, CH of olefin), 7.19 (d, 1H, J = 14.5 Hz, CH of olefin), 6.99 (m, 2H), 6.95 (m, 1H), 3.80 (s, 3H, CH3), 3.789 (s, 3H, CH3); 13C NMR (DMSO-d6): δ 188.5, 159.5, 159.4, 146.5, 138.9, 136.1,135.8, 134.2, 130.6, 129.9, 129.9, 129.3, 121.5, 121.1, 120.8, 119.1, 114.6, 113.7, 112.8, 55.2, 39.5; MS: m/z 351.10 (M+1). Anal. Calcd for C21H18O3S: C, 71.98; H, 5.18. Found: C, 72.01; H, 5.21.
1-(4-methoxyphenyl)-3-(3-(3-methoxyphenyl)thiophen-2-yl)prop-2-en-1-one (5c)
Yield 92%; mp 85°C; IR: ν 1658 (C=O), 1565 (C=C), 1480, 1260 cm-1; 1H NMR (DMSO-d6): δ 8.29 (m, 2H), 7.85 (d, 1H), 7.50 (m, 4H), 7.45 (d, 1H, J = 14.5 Hz, CH of olefin), 7.21 (d, 1H, J = 14.5 Hz, CH of olefin), 6.98 (m, 3H), 3.80 (s, 3H, OCH3), 3.79 (s, 3H, OCH3); 13C NMR (DMSO-d6): δ 188.4, 159.6, 159.3, 146.5, 138.8, 136.1, 135.8, 134.3, 130.6, 129.9, 129.9, 129.3, 121.5, 121.1, 120.8, 119.1, 114.6, 113.7, 112.8, 79.1, 55.3, 55.1; MS: m/z 351.20 (M+1). Anal. Calcd for C21H18O3S: C, 71.98; H, 5.18. Found: C, 71.81; H, 5.16.
1-(4-Bromophenyl)-3-(3-(3-methoxyphenyl)thiophen-2-yl)prop-2-en-1-one (5d)
Yield 92%; mp 136°C; IR: (KBr, cm-1) ν: 1652 (C=O), 1567 (C=C), 1472, 1241 cm-1; 1H NMR (DMSO-d6): δ 8.013 (d, 2H), 7.86 (d, 2H), 7.75 (d, 2H), 7.54 (1H, s), 7.50 (1H, s), 7.44 (d, 1H, J = 14.5 Hz, CH of olefin), 7.33 (d, 1H, J = 14.5 Hz, CH of olefin), 6.9 (m, 2H), 3.82 (s, 3H, CH3); 13C NMR (DMSO-d6): δ 187.7, 159.4, 146.7, 136.4, 136.2, 136.0, 134.2, 131.8, 130.6, 130.4, 129.9, 129.2, 127.2, 121.6, 120.5, 114.7, 113.7, 112.5, 55.2, 39.9. MS: m/z 399.18, 401.16 (M+2). Anal. Calcd for C20H15BrO2S: C, 60.16; H, 3.79. Found: C, 60.24; H, 3.82.
1-(4-Chlorophenyl)-3-[3-(3-methoxyphenyl)thiophen-2-yl]prop-2-en-1-one (5e)
Yield 94%; mp 123°C; IR: ν 1685 (C=O), 1557 (C=C), 1482, 1261 cm-1; 1H NMR (DMSO-d6): δ 8.09 (d, 2H), 7.87 (d, 2H), 7.61 (m, 2H), 7.55 (d, 1H, J = 14.0 Hz, CH of olefin), 7.51 (m, 1H), 7.44 (d, 1H, J = 14.0 Hz, CH of olefin), 7.33 (3H, m), 3.8 (s, 3H, OCH3); 13C NMR (DMSO-d6): δ 187.5, 159.4, 146.7, 138.0, 136.1, 136.0, 136.0,134.2, 130.6, 130.2, 129.9, 129.5, 128.8, 121.5, 120.6, 114.6, 113.7, 55.2, 39.9; MS: m/z 355.11 (M+1). Anal. Calcd for C20H15ClO2S: C, 67.69; H, 4.28. Found: C, 67.62; H, 4.31.
1-(4-Iodophenyl)-3-(3-(3-methoxyphenyl)thiophen-2-yl)prop-2-en-1-one (5f)
Yield 95%; mp 97°C; IR: ν 1662 (C=O), 1557 (C=C), 1482, 1261 cm-1; 1H NMR (DMSO-d6): δ 8.86 (s, 1H), 8.21 (d, 1H), 8.05 (m, 2H), 7.97 (m, 1H), 7.88 (s, 1H), 7.77 (m, 2H), 7.72 (d, 1H, J = 14.0 Hz, CH of olefin), 7.70 (d, 1H, J = 14.0 Hz, CH of olefin), 7.68 (m, 2H), 3.82 (s, 3H); 13C NMR (DMSO-d6): δ 188.4, 159.4, 146.5, 136.1, 135.7, 134.5, 134.4, 132.2, 130.6, 130.1, 129.9, 129.6, 129.2, 128.4, 127.6, 124.0, 121.6, 121.1, 114.7, 55.2; MS: m/z 446.30 (M+1). Anal. Calcd for C20H15IO2S: C, 53.82; H, 3.39. Found: C, 53.98; H, 3.43.
3-(3-(3-Methoxyphenyl)thiophen-2-yl)-1-(2,4,6-trimethylphenyl)prop-2-en-1-one (5g)
Yield 93%; mp 120°C; IR: ν 1663 (C=O), 1560 (C=C), 1462, 1251 cm-1; 1H NMR (DMSO-d6): δ 7.86 (s, 1H), 7.30 (d, 1H, J = 14.0 Hz, CH of olefin), 6.94 (d, 1H, J = 14.0 Hz, CH of olefin), 6.85 (m, 6H, Ar-H), 3.73 (s, 3H, CH3), 2.22 (s, 9H); 13C NMR (DMSO-d6): δ 199.3, 159.3, 146.1, 138.0 137.7, 136.7, 135.6, 133.3, 133.2, 130.6, 129.9, 129.7, 128.0, 126.9, 121.2, 114.2, 113.9, 55.1, 20.6, 18.7; MS: m/z 363.28 (M+1). Anal. Calcd for C23H22O2S: C, 76.21; H, 6.12. Found: C, 76.12; H, 6.21.
3-(3-(3-methoxyphenyl)thiophen-2-yl)-1-(napthalene-1-yl)prop-2-en-1-one (5h)
Yield 94%; mp 119°C; IR: ν 1662 (C=O), 1545 (C=C), 1462, 1241 cm-1; 1H NMR (DMSO-d6): δ 8.12 (s, 1H), 8.01 (d, 1H, Ar-H), 7.90 (d, 2H, Ar-H), 7.81 (d, 1H, J = 14.0 Hz, CH of olefin), 7.58 (d, 3H, Ar-H), 7.46 (d, 4H, Ar-H), 7.33 (d, 1H, J = 14.0 Hz, CH of olefin), 7.01 (s, 1H, Ar-H), 3.82 (s, 3H, CH3); 13C NMR (DMSO-d6): δ 196.0, 187.9, 159.4, 159.0, 153.2, 149.4, 141.4, 138.1, 136.1, 136.0, 132.7, 130.4, 129.8, 129.6, 129.2, 127.2, 121.5, 120.7, 114.1, 113.6, 55.2, 45.8; MS: m/z 371.15 (M+1). Anal. Calcd for C24H18O2S: C, 77.81; H, 4.90. Found: C, 78.31; H, 4.94.
3-(3-(3-methoxyphenyl)thiophen-2-yl)-1-(napthalene-2-yl)-prop-2-en-1-one (5i)
Yield 94%; mp 119°C; IR: ν 1662 (C=O), 1545 (C=C), 1462, 1241 cm-1; 1H NMR (DMSO-d6): δ 8.07 (s, 1H), 8.02 (d, 1H, Ar-H), 7.94 (d, 2H, Ar-H), 7.83 (d,2H), 7.54 (d, 3H, Ar-H), 7.42 (d, 3H, Ar-H), 7.32 (d, 1H, J = 14.0 Hz, CH of olefin), 7.01 (d, 1H, J = 14.0 Hz, CH of olefin), 3.82 (s, 3H, CH3); 13C NMR (DMSO-d6): δ 197.0, 187.5, 159.4, 159.4, 159.1, 146.8, 141.4, 139.3, 138.1, 136.4, 136.0, 134.2, 130.8, 130.6, 130.6, 129.9, 129.6, 129.2, 127.7, 127.1, 120.9, 114.6, 113.7, 95.2, 55.1, 54.9, 45.8. MS: m/z 371.15, 372.17 (M+1). Anal. Calcd for C24H18O2S: C, 77.81; H, 4.90. Found: C, 77.62; H, 4.87.
3-(3-(3-methoxyphenyl)thiophen-2-yl)-1-(pyridin-2-yl)-prop-2-en-1-one (5j)
Yield 92%; mp 158°C; IR: ν 1675 (C=O), 1576, 1525 (C=C), 1482, 1224, 970 cm-1; 1H NMR (DMSO-d6): δ 8.81 (d, 1H, Ar-H), 8.04 (m, 3H, Ar-H), 7.94 (s, 1H), 7.85 (d, 1H, J = 14.3 Hz, CH of olefin), 7.68 (d, 1H, J = 14.3 Hz, CH of olefin), 7.46 (s, 1H), 7.34 (d, 1H, Ar-H), 7.034 (m, 3H, Ar-H), 3.83 (s, 3H, CH3); 13C NMR (DMSO-d6): δ 187.9, 159.4, 153.1, 149.1, 146.7, 137.7, 136.0, 135.5, 134.5, 130.8, 129.9, 129.4, 127.6, 122.3, 121.5, 119.8, 114.6, 113.8, 55.2; MS: m/z 322.0 (M+1). Anal. Calcd for C19H15NO2S: C, 71.00; H, 4.70; N, 4.36. Found: C, 71.12; H, 4.65; N, 4.28.
3-(3-(3-methoxyphenyl)thiophen-2-yl)-1-(pyridin-3-yl)prop-2-en-1-one (5k)
Yield 92%; mp 140°C; IR: ν 1682 (C=O), 1567 (C=C), 1482, 1261, 975 cm-1; 1H NMR (DMSO-d6): δ 9.23 (S, 1H Py-H), 8.80 (d, 1H, Py-H), 8.40 (d, 1H, Py-H), 7.90 (2H, m), 7.65 (m, 2H), 7.43-7.09 (m, 3H), 7.42 (d, 1H, J = 14.0 Hz, CH of olefin), 7.30 (d, 1H, J = 14.0 Hz, CH of olefin), 3.8 (s, 3H, OCH3); 13C NMR (DMSO-d6): δ 187.9, 159.4, 153.1, 149.1, 146.7, 137.7, 136.0, 135.5, 134.6, 130.8, 129.4, 127.6, 122.3, 121.5, 119.8, 114.6, 113.8, 55.2; MS: m/z 322.0 (M+1). Anal. Calcd for C19H15NO2S: C, 71.00; H, 4.70; N, 4.36. Found: C, 71.15; H, 4.61; N, 4.30.
3-(3-(3-Methoxyphenyl)thiophen-2-yl)-1-(thiophen-2-yl)-prop-2-en-1-one (5l)
Yield 94%; mp 129°C; IR: ν 1642 (C=O), 1546 (C=C), 1462, 1261 cm-1; 1H NMR (DMSO-d6): δ 8.25 (s, 1H), 8.24 (d, 1H, Ar-H), 7.85 (d, 2H), 7.53 (m, 2H, Ar-H), 7.49 (d, 1H, J = 14.5 Hz, CH of olefin), 7.49 (d, 1H, J = 14.5 Hz, CH of olefin), 7.43 (m, 3H), 3.82 (s, 3H, OCH3); 13C NMR (DMSO-d6): δ 180.9, 159.4, 146.5, 145.1, 136.1, 135.4, 134.8, 134.1, 133.4, 130.6, 129.9, 129.3, 128.9, 121.5, 120.8, 114.6, 113.7, 55.2; MS: m/z 327.10 (M+1). Anal. Calcd for C18H14O2S2: C, 66.23; H, 4.32. Found: C, 66.18; H, 4.28.
This publication is dedicated to Professor G.S.R. Subba Rao.
Acknowledgments
The authors would like to express their gratitude and thanks to the Centre for Biotechnology and Centre for Chemical Sciences and Technology, Institute of Science and Technology, Jawaharlal Nehru Technological University Hyderabad, Kukatpally, Hyderabad – 500 085, Telangana, India for doing the antitumor testing.
References
[1] Sing, P.; Anand, A.; Kumar, V. Recent developments in biological activities of chalcones: A mini review. Eur. J. Med. Chem. 2014, 85, 758–777.10.1016/j.ejmech.2014.08.033Suche in Google Scholar
[2] Vasiliou, V.; Vasiliou, K.; Nebert, D. W. Human ATP-binding cassette (ABC) transporter family. Hum. Genomics2009, 3, 281–290.10.1186/1479-7364-3-3-281Suche in Google Scholar
[3] Stoll, R.; Renner, C.; Hansen, S.; Palme, S.; Klein, C.; Belling, A.; Zeslawski, W.; Kamionka, K.; Rehm, T.; Muhlhahn, P.; et al. Chalcone derivatives antagonize interactions between the human oncoprotein MDM2 and p53. Biochemistry2001, 40, 336–344.10.1021/bi000930vSuche in Google Scholar
[4] Pinz, S.; Unser, S.; Brueggemann, S.; Besel, E.; Al-Rifai, N.; Petkes, H.; Amslinger, S.; Rascle, A. The synthetic α-bromo-2′,3,4,4′-tetramethoxychalcone inhibits the JAK/STAT signaling pathway. PLoS One2014, 9, e90275.10.1371/journal.pone.0090275Suche in Google Scholar PubMed PubMed Central
[5] Baeriswyl, V.; Christofori, G. The angiogenic switch in carcinogenesis. Sem. Cancer Biol. 2009, 19, 329–337.10.1016/j.semcancer.2009.05.003Suche in Google Scholar
[6] Rizvi, S. U. F.; Siddiqui, H. L.; Nisar, M.; Khan, N.; Khan, I. Discovery and molecular docking of quinolyl-thienyl chalcones as anti-angiogenic agents targeting VEGFR-2 tyrosine kinase. Biorg. Med. Chem. Lett. 2012, 22, 942–944.10.1016/j.bmcl.2011.12.017Suche in Google Scholar
[7] Anna-Maria, K.; Hadjipavlou-Litina, D. Recent progress in therapeutic applications of chalcones. Expert Opin. Ther. Pat. 2011, 21, 1575–1596.10.1517/13543776.2011.596529Suche in Google Scholar
[8] Zhang, H. J.; Qian, Y.; Zhu, D. D.; Yang, X. Z.; Zhu, H. L. Synthesis, molecular modelling and biological evaluation of chalcone thiosemicarbazide derivatives as novel anticancer agents. Eur. J. Med. Chem. 2011, 46, 4702–4708.10.1016/j.ejmech.2011.07.016Suche in Google Scholar
[9] Meng, Q. C.; Liming, N.; Worsencroft, J. K.; Ye, Z.; Weingarten, D. M.; Simpson, E. J.; Skudlared, W. J.; Marino, M. E.; Ki-Ling, S.; Kunsch, C.; et al. Carboxylated, heteroaryl-substituted chalcones as inhibitors of vascular cell adhesion molecule-1 expression for use in chronic inflammatory diseases. A. J. Med. Chem. 2007, 50, 1304–1315.10.1021/jm0614230Suche in Google Scholar
[10] Vasconcelos, A. D.; Campos, V. F.; Nedel, F.; Seixas, F. K.; Dellagostin, O. A.; Smith, K. R.; Pereira, C. M. P.; Stefanello, F. M.; Collares, T.; Barschak, A. G. Cytotoxic and apoptotic effects of chalcone derivatives of 2-acetyl thiophene on human colon adenocarcinoma cells. Cell. Biochem. Funct. 2013, 31, 289–297.10.1002/cbf.2897Suche in Google Scholar
[11] Wu, C.; Lin, K.; Teng, C.; Huang, A.; Chen, Y.; Yen, M.; Wu, W.; Pu, Y.; Lin, C. Chalcone derivatives inhibit human platelet aggregation and inhibit growth in human bladder cancer cells. Biol. Pharm. Bull. 2014, 37, 1191–1198.10.1248/bpb.b14-00099Suche in Google Scholar
[12] Miyaura, N.; Yanagi, T.; Suzuki, A. The palladium catalyzed cross-coupling reactions of phenylboronic acid with haloarenes. Synth. Commun. 1981, 11, 513–519.10.1080/00397918108063618Suche in Google Scholar
[13] Li, J. J.; Gribble, G. W. Palladium in Heterocyclic Chemistry: A Guide for Synthetic Chemist. Pergamon Publishers: Amsterdam, 2000.Suche in Google Scholar
[14] Ohta, A.; Akita, Y.; Ohkuwa, T.; Chiba, M.; Fukunaga, R.; Miyafuji, A.; Nakata, T.; Tani, N.; Aoyagi, Y. Palladium-catalyse arylation of furan, thiophene, benzo[b]furan and benzo[b]thiophene. Heterocyles1990, 31, 1951–1958.10.3987/COM-90-5467Suche in Google Scholar
[15] Roger, J.; Aditya, L. G.; Doucet, H. Palladium-catalysed C3 or C4 direct arylation of heteromatic compounds with aryl halides by C-H bond activation. ChemCatChem. 2010, 2, 20–40.10.1002/cctc.200900074Suche in Google Scholar
[16] Rizwan, K.; Zubair, M.; Rasool, N.; Ali, S.; Zahoor, A. F.; Rana, A. U.; Khan, S. U. D.; Shahid, M.; Zia-Ul-Haq, M.; Jaafar, H. Z. Regioselective synthesis of 2-(bromomethyl)-5-aryl-thiophene derivatives via palladium (0) catalyzed Suzuki cross-coupling reactions: as antithrombotic and haemolytically active molecules. Chem. Cent. J. 2014, 8, 74.10.1186/s13065-014-0074-zSuche in Google Scholar
[17] William, J. K.; Nord, F. F. Preparation of thiophene-2-aldehyde and some substituted thiophene aldehydes. J. Org. Chem. 1948, 13, 635–640.10.1021/jo01163a003Suche in Google Scholar
[18] Mosmann, T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Meth. 1983, 65, 55–63.10.1016/0022-1759(83)90303-4Suche in Google Scholar
©2016 Walter de Gruyter GmbH, Berlin/Boston
Artikel in diesem Heft
- Frontmatter
- Preliminary Communication
- Design, synthesis, and anticancer activity of novel aryl/heteroaryl chalcone derivatives
- Research Articles
- A simple and convenient method for the synthesis of 1,3,5-triazine-nitrolic acids. The first X-ray investigation of Z-isomeric nitrolic acid
- Pot, atom and step-economic (PASE) synthesis of medicinally relevant spiro[oxindole-3,4′-pyrano[4,3-b]pyran] scaffold
- An efficient asymmetric approach to the R-enantiomer impurity of esomeprazole
- Synthesis, optical and electrochemical properties of 2-[(9H-fluoren-2-yl)aryl]-1H-benz[d]imidazole and 2,7-bis[(1H-benz[d]imidazol-2-yl)aryl]- 9H-fluorene derivatives
- Synthesis and fluorescence of pyrazolines substituted with pyrimidine and ferrocene subunits
- Design and synthesis of a novel rhodamine-based chemosensor and recognition study to Fe3+
- An efficient, one-pot three-component synthesis of 4H-thiazolo[3,2-a][1,3,5]triazin-6-one derivatives
- Microwave-assisted synthesis and antibacterial evaluation of new derivatives of 1,2-dihydro-3H-pyrazolo[3,4-d]pyrimidin-3-one
- Efficient assembly of quinoxaline derivatives from benzene-1,2-diamines, dialkyl acetylenedicarboxylates and ninhydrin
Artikel in diesem Heft
- Frontmatter
- Preliminary Communication
- Design, synthesis, and anticancer activity of novel aryl/heteroaryl chalcone derivatives
- Research Articles
- A simple and convenient method for the synthesis of 1,3,5-triazine-nitrolic acids. The first X-ray investigation of Z-isomeric nitrolic acid
- Pot, atom and step-economic (PASE) synthesis of medicinally relevant spiro[oxindole-3,4′-pyrano[4,3-b]pyran] scaffold
- An efficient asymmetric approach to the R-enantiomer impurity of esomeprazole
- Synthesis, optical and electrochemical properties of 2-[(9H-fluoren-2-yl)aryl]-1H-benz[d]imidazole and 2,7-bis[(1H-benz[d]imidazol-2-yl)aryl]- 9H-fluorene derivatives
- Synthesis and fluorescence of pyrazolines substituted with pyrimidine and ferrocene subunits
- Design and synthesis of a novel rhodamine-based chemosensor and recognition study to Fe3+
- An efficient, one-pot three-component synthesis of 4H-thiazolo[3,2-a][1,3,5]triazin-6-one derivatives
- Microwave-assisted synthesis and antibacterial evaluation of new derivatives of 1,2-dihydro-3H-pyrazolo[3,4-d]pyrimidin-3-one
- Efficient assembly of quinoxaline derivatives from benzene-1,2-diamines, dialkyl acetylenedicarboxylates and ninhydrin

