Abstract
3′-Deoxy-3-azidothymidine (AZT, zidovudine) is a nucleoside-analog reverse transcriptase inhibitor, successfully used against the human immunodeficiency virus (HIV). Its structure contains an azide function, which makes it a useful substrate for 1,2,3-triazole synthesis, using the copper-catalyzed azide-alkyne cycloaddition, the flagship reaction of ‘click chemistry’. Herein we present the synthesis and spectral characterization of its 1,2,3-triazole derivative containing a fluorenylmethyloxycarbonyl (fmoc) fluorescent fragment. The preparation and characteristics of a novel fluorescent probe, 9H-fluoren-9-ylmethyl prop-2-yn-1-yl carbonate (propargyl-fmoc) is also presented.
3′-Deoxy-3-azidothymidine (AZT, zidovudine) is a nucleoside-analog reverse transcriptase inhibitor [1]. Nucleoside analogs are well known as potent antiviral agents used against HIV [1], HSV [2] or HCV [3]. Zidovudine was the first efficient anti-HIV medication, initially used as a ‘stand-alone’ drug and later, as an ingredient of highly-active anti-retroviral therapy (HAART) compositions, together with lamivudine and abacavir. Apart from the usage in HIV therapy, AZT has also found potential application in the treatment of advanced colon cancer [4, 5] and breast cancer [6].
The azide group found in the structure of AZT makes it a ready-to-use click chemistry [7] substrate, able to undergo the copper-catalyzed Huisgen cycloaddition with terminal alkynes to easily yield 1,4disubstituted 1,2,3-triazoles. This reaction has found numerous applications in pharmaceutical sciences and chemical biology, including fluorescence labelling or bioconjugation [8, 9]. Examples of cycloaddition using AZT are known in the literature. The Wang group has developed 1,2,3-triazole derivatives of AZT effective against HIV [10], which upon silylation of the 5′ hydroxyl group became efficient inhibitors of Dengue and West Nile viruses [11]. In 2007 Lee and coworkers developed an efficient AZT 18F labeling technique for PET studies [12]. The first fluorescent 1,2,3-triazole derivatives of zidovudine were obtained by the Celewicz group in 2011 [13]. This initial study used propargyl derivatives of Cinhona alkaloids as the fluorescent alkyne counterpart and investigated also the cytostatic potency of the triazole products. In 2011, a study by the Park group revealed another fluorescent derivative of AZT, prepared using a custom-made fluorescent probe, and its application for ratiometric analysis of zidovudine incorporation by polymerases and reverse transcriptases [14]. Nevertheless, the efficient synthesis of fluorescent AZT derivatives still remains an open field with numerous potential applications in chemical biology and pharmaceutical sciences.
Herein, we report the synthesis of a 1,2,3-triazole derivative of AZT containing a fluorescent fragment and its UV-VIS absorption and emission spectra. The synthesis of a novel alkyne-containing fluorescent probe, 9H-fluoren-9-ylmethyl prop-2-yn-1-yl carbonate (propargyl-fmoc) and its copper-catalyzed cycloaddition reactions with two model azides, trans-2-azidocyclohexanol, and benzyl azide, are also described. For these reactions, a ligand-assisted protocol, using 2-{4-[(dimethylamino)methyl]-1,2,3-triazol-1-yl}cyclohexan-1-ol (AMTC) as a copper-stabilizing ligand, developed in our research group, was applied [15] and additional solvent composition studies were performed to develop an efficient cycloaddition procedure for propargyl-fmoc (1) and zidovudine. 9H-Fluoren-9-ylmethyl prop-2-yn-1-yl carbonate (propargyl-fmoc, 1) was efficiently prepared from fmoc-chloride and propargyl alcohol (Scheme 1). The detailed synthetic procedure is given in the experimental section.
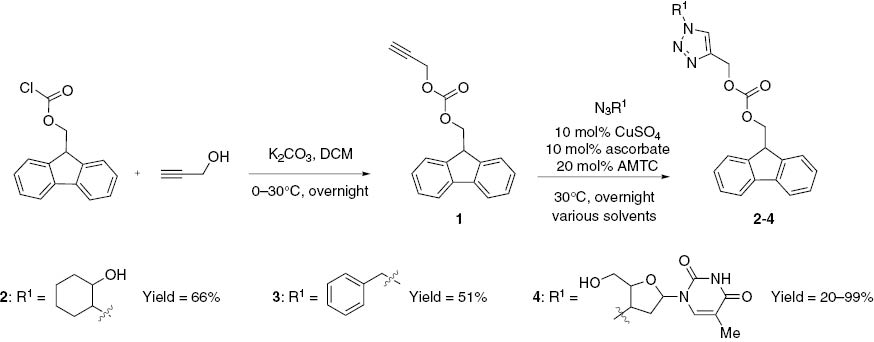
Synthesis of propargyl-fmoc (1) and its conjugates with 2-azidocyclohexanol (2), benzyl azide (3) and AZT (4).
To provide initial information on the reactivity of 1 in the copper-catalyzed azide-alkyne cycloaddition, the reactions with two model azides, namely benzyl azide and trans-2-azidocyclohexanol were performed (Scheme 1). A ligand-assisted cycloaddition protocol, developed in our laboratory [15], with a high copper loading (10 mol%) and long reaction time to ensure high conversion was used. The general procedure is given below. The triazoles 2 and 3 were prepared with 66% and 46% yields, respectively.
In spite of satisfactory yields obtained for 2 and 3, the low solubility of 1 in ethanol and water was an inconvenience that pointed towards problems in developing the cycloaddition procedures with AZT. The initial cycloaddition reaction between AZT and 1 performed in the 2:1 water:ethanol system with 10 mol% of copper and 20 mol% of AMTC proceeded with a low yield of 21%. Therefore, it was decided to test several other solvent systems, to find the optimal reaction conditions overcoming the solubility problem. We used water, 2:1 water-isopropanol, 2:1 water-tert-butanol and dichloromethane as solvents. The amounts of copper, ascorbate and AMTC were not changed. The products were isolated by extraction to dichloromethane and chromatographic purification was not necessary. The isolated products were analyzed by HPLC-MS to determine the purity and the presence of the starting material 1; the results are given in Table 1.
Yields and purity of 4 synthesized in various solvent systems.
| Solvent | Isolated yield | HPLC purity |
|---|---|---|
| 2:1 water/ethanol | 21%a | 99%a |
| water | 76% | 6.5% |
| Dichloromethane | 96% | 98.4% |
| 2:1 water/tert-butanol | >99%b | 92.3% |
| 2:1 water/2-propanol | >99%b | 82.7% |
aThe product was purified by flash chromatography, the final yield is given.
bThe samples contained residual solvent, difficult to remove.
The on-water reaction was inefficient – the isolated product contained mostly 1 (78%) and only a small amount (6.5%) of 4 was formed, which is also consistent with the low yield obtained in the water-ethanol system. By contrast, the reactions conducted in the remaining solvents proceeded efficiently, with almost quantitative yield and high purity of the product. The best result was obtained using dichloromethane. The reaction conducted in tert-butanol furnished the product in high yield, but the HPLC purity was slightly lower and the purified product contained a small amount of residual solvent. The reaction in the isopropanol-water mixture was also efficient in terms of isolated yield, but the purity of the product was significantly lower (82%) and small amounts of catalytic ligand and AZT could be detected by HPLC-MS. It is important to note that in all three cases, the conversion of 1 was complete (only trace amounts could be detected by HPLC-MS). A detailed procedure for the synthesis of 4 in dichloromethane is given in the experimental section.
The UV-VIS absorption and emission spectra were recorded for acetonitrile solutions of compounds 1-4 (Figure 1). The absorption spectra display three important bands around 208, 266.5 and 300 nm. The maxima vary only slightly (see Table 2), whereas the absorption intensities for compounds 2 and 3 are greater than the values for 1 and 4. In the case of fluorescence spectra, recorded at 266 nm excitation wavelength, the relative quantum yields (Table 2) obtained for the triazoles 2-4 are significantly lower than that for 1. The emission maxima vary only slightly between the derivatives and are within the range of 2 nm.

UV-VIS absorption (left) and fluorescence (right) spectra measured for 10 μm solutions of 1-4 in acetonitrile.
UV-VIS absorption and emission properties of compounds 1-4.
| Compound | Absorption maxima (nm) | Emission maxima (nm) | Relative quantum yield |
|---|---|---|---|
| 1 | 207 | ||
| 266.5 | 302.5 | Reference | |
| 290 | 312.5 | ||
| 300.5 | |||
| 2 | 208 | ||
| 266.5 | 304 | 0.240 | |
| 290 | 314 | ||
| 300.5 | |||
| 3 | 208 | ||
| 266.5 | 303 | 0.757 | |
| 290 | 311.5 | ||
| 300.5 | |||
| 4 | 210 | 303 | 0.257 |
| 266.5 | 312.5 | ||
| 300.5 |
The studies presented herein provide an efficient protocol for the synthesis of a novel fluorescent 1,2,3triazole derivative 4 of 3′-deoxy-3-azidothymidine as well as its UV-VIS and fluorescence spectra. Water and a water-ethanol mixture proved inefficient for the reaction, whereas almost quantitative yields were obtained using 2:1 water-isopropanol and water-tert-butanol mixtures or dichloromethane. Simple and efficient preparation of fluoren-9-ylmethyl prop-2-yn-1-yl carbonate (propargyl-fmoc, 1) is also presented, together with its UV-VIS absorption and fluorescence spectra and examples of copper-catalyzed azide-alkyne cycloaddition reactions.
Experimental
Starting materials and reagents were purchased from Sigma-Aldrich, solvents were purchased from Sigma-Aldrich and CHEMPUR. Dichloromethane for the synthesis of 1 was dried over calcium hydride overnight and distilled. Proton (300 MHz) and carbon (75 MHz) NMR spectra were taken in CDCl3 using a Varian Mercury spectrometer, UV-VIS absorption spectra at 200–400 nm were recorded in acetonitrile, using a Cecil CE7200 spectrometer, and emission spectra were recorded in acetonitrile using a Perkin-Elmer LS50b spectrofluorimeter with excitation at 266 nm. The HPLC-MS analyses were performed using an Acquity TQD apparatus manufactured by Waters, equipped with a Acquity UPLC BEH C18 1.7 μm 2.1×100 mm column. The separations were carried out at 0.3 mL/min flow rate, eluting with a water/acetonitrile gradient (linear, 0–99% acetonitrile over 10 min) with 0.1% HCOOH. The separation was monitored using a DAD e(lambda) detector within a 200–700 nm range and an MS detector with electrospray ionization. LC-HRMS analyses were performed using a QTRAP 4000 spectrometer manufactured by Applied Biosystems, coupled to a Prominence HPLC system manufactured by Shimadzu. For HRMS measurements, ESI ionization was used. trans2Azidocyclohexanol and benzyl azide were prepared using literature procedures, from cyclohexene oxide [16] and benzyl bromide [17], respectively. AMTC (the catalytic ligand for copper-catalyzed azide-alkyne cycloaddition), was prepared according to a procedure developed by our group [15].
9H-Fluoren-9-ylmethyl prop-2-yn-1-yl carbonate (1)
Anhydrous potassium carbonate (0.53 g, 2 equiv.) was suspended in 5 mL of anhydrous dichloromethane and treated with propargyl alcohol (0.54 mL, 6 equiv.) and fmoc-chloride (0.5 g, 1 equiv.) under vigorous stirring. The stirring was continued overnight. Then the mixture was diluted with water to dissolve K2CO3 and extracted with dichloromethane. The combined organic layers were washed with water, dried over anhydrous magnesium sulfate and concentrated under reduced pressure to yield 0.5 g of crude product 1. Purification by flash chromatography (silica gel/dichloromethane) yielded 0.49 g (91%) of the pure product as a colorless solid; m p 66–67°C; 1H NMR: δ 7.78 (d, J = 7.5 Hz, 2 H), 7.63 (d, J = 7.5 Hz, 2 H), 7.42 (td, J = 7.5, J = 0.5 Hz, 2 H), 7.33 (td, J = 7.5, J = 1.5 Hz, 2 H), 4.78 (d, J = 2.4 Hz, 2 H), 4.46 (d, J = 7.5 Hz, 2 H), 4.28 (t, J = 7.5 Hz, 1 H), 2.56 (t, J = 2.5 Hz, 1 H); 13C NMR: δ 154.6, 143.2, 141.3, 127.9, 127.2, 125.2, 120.1, 75.8, 70.3, 55.4, 46.7; MS (ESI): m/z 179 (fragmentation occurred upon ionization).
A general procedure for triazoles 2 and 3
Compound 1 (50 mg, 0.18 mmol) was suspended in 1 mL of ethanol. Next, under vigorous stirring, the mixture was treated with 0.18 mmol of the azide, followed by 8 mg of AMTC (20 mol%), 0.36 mL of 0.05 m CuSO4 (10 mol% Cu) and 1.28 mL of water. The reaction was initiated by addition of 0.36 mL of 10 mg/mL solution of sodium ascorbate (10 mol%) and proceeded overnight at room temperature (30°C) under vigorous stirring. Upon completion, ethanol was evaporated under reduced pressure. The residue was diluted with 25 mL of 2 mg/mL aqueous EDTA solution and extracted with 3×20 mL of dichloromethane. The combined organic layers were washed with 1×15 mL of aqueous EDTA and 1×15 mL of water and dried over anhydrous MgSO4. Concentration under reduced pressure yielded the crude product, which was purified by flash chromatography on silica gel eluting with dichloromethane/ethyl acetate to yield the pure triazole 2 or 3 as a colorless solid.
(9H-Fluoren-9-yl)methyl [1-(trans-2-hydroxycyclohexyl)-1H-1,2,3-triazol-4-yl]methyl carbonate (2)
Yield 49.5 mg (66%); mp 153°C; 1H NMR: δ 7.75 (d, J = 7.5 Hz, 2 H), 7.64 (s, 1 H), 7.59 (d, J = 7.5 Hz, 2 H), 7.39 (t, J = 7.5 Hz, 2 H), 7.30 (t, J = 7.5 Hz, 2 H), 5.23 (s, 2 H), 4.43 (d, J = 7.5 Hz, 2 H), 4.24 (t, J = 7.5 Hz, 1 H), 4.06–4.18 (m, 1 H), 3.92–4.04 (m, 1 H), 2.10–2.24 (m, 2 H), 2.04 (s, 1 H), 1.79–1.97 (m, 3 H), 1.21–1.55 (m, 3 H); 13C NMR: δ 155.0, 143.2, 141.3, 127.9, 127.2, 125.1, 120.0, 72.5, 70.0, 67.0, 60.9, 46.7, 33.8, 31.7, 24.7, 24.0; MS (ESI): m/z 520 (M+H+). HRMS. Calcd for C24H26N3O4(M+H+): m/z 420.1923. Found: m/z 420.1921.
(9H-fluoren-9-yl)methyl [1-(benzyl)-1H-1,2,3-triazol-4-yl]methyl carbonate (3)
Yield 34 mg (46%); mp (decomposition at 152°C); 1H NMR: δ 7.75 (d, J = 7.5 Hz, 2 H), 7.58 (d, J = 7.5 Hz, 2 H), 7.54 (s, 1 H), 7.40 (t, J = 7.0 Hz, 2 H), 7.22–7.37 (m, 7 H), 5.52 (s, 2 H), 5.28 (s, 2 H), 4.41 (d, J = 7.5 Hz, 2 H), 4.18–4.27 (m, 1 H); 13C NMR: δ 155.0, 143.2, 141.3, 134.3, 129.2, 128.9, 128.1, 127.9, 127.2, 125.1, 120.0, 70.0, 61.0, 54.3, 46.7; MS (ESI): m/z 512 (M+H+). HRMS. Calcd for C25H22N3O3(M+H+): m/z 412.1661. Found: m/z 412.1671.
(9H-Fluoren-9-yl)methyl {1-[2-(hydroxymethyl)-5-(methyl-2,4-dioxo-1,2,3,4-tetrahydropyrimidin-1-yl)oxolan-3-yl]-1H-1,2,3-triazol-4-yl]methyl} carbonate (fmocAZT, 4)
AMTC (16 mg, 20 mol%) was added to 0.75 mL of 0.05 m aqueous CuSO4 (10 mol% Cu), followed by 100 mg of AZT and 104 mg (1 equiv.) of 1, and this mixture was treated with 3 mL of dichloromethane and an aqueous solution (0.03 mL) of sodium ascorbate (7.4 mg, 10 mol%). The reaction proceeded overnight at room temperature (30°C), under vigorous stirring, then the mixture was extracted with 3×15 mL of dichloromethane. The combined organic layers were washed with 2×15 mL of brine, dried over anhydrous MgSO4 and concentrated under reduced pressure to yield 0.196 g (96%) of a colorless solid of 4: mp 122°C; 1H NMR: δ 9.65 (br. s., 1 H), 7.80 (s, 2 H), 7.73 (d, J = 7.5 Hz, 2 H), 7.56 (d, J = 7.5 Hz, 2 H), 7.44 (s, 1 H), 7.37 (t, J = 7.5 Hz, 2 H), 7.27 (t, J = 7.5 Hz, 2 H), 6.20 (t, J = 6.5 Hz, 1 H), 5.37–5.49 (m, 1 H), 5.26 (s, 2 H), 4.43 (d, J = 7.5 Hz, 2 H), 4.35–4.40 (m, 1 H), 4.17–4.26 (m, 1 H), 3.90–4.01 (m, 1 H), 3.75 (d, J = 10.3 Hz, 1 H), 2.91 (t, J = 6.5 Hz, 2 H), 1.86 (s, 3 H); 13C NMR: δ 164.0, 155.0, 150.6, 143.2, 142.4, 141.2, 137.8, 127.9, 127.2, 125.1, 124.3, 120.1, 111.2, 88.3, 85.1, 70.0, 61.4, 60.7, 59.4, 46.7, 37.5, 12.4; MS (ESI): m/z 546 (M+H+). HRMS. Calcd for C28H28N5O7(M+H+): m/z 546.1989. Found: m/z 546.2001.
References
[1] Oates, J.; Wood, A. Clinical pharmacology of 3′-azido-2′, 3′-dideoxythymidine (zidovudine) and related dideoxynucleosides. N. Engl. J. Med. 1989, 321, 726–738.10.1056/NEJM198909143211106Suche in Google Scholar
[2] Watanabe, K. A.; Su, T. L.; Klein, R. S.; Chu, C. K.; Matsuda, A.; Chun, M. W. Nucleosides. 123. Synthesis of antiviral nucleosides: 5-substituted 1-(2-deoxy-2-halogeno-β-D-arabinofuranosyl)cytosines and -uracils. Some structure-activity relationships. J. Med. Chem. 1983, 26, 152–156.10.1021/jm00356a007Suche in Google Scholar
[3] Clark, J. L.; Hollecker, L.; Mason, J. C.; Stuyver, L. J.; Tharnish, P. M.; Lostia, S. Design, synthesis, and antiviral activity of 2′-deoxy-2′-fluoro-2′-C-methylcytidine, a potent inhibitor of hepatitis C virus replication. J. Med. Chem. 2005, 48, 5504–5508.10.1021/jm0502788Suche in Google Scholar
[4] Brunetti, I.; Falcone, A.; Calabresi, P.; Goulette, F. A.; Darnowski, J. W. 5-Fluorouracil enhances azidothymidine cytotoxicity: in vitro, in vivo, and biochemical studies. Cancer Res. 1990, 50, 4026–4031.Suche in Google Scholar
[5] Darnowski, J. W.; Goulette, F. A. 3′-Azido-3′-deoxythymidine cytotoxicity and metabolism in the human colon tumor cell line HCT-8. Biochem. Pharmacol. 1994, 48, 1797–1805.10.1016/0006-2952(94)90466-9Suche in Google Scholar
[6] Iyer, V. V.; Griesgraber, G. W.; Radmer, M. R.; McIntee, E. J.; Wagner, C. R. Synthesis, in vitro anti-breast cancer activity, and intracellular decomposition of amino acid methyl ester and alkyl amide phosphoramidate monoesters of 3′-azido-3′-deoxythymidine (AZT). J. Med. Chem. 2000, 43, 2266–2274.10.1021/jm000110gSuche in Google Scholar
[7] Kolb, H. C.; Finn, M. G.; Sharpless, K. B. Click chemistry: diverse chemical function from a few good reactions. Angew. Chem. Int. Ed. Engl. 2001, 40, 2004–2021.10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5Suche in Google Scholar
[8] Thirumurugan, P.; Matosiuk, D.; Jozwiak, K. Click chemistry for drug development and diverse chemical-biology applications. Chem. Rev. 2013, 113, 4905–4979.10.1021/cr200409fSuche in Google Scholar
[9] Moses, J. E.; Moorhouse, A. D. The growing applications of click chemistry. Chem. Soc. Rev. 2007, 36, 1249–1262.10.1039/B613014NSuche in Google Scholar
[10] Sirivolu, V. R.; Vernekar, S. K.; Ilina, T.; Myshakina, N. S.;. Parniak, M. A.; Wang, Z. Clicking 3′-azidothymidine into novel potent inhibitors of human immunodeficiency virus. J. Med. Chem. 2013, 56, 8765–8780.10.1021/jm401232vSuche in Google Scholar
[11] Vernekar, S. K.; Qiu, L.; Zhang, J.; Kankanala, J.; Li, H.; Geraghty, R. J. 5′-Silylated 3′-1,2,3-triazolyl thymidine analogues as inhibitors of West Nile virus and Dengue virus. J. Med. Chem. 2015, 58, 4016–4028.10.1021/acs.jmedchem.5b00327Suche in Google Scholar
[12] Sirion, U.; Kim, H. J.; Lee, J. H.; Seo, J. W.; Lee, B. S.; Lee, S. J. An efficient F-18 labeling method for PET study: Huisgen 1,3-dipolar cycloaddition of bioactive substances and F-18-labeled compounds. Tetrahedron Lett. 2007, 48, 3953–3957.10.1016/j.tetlet.2007.04.048Suche in Google Scholar
[13] Baraniak, D.; Kacprzak, K.; Celewicz, L. Synthesis of 3′-azido-3′-deoxythymidine (AZT)-Cinchona alkaloid conjugates via click chemistry: toward novel fluorescent markers and cytostatic agents. Bioorg. Med. Chem. Lett. 2011, 21, 723–726.10.1016/j.bmcl.2010.11.127Suche in Google Scholar
[14] Koh, M.; Park, J.; An, H.; Park, S. B. Ratiometric analysis of zidovudine (ZDV) incorporation by reverse transcriptases or polymerases via bio-orthogonal click chemistry. Chem. Commun. (Camb). 2011, 47, 7614–7616.10.1039/c1cc12518dSuche in Google Scholar
[15] Szafrański, P. W.; Kasza, P.; Cegła, M. T. A new water-soluble ligand for efficient copper-catalyzed Huisgen cycloaddition of aliphatic azides and alkynes. Tetrahedron Lett. 2015, published online ahead of print. http://dx.doi.org/10.1016/j.tetlet.2015.09.110.10.1016/j.tetlet.2015.09.110Suche in Google Scholar
[16] Christoffers, J.; Schulze, Y.; Pickardt, J. Synthesis, resolution, and absolute configuration of trans-1-amino-2-dimethylaminocyclohexane. Tetrahedron2001, 57, 1765–1769.10.1016/S0040-4020(00)01172-8Suche in Google Scholar
[17] Campbell-Verduyn, L.; Elsinga, P. H.; Mirfeizi, L.; Dierckx, R. A.; Feringa, B. L. Copper-free “click”: 1,3-dipolar cycloaddition of azides and arynes. Org. Biomol. Chem. 2008, 6, 3461–3463.10.1039/b812403eSuche in Google Scholar
©2015 by De Gruyter
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Artikel in diesem Heft
- Frontmatter
- Guest Editorial
- Dedication to Kyoichi A. Watanabe
- Review
- From ribavirin to NAD analogues and back to ribavirin in search for anticancer agents
- Preliminary Communications
- 5′-Norcarbocyclic analogues of furano[2,3-d]pyrimidine nucleosides
- Fluorescent 1,2,3-triazole derivative of 3′-deoxy-3-azidothymidine: synthesis and absorption/emission spectra
- Research Articles
- Synthesis and characterization of N-glucosylated dithiadiazepine derivatives through carbon-sulfur bond formation
- Synthesis of 8-alkoxy-1,3-dimethyl-2, 6-dioxopurin-7-yl-substituted acetohydrazides and butanehydrazides as analgesic and anti-inflammatory agents
- 13C NMR spectroscopy of heterocycles: 3,5-diaryl-4-bromoisoxazoles
- Synthesis and anti-proliferative activity of pyridine O-galactosides and 4-fluorobenzoyl analogues
- Optimized synthesis of 3′-O-aminothymidine and evaluation of its oxime derivative as an anti-HIV agent
- Synthesis and antimicrobial properties of 5,5′-modified 2′,5′-dideoxyuridines
- Acyclic analogs of nucleosides based on tris(hydroxymethyl)phosphine oxide: synthesis and incorporation into short DNA oligomers
- Synthesis and antiviral evaluation of 2′,3′-dideoxy-2′,3′-difluoro-D-arabinofuranosyl 2,6-disubstituted purine nucleosides
Artikel in diesem Heft
- Frontmatter
- Guest Editorial
- Dedication to Kyoichi A. Watanabe
- Review
- From ribavirin to NAD analogues and back to ribavirin in search for anticancer agents
- Preliminary Communications
- 5′-Norcarbocyclic analogues of furano[2,3-d]pyrimidine nucleosides
- Fluorescent 1,2,3-triazole derivative of 3′-deoxy-3-azidothymidine: synthesis and absorption/emission spectra
- Research Articles
- Synthesis and characterization of N-glucosylated dithiadiazepine derivatives through carbon-sulfur bond formation
- Synthesis of 8-alkoxy-1,3-dimethyl-2, 6-dioxopurin-7-yl-substituted acetohydrazides and butanehydrazides as analgesic and anti-inflammatory agents
- 13C NMR spectroscopy of heterocycles: 3,5-diaryl-4-bromoisoxazoles
- Synthesis and anti-proliferative activity of pyridine O-galactosides and 4-fluorobenzoyl analogues
- Optimized synthesis of 3′-O-aminothymidine and evaluation of its oxime derivative as an anti-HIV agent
- Synthesis and antimicrobial properties of 5,5′-modified 2′,5′-dideoxyuridines
- Acyclic analogs of nucleosides based on tris(hydroxymethyl)phosphine oxide: synthesis and incorporation into short DNA oligomers
- Synthesis and antiviral evaluation of 2′,3′-dideoxy-2′,3′-difluoro-D-arabinofuranosyl 2,6-disubstituted purine nucleosides

