Synthesis of 8-alkoxy-1,3-dimethyl-2, 6-dioxopurin-7-yl-substituted acetohydrazides and butanehydrazides as analgesic and anti-inflammatory agents
-
Grażyna Chłoń-Rzepa
, Agnieszka W. Jankowska
Abstract
A series of new 8-alkoxy-1,3-dimethyl-2,6-dioxopurin-7-yl-substituted acetohydrazides and butanehydrazides 6–12 was synthesized and evaluated for the analgesic activity in two in vivo models: the writhing syndrome and the hot-plate tests. Among the investigated derivatives, compounds with N′-arylidenehydrazide moiety 9–12 show analgesic activity significantly higher than that of acetylsalicylic acid, which may indicate the importance of this structural element for analgesic properties. The lack of the activity in the hot-plate test may suggest that the analgesic activity of the newly synthesized compounds is mediated by a peripheral mechanism. The selected compounds 7 and 12 inhibit tumor necrosis factor α production in a rat model of lipopolysaccharide-induced endotoxemia, similarly to theophylline, which may confirm their anti-inflammatory properties.
Introduction
Non-steroidal anti-inflammatory drugs (NSAIDs) are a class of non-opioid anti-nociceptive agents very often used in the treatment of pain and inflammation. However, the clinical use of NSAIDs is limited by a variety of side effects, with gastroduodenal ulceration and bleeding being the most serious. Therefore, the search for new potent analgesic agents with minimal side effects is an important line of research in the pharmaceutical industry [1–4]. It is well known that leukotrienes (LT), particularly LTB4, are involved in the acute ulceration induced by NSAID’s. Evidence has been accumulating that compounds that are dual inhibitors of cyclooxygenase (COX) and 5-lipoxygenase (5-LO) may show a safer profile of activity and enhanced efficacy as analgesic agents in inflammatory diseases.
Hydrazides are a group of compounds possessing several different biological activities including anti-convulsant, antidepressant, antimalarial, antimicrobial, and antimycobacterial properties [4–8]. Moreover, some hydrazides have been also reported as potent anti-inflammatory and/or analgesic agents with potency comparable or even greater than currently used NSAIDs, such as salicylic acid derivatives (aspirin, salicylamide), ibuprofen, mefenamic acid, or indomethacin [4–6]. Literature data suggest that the hydrazone moiety is a pharmacophore group for the inhibition of COX and 5-LO [4].
For several years, we have been interested in developing purine-2,6-dione derivatives with diverse biological activity, such as antidepressant, anxiolytic, antipsychotic [9–11], antiarrhythmic, and hypotensive activity [12, 13] for the potential treatment of central nervous and cardiovascular systems disorders. Searching for new analgesic agents in a group of purine-2,6-dione derivatives, we synthesized and pharmacologically evaluated series of 3,7-dimethyl-2,6-dioxopurin-1-yl I and 1,3-dimethyl-2,6-dioxopurin-7-yl II and III derivatives (Figure 1). The tested compounds show significant analgesic and/or anti-inflammatory activity as evidenced in some behavioral models [14–16]. The most interesting compounds are up to 36-fold more active than acetylsalicylic acid (ASA) used as a reference drug [16]. The obtained data suggest that the N′-benzylidenehydrazide moiety is strongly beneficial for analgesic activity [14].
![Figure 1 Structures of the analgesic agents (series I–III) from our previous studies [14–16].](/document/doi/10.1515/hc-2015-0100/asset/graphic/j_hc-2015-0100_fig_001.jpg)
Structures of the analgesic agents (series I–III) from our previous studies [14–16].
In this study, new 8-alkoxy-1,3-dimethyl-2,6-dioxopurin-7-yl derivatives of acetic and butanoic acids with a hydrazide moiety were designed and synthesized. In comparison with previously reported 8-methoxypurines [16], the new analogues contain a longer alkoxy substituent in the 8 position. The obtained compounds 6–12 were tested in vivo for their potential analgesic activity in the writhing syndrome and the hot-plate tests. In addition, anti-inflammatory properties for the selected compounds 7 and 12 were evaluated in an animal model of endotoxemia.
Results and discussion
Synthesis of compounds 6–12 is presented in Scheme 1. Ethyl 2-[8-bromo-1,3-dimethyl-2,6-dioxo-2,3-dihydro-1H- purin7(6H)-yl]acetate (1) and its higher butanoate homologue 2 were prepared by a reaction of 8-bromo-1,3-dimethylpurine-2,6-dione with ethyl 2-chloroacetate or ethyl 4-bromobutyrate, respectively, according to the previously described method [17–19]. In the next step, ethyl 4-[8-ethoxy-1,3-dimethyl-2,6-dioxo-2,3-dihydro-1H-purin7(6H)-yl] butanoate (3), propyl 2-[1,3-dimethyl-2,6-dioxo-8-propoxy-2,3-dihydro-1H-purin-7(6H)-yl]acetate (4), and its higher butanoate homologue 5 were obtained by treatment of 1 or 2 with a corresponding sodium alkoxide. Subsequently, ω-(8-alkoxy-1,3-dimethyl-2,6-dioxo-2,3-dihydro-1H-purin-7-yl)alkanehydrazides 6–8 were prepared by treatment of 3–5 with a 5-fold molar excess of hydrazine hydrate in anhydrous ethanol. The final hydrazinylidene derivatives 9–12 were prepared by condensation reaction of 6–8 with benzaldehyde or acetophenone at room temperature in the presence of a catalytic amount of concentrated hydrochloric acid. The structures of the newly synthesized compounds 6–12 were fully consistent with their 1H-NMR spectra, LC/MS data, and elemental analysis.
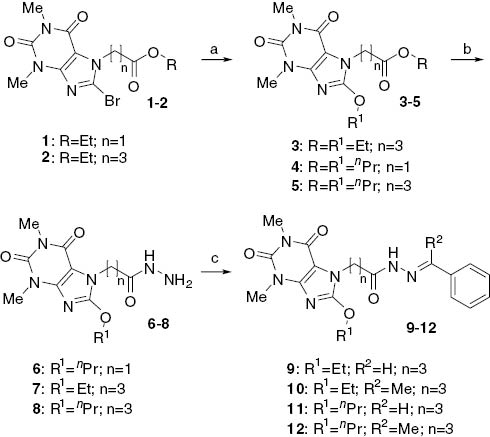
Reagents and conditions: (a) RONa, ROH, reflux; (b) hydrazine hydrate, ethanol, reflux; (c) benzaldehyde or acetophenone, HCl, methanol, room temperature.
The analgesic activity of the newly synthesized hydrazides 6–12 was tested using a writhing syndrome assay with ASA as a reference agent. Based on the available literature data, this assay reveals anti-inflammatory properties [20]. On the basis of the calculated ED50 values, in a range of 12.2–51.3 mg/kg (see Supplementary Material), it can be seen that the investigated compounds 6–12 show diverse analgesic properties. Compounds with N′-arylidenehydrazide moiety 9–12 show higher analgesic activity than ASA (ED50=39.1 mg/kg). The strongest analgesic effect (2- and 3-fold stronger than that of ASA) is observed for compounds 10 and 12 that contain a N′-(1-phenylethylidene)butanehydrazide fragment, which may indicate the importance of this group for analgesic activity. The same effect has been observed previously for (3,7-dimethylpurine-2,6-dioxo-1-yl)-substituted acetohydrazide and butanehydrazide [14]. The elongation of an alkyl chain in the alkoxy substituent in the 8-position of purine-2,6-dione slightly increases the analgesic activity, as can be seen from comparison of 9 with 11 and 10 with 12. Compounds possessing more hydrophilic N′-unsubstituted hydrazide moiety show lower activity than their N-arylidene derivatives, as seen from comparison within the series 6–8 vs. 9–12. In the case of 7 and 8, their analgesic activity is less pronounced than that of ASA.
In the hot-plate test, which is used to determine activity of centrally acting analgesic compounds, for example, morphine and its analogues, the investigated compounds 6–12 did not show any analgesic activity (see Supplementary material). It can be suggested that analgesic activity of the newly synthesized compounds is mediated by a peripheral mechanism. None of the tested derivatives 6–12 significantly change the latency to fall from the rota-rod (data not shown). These compounds do not affect a motor performance in the rota-rod test [21].
Taking into account that the structures of compounds 6–12 correspond to theophylline (Th) and pentoxifylline – the well-known tumor necrosis factor α (TNF-α) inhibitors, their ability to inhibit TNF-α production in vivo in lipopolysaccharide (LPS)-induced model of endotoxemia was assessed for one representative compound from each group, namely 7 (with an unsubstituted hydrazide moiety) and 12 [with N′-(11-phenylethylidene)butanehydrazide fragment]. The results of the in vivo experiment in rats with LPS-induced endotoxemia indicate that compounds 7 and 12 exert a significant anti-inflammatory activity that is similar to that of Th (Figure 2).
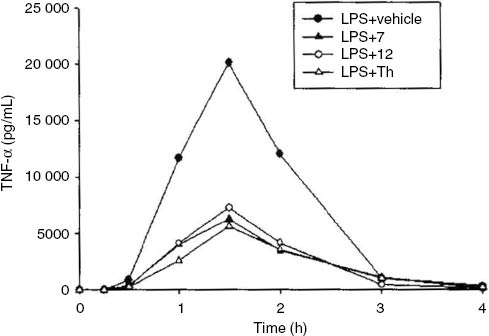
TNF-α concentration vs. time profiles following intraperitoneal administration of compounds 7 and 12 and Th as a reference drug (50 mg/kg) to rats (n=4–5) with endotoxemia induced by administration of LPS (1 mg/kg iv).
This effect is probably caused, at least in part, by an increased cyclic adenosine monophosphate level. This nucleotide acting as an inhibitor of the nuclear factor κB pathway is assumed to be the most important player in the mechanism of action of most xanthines with anti-inflammatory properties [22].
Conclusion
New 8-alkoxy-1,3-dimethyl-2,6-dioxopurin-7-yl-substituted acetohydrazides and butanehydrazides 6–12 were synthesized and their analgesic activity was determined in pharmacological models in vivo. The tested compounds show a significant analgesic activity in the writhing syndrome test. The strongest analgesic effect is observed for compounds 10 and 12 with N′-(1-phenylethylidene)butanehydrazide moiety, which may indicate that this structural element is important for analgesic properties. The lack of the activity in the hot-plate test may suggest that analgesic activity of the newly synthesized compounds is mediated by a peripheral mechanism and may be connected with their anti-inflammatory properties. The selected compounds 7 and 12 suppress TNF-α release in rat plasma similarly to Th, which may confirm their anti-inflammatory properties.
Further studies are warranted to determine the effect of the evaluated compounds on the activity of COX, the acid-sensing ion channels, and the transient receptor potential V1 channel to clarify the exact mechanism of their analgesic and anti-inflammatory activity.
Experimental
Melting points (mp) were determined with a Büchi Melting Point B-545 apparatus and are uncorrected. 1H NMR spectra were taken with a Varian Mercury-VX spectrometer at 300 MHz in CDCl3 (3–5, 7–12) or DMSO-d6 (6, 7) solutions. LC/MS analyses were performed on a Waters Acquity TQD apparatus with an eλ DAD detector. For mass spectrometry, ESI+ (electrospray-positive) ionization mode was used. The UPLC/MS purity of all the investigated compounds was determined to be over 98%. Purity of all compounds was checked by TLC using Merck Kieselgel 60 F254 sheets eluting with dichloromethane/methanol (95:5). Spots were detected by UV irradiation. Elemental analyses were taken with an Elementar Vario EL III apparatus.
General procedure for preparation ethyl and propyl ω-[8-alkoxy-1,3-dimethyl-2,6-dioxo-2,3-dihydro-1H-purin-7(6H)-yl]alkanecarboxylates 3–5
The equimolar amounts (20 mmol) of compound 1 or 2 and sodium ethoxide or sodium propoxide were heated under reflux in the corresponding alcohol (100 mL) for 6 h. The mixture was filtered, and the solvent was evaporated under reduced pressure. The oily residue was washed with water until it solidified, then crystallized from ethanol or propan-1-ol.
Ethyl 4-[8-ethoxy-1,3-dimethyl-2,6-dioxo-2,3-dihydro-1H-purin-7 (6H)-yl]butanoate (3)
This compound was obtained from 2in 76% yield; mp 59–61°C; Rf=0.62; 1H NMR (CDCl3): δ 1.21 (t, 3J=6.0 Hz, 3H), 1.42 (t, 3J=7.1 Hz, 3H), 2.05–2.11 (m, 2H), 2.27–2.36 (m, 2H), 3.36 (s, 3H), 3.49 (s, 3H), 4.04–4.16 (m, 4H), 4.51 (q, 3J=7.1 Hz, 2H); LC/MS: m/z 339.26 [(M+H)+]. Anal. Calcd for C15H22N4O5: C, 55.89; H, 6.88; N, 17.38. Found: C, 55.80; H, 6.93; N, 17.34.
Propyl 2-[1,3-dimethyl-2,6-dioxo-8-propoxy-2,3-dihydro-1H-purin-7 (6H)-yl]acetate (4)
This compound was obtained from 1in 71% yield; mp 68–70°C; Rf=0.66; 1H NMR (CDCl3): δ 0.92 (t, 3J=7.4 Hz, 3H), 0.99 (t, 3J=7.4 Hz, 3H), 1.62–1.71 (m, 2H), 1.81 (m, 2H), 3.35 (s, 3H), 3.52 (s, 3H), 4.13 (t, 3J=6.6 Hz, 2H), 4.43 (t, 3J=6.6 Hz, 2H), 4.87 (s, 2H); LC/MS: m/z 339.20 [(M+H)+]. Anal. Calcd for C15H22N4O5: C, 55.89; H, 6.88 N, 17.38. Found: C, 55.82; H, 6.90; N, 17.41.
Propyl 4-[1,3-dimethyl-2,6-dioxo-8-propoxy-2,3-dihydro-1H-purin-7 (6H)-yl]butanoate (5)
This compound was obtained from 2in 68% yield; mp 53–55°C; Rf=0.66; 1H NMR (CDCl3): δ 0.91 (t, 3J=7.4 Hz, 3H), 1.02 (t, 3J=7.4 Hz, 3H), 1.55–1.67 (m, 2H), 1.77–1.89 (m, 2H), 2.05–2.15 (m, 2H), 2.29–2.37 (m, 2H), 3.37 (s, 3H), 3.50 (s, 3H), 3.99 (t, 3J=6.6 Hz, 2H), 4.15 (t, 3J=6.8 Hz, 2H), 4.41 (t, 3J=6.5 Hz, 2H); LC/MS: m/z 367.32 [(M+H)+]. Anal. Calcd for C17H26N4O5: C, 58.27; H, 7.48; N, 15.99. Found: C, 58.31; H, 7.42; N, 16.09.
General procedure for ω-(8-alkoxy-1,3-dimethyl-2,6-dioxo-2,3-dihydro-1H-purin-7-yl)alkanehydrazides 6–8
A mixture of ester 3–5 (10 mmol) and hydrazine hydrate (50 mmol) in anhydrous ethanol (30 mL) was heated under reflux for 10 h. Afterward, the reaction mixture was cooled and the resultant precipitate of product was filtered off, washed with a small amount of water, and crystallized from methanol.
2-[1,3-Dimethyl-2,6-dioxo-8-propoxy-2,3-dihydro-1H-purin-7(6H)-yl]acetohydrazide (6)
This compound was obtained from 4 in 65% yield; mp 191–193°C; Rf=0.46; 1H NMR (DMSO-d6): δ 0.91 (t, 3J=7.4 Hz, 3H,), 1.65–1.75 (m, 2H), 3.16 (s, 3H), 3.37 (s, 3H), 4.24 (bs, 2H), 4.36 (t, 3J=6.4 Hz, 2H), 4.62 (s, 2H), 9.25 (bs, 1H); LC/MS: m/z 311.15 [(M+H)+]. Anal. Calcd for C12H18N6O4: C, 46.45; H, 5.85; N, 27.08. Found: C, 46.48; H, 5.90; N, 27.02.
4-[8-Ethoxy-1,3-dimethyl-2,6-dioxo-2,3-dihydro-1H-purin-7(6H)-yl]butanehydrazide (7)
This compound was obtained from 3 in 62%; mp 174–176°C; Rf=0.55; 1H NMR (CDCl3): δ 1.44 (t, 3J=7.1 Hz, 3H), 2.11 (m, 2H), 2.22 (t, 3J=6.5 Hz, 2H), 3.38 (s, 3H), 3.51 (s, 3H), 4.11 (t, 3J=6.5 Hz, 2H), 4.52 (q, 3J=7.1 Hz, 2H), 7.60 (bs, 1H); 1H NMR (DMSO-d6): δ 1.35 (t, J=7.1 Hz, 3H), 1.86–1.98 (m, 4H), 3.18 (s, 3H), 3.35 (s, 3H), 3.97 (t, 3J=6.5 Hz, 2H), 4.14 (bs, 2H), 4.43–4.50 (q, 3J=7.1 Hz, 2H), 8.87 (bs, 1H); LC/MS: m/z 325.30 [(M+H)+]. Anal. Calcd for C13H20N6O4: C, 48.14; H, 6.22; N, 25.91. Found: C, 48.11; H, 6.19; N, 25.96.
4-[1,3-Dimethyl-2,6-dioxo-8-propoxy-2,3-dihydro-1H-purin-7(6H)-yl]butanehydrazide (8)
This compound was obtained from 5 in 67% yield; mp 130–132°C; Rf=0.46; 1H NMR (CDCl3): δ 1.02 (t, 3J=7.4 Hz, 3H), 1.80–1.87 (m, 2H), 2.08–2.12 (q,3J=7.4 Hz, 2H), 2.22 (t, 3J=7.0 Hz, 2H), 3.05 (bs, 2H), 3.38 (s, 3H), 3.51 (s, 3H), 4.11 (t, 3J=6.5 Hz, 2H), 4.42 (t, 3J=6.5 Hz, 2H), 7.60 (bs, 1H); LC/MS: m/z 339.26. Anal. Calcd for C14H22N6O4: C, 49.70; H, 6.55; N, 24.84. Found: C, 49.67; H, 6.50; N, 24.87.
General procedure for N′-arylidene-4-[8-alkoxy-1,3-dimethyl-2,6-dioxo-2,3-dihydro-1H-purin-7(6H)-yl]butanehydrazides 9–12
A mixture of compound 7 or 8 (1 mmol) and benzaldehyde or acetophenone (1 mmol) was stirred in methanol (10 mL) in the presence of a catalytic amount of HCl (2 drops of concentrated acid) at room temperature for 2 days. Afterward, water was added and the resultant precipitate of the product was filtered and purified by silica gel chromatography eluting with dichloromethane/methanol (95:5).
N′-Benzylidene-4-[8-ethoxy-1,3-dimethyl-2,6-dioxo-2,3-dihydro-1H-purin-7(6H)-yl]butanehydrazide (9)
This compound was obtained from 7 in 75% yield; mp 206–208°C; Rf=0.40; 1H NMR (CDCl3): δ 1.41 (t, 3J=7.0 Hz, 3H), 2.20 (m, 2H), 2.79 (t, 3J=7.4 Hz, 2H), 3.34 (s, 3H), 3.50 (s, 3H), 4.23 (t, 3J=7.0 Hz, 2H), 4.50 (q, 3J=7.0 Hz, 2H), 7.36–7.40 (m, 3H), 7.57–7.60 (m, 2H), 7.73 (s, 1H), 9.29 (bs, 1H); LC/MS: m/z 413.25 [(M+H)+]. Anal. Calcd for C20H24N6O4: C, 58.24; H, 5.87; N, 20.38. Found: C, 58.26; H, 5.81; N, 20.41.
4-[8-Ethoxy-1,3-dimethyl-2,6-dioxo-2,3-dihydro-1H-purin-7(6H)-yl)-N′-(1-phenylethylidene]butanehydrazide (10)
This compound was obtained from 7 in 73% yield; mp 183–185°C; Rf=0.40; 1H NMR (CDCl3): δ 1.40 (t, 3J=7.1 Hz, 3H), 2.16–2.25 (q, 3J=7.0 Hz, 2H), 2.18 (s, 3H), 2.81 (t, 3J=7.4 Hz, 2H), 3.32 (s, 3H), 3.50 (s, 3H), 4.23 (t, 3J=6.6 Hz, 2H), 4.45–4.52 (q, 3J=7.1 Hz, 2H), 7.35–7.43 (m, 3H), 7.66–7.69 (m, 2H), 8.58 (bs, 1H); LC/MS: m/z 427.20 [(M+H)+]. Anal. Calcd for C21H26N6O4: C, 59.14; H, 6.14; N, 19.71. Found: C, 59.18; H, 6.19; N, 19.78.
N′-Benzylidene-4-[1,3-dimethyl-2,6-dioxo-8-propoxy-2,3-dihydro-1H-purin-7(6H)-yl]butanehydrazide (11)
This compound was obtained from 8 in 71% yield; mp 166–169°C; Rf=0.44; 1H NMR (CDCl3): δ 0.97 (t, 3J=7.0 Hz, 3H), 1.79 (m, 2H), 2.16–2.26 (q, 3J=7.0 Hz, 2H), 2.78 (t, 3J=7.0 Hz, 2H), 3.32 (s, 3H), 3.49 (s, 3H), 4.23 (t, 3J=7.0 Hz, 2H), 4.38 (t, 3J=7.0 Hz, 2H), 7.35–7.39 (m, 3H), 7.58 (m, 2H), 7.79 (s, 1H), 10.55 (s, 1H); LC/MS: m/z 427.45 [(M+H)+]. Anal. Calcd for C21H26N6O4: C, 59.14; H, 6.14; N, 19.71. Found: C, 59.12; H, 6.17; N, 19.75.
4-[1,3-Dimethyl-2,6-dioxo-8-propoxy-2,3-dihydro-1H-purin-7(6H)-yl)-N′-(1-phenylethylidene]butanehydrazide (12)
This compound was obtained from 8 in 69% yield; mp 156–158°C; Rf=0.52; 1H NMR (CDCl3): δ 0.97 (t, 3J=7.4 Hz, 3H), 1.71–1.84 (m, 2H), 2.15–2.22 (q, 3J=5.2 Hz, 2H), 2.19 (s, 3H), 2.81 (t, 3J=7.4 Hz, 2H), 3.31 (s, 3H), 3.49 (s, 3H), 4.22 (t, 3J=6.7 Hz, 2H), 4.39 (t, 3J=6.6 Hz, 2H), 7.34–7.37 (m, 3H), 7.64–7.68 (m, 2H), 8.96 (s, 1H); LC/MS: m/z 441.21 [(M+H)+]. Anal. Calcd for C22H28N6O4: C, 59.99; H, 6.41; N, 19.08. Found: C, 59.92; H, 6.35; N, 19.12.
Pharmacological evaluation
The writhing syndrome and rota-rod tests were carried out using the methods previously published by Zygmunt et al. [16]. The hot-plate test was performed according to the method described by Śladowska et al. [23]. Experimental endotoxemia was induced by systemic injection of LPS (1 mg/kg) from Escherichia coli serotype 055:B5 (Sigma Aldrich, St Louis, MO, USA) to male Wistar rats cannulated in the jugular vein. Compounds 7, 12, and Th suspended in PEG400/saline (50:50, v/v) were given to rats (n=4–5) at a dose of 50 mg/kg ip simultaneously with LPS. Control animals received LPS and a respective volume of vehicle by the same routes of administration as the treatment groups. Blood samples were collected into heparinized tubes at different time points after LPS and compound administration. Blood was centrifuged at 4°C for 20 min (1500×g), and plasma was stored at -80°C until assayed. Concentrations of TNF-α were measured using ELISA (R&D Systems, Minneapolis, MN, USA).
Statistical analysis
The data expressed as mean±standard error were evaluated by one-way analysis of variance followed by Duncan or Tukey HSD test (Statistica, Statsoft, USA). The difference of means was considered significant if p<0.05. The areas under effect curve were calculated using the trapezoidal rule (Phoenix WinNonlin v. 6.3; Pharsight, Mountain View, CA, USA).
Acknowledgments
The study was supported by statutory funds from Jagiellonian University Medical College (K/ZDS/003299 and K/ZDS/005535).
References
[1] Manrique-Moreno, M.; Howe, J.; Suwalsky, M.; Garidel, P.; Brandenburg, K. Physicochemical interaction study of non-steroidal anti-inflammatory drugs with dimyristoylphosphatidylethanolamine liposomes. Lett. Drug Des. Discov. 2010, 7, 50–56.10.2174/157018010789869280Search in Google Scholar
[2] Nassiri Koopaei, M.; Assarzadeh, M. J.; Almasirad, A.; Ghasemi-Niri, S. F.; Amini, M.; Kebriaeezadeh, A.; Nassiri Koopaei, N.; Ghadimi, M.; Tabei, A. Synthesis and analgesic activity of novel hydrazide and hydrazine derivatives. Iran. J. Pharm. Res. 2013, 12, 721–727.Search in Google Scholar
[3] Shau, W. Y.; Chen, H. C.; Chen, S. T.; Chou, H. W.; Chang, C. H.; Kuo, C. W.; Lai, M. S. Risk of new acute myocardial infarction hospitalization associated with use of oral and parenteral non-steroidal anti-inflammation drugs (NSAIDs): a case-crossover study of Taiwan’s National Health Insurance claims database and review of current evidence. BMC Cardiovasc. Disord. 2012, 12, 4–16.10.1186/1471-2261-12-4Search in Google Scholar PubMed PubMed Central
[4] Cuadro, A. M.; Valneciano, J.; Vaquero, J. J.; Alvarez-Builla, J.; Sunkel, C.; Fau de Casa-Juana, M.; Pilar, M. Synthesis and biological evaluation of 2,6-di-tert-butylphenol hydrazones as 5-lipoxygenase inhibitors. Bioorg. Med. Chem.1998, 6, 173–180.10.1016/S0968-0896(97)10018-9Search in Google Scholar PubMed
[5] Dimmock, J. R.; Vashishtha, S. C.; Stables, J. P. Anticonvulsant properties of various acetylhydrazones, oxamoylhydrazones and semicarbazones derived from aromatic and unsaturated carbonyl compounds. Eur. J. Med. Chem.2000, 32, 241–248.10.1016/S0223-5234(00)00123-9Search in Google Scholar
[6] Egrenç, N.; Günay, N. S. Synthesis and antidepressant evaluation of new 3-phenyl-5-sulfonamidoindole derivatives. Eur. J. Med. Chem.1998, 33, 143–148.10.1016/S0223-5234(98)80039-1Search in Google Scholar
[7] Khan, S. S.; Hasan, A. Synthesis of some new bioactive 1-N-acid hydrazide substituted pyrazolines. Heterocycl. Commun. 2006, 12, 377–382.10.1515/HC.2006.12.5.377Search in Google Scholar
[8] Sahan, E.; Yildirim, I.; Albayak, S. Synthesis of 4-benzoyl-1,5-diphenyl-1H-pyrazole-3-carboxylic acid derivatives and their antimicrobial activities. Heterocycl. Commun. 2013, 19, 183–187.10.1515/hc-2012-0167Search in Google Scholar
[9] Żmudzki, P.; Satała, G.; Chłoń-Rzepa, G.; Bojarski, A. J.; Popik, P.; Zajdel, P. N-(4-Arylpiperazinoalkyl)acetamide derivatives of 1,3-and 3,7-dimethyl-1H-purine-2,6(3H,7H)-diones and their 5-HT6, 5-HT7, and D2 receptors affinity. Heterocycl. Commun. 2015, 21, 13–18.10.1515/hc-2014-0200Search in Google Scholar
[10] Chłoń-Rzepa, G.; Żmudzki, P.; Pawłowski, M.; Wesołowska, A.; Satała, G.; Bojarski, A. J.; Jabłoński, M.; Kalinowska-Tłuścik, J. New 7-arylpiperazinylalkyl-8-morpholin-4-yl-purine-2,6-dione derivatives with anxiolytic activity – synthesis, crystal structure and structure-activity study. J. Mol. Struct. 2014, 1067, 243–251.10.1016/j.molstruc.2014.03.018Search in Google Scholar
[11] Partyka, A.; Chłoń-Rzepa, G.; Wasik, A.; Jastrzębska-Więsek, M.; Bucki, A.; Kołaczkowski, M.; Satała, G.; Bojarski, A. J.; Wesołowska, A. Antidepressant- and anxiolytic-like activity of 7-phenylpiperazinylalkyl-1,3-dimethyl-purine-2,6-dione derivatives with diversified 5-HT1A receptor functional profile. Bioorg. Med. Chem. 2015, 23, 212–221.10.1016/j.bmc.2014.11.008Search in Google Scholar PubMed
[12] Chłoń-Rzepa, G.; Pawłowski, M.; Zygmunt, M.; Filipek, B.; Maciąg. D. Synthesis and cardiovascular activity of new 8-alkylamino-1,3-dimethyl-7-(2-hydroxy-3-piperazinopropyl)-3,7-dihydro-1H-purine-2,6-diones. Pol. J. Pharmacol. 2004, 56, 755–766.Search in Google Scholar
[13] Chłoń-Rzepa, G.; Żmudzki, P.; Pawłowski, M.; Zygmunt, M.; Filipek, B. Structure-cardiovascular activity relationships in a group of new 8-alkylamino-1,3-dimethyl-7-(2-hydroxy-3-aminopropyl)-3,7-dihydro-1H-purine-2,6-diones. Pharm. Rep. 2011, 63, 476–486.10.1016/S1734-1140(11)70514-XSearch in Google Scholar PubMed
[14] Zygmunt, M.; Żmudzki, P.; Chłoń-Rzepa, G.; Sapa, J.; Pawłowski, M. Synthesis and analgesic activity of 3,7-dimethylpurine-2,6-dion-1-yl derivatives of acetic and butanoic acid. Lett. Drug Des. Discov. 2014, 11, 1204–1213.10.2174/1570180811666140718162449Search in Google Scholar
[15] Zygmunt, M.; Żmudzki, P.; Chłoń-Rzepa, G.; Sapa, J. Analgesic and anti-inflammatory activity of 7-substituted purine-2,6-diones. Pharm. Rep. 2014, 66, 996–1002.10.1016/j.pharep.2014.06.015Search in Google Scholar PubMed
[16] Zygmunt, M.; Chłoń-Rzepa, G.; Sapa, J.; Pawłowski, M. Analgesic activity of new 8-methoxy-1,3-dimethyl-2,6-dioxo-purin-7-yl derivatives with carboxylic, ester or amide moieties. Pharm. Rep. 2015, 67, 9–16.10.1016/j.pharep.2014.07.018Search in Google Scholar PubMed
[17] Cacace, F.; Crisera, R.; Zifferero, M. Preparazione di alcuni derivati degli acidi 8-Br-teofilin 7-acetico e 8-alchilammino-teofilin-7-acetici. Ann. Chim. (Roma) 1956, 46, 99–104.Search in Google Scholar
[18] Karolak-Wojciechowska, J.; Pawłowski, M. Synthesis, crystal and molecular structure of 1,3-dimethyl-10-benzyl-2,4,9-trioxo-1,3,6,7,8,10-hexahydro-1,3-diazepino-[2,1-f]-purine. J. Crystallogr. Spectrosc. Res. 1990, 20, 477–482.10.1007/BF01180116Search in Google Scholar
[19] Pawłowski, M.; Chłoń, G.; Obniska, J.; Zejc, A.; Charakchieva-Minol, S.; Mokrosz, M. J. Synthesis, 5-HT1A and 5-HT2A receptor affinity of new 1-phenylpiperazinylpropyl derivatives of purine-2,6- and pyrrolidine-2,5-diones. Il Farmaco2000, 55, 461–468.10.1016/S0014-827X(00)00069-0Search in Google Scholar PubMed
[20] Hendershot, L. C.; Forsaith, J. Antagonism of the frequency of phenylbenzoquinone-induced writhing in the mouse by weak analgesics and nonanalgesics. J. Pharmacol. Exp. Ther. 1959, 125, 237–240.Search in Google Scholar
[21] Malvar, D. C.; Fereira, R. T.; de Castro, R. A.; Castro, L. L.; Freitas, A. C.; Costa, E. A.; Florentino, I. F.; Mafra, J. C.; de Souza, G. E.; Vanderlinde, F. A. Antinociceptive, anti-inflammatory and antipyretic effects of 1,5-phenyl-1H-pyrazole-3-carbohydrazide, a new heterocyclic pyrazole derivative. Life Sci. 2014, 95, 81–88.10.1016/j.lfs.2013.12.005Search in Google Scholar PubMed
[22] Sinha, B.; Semmler, J.; Eisenhut, T.; Eigler, A.; Endres, S. Enhanced tumor necrosis factor suppression and cyclic adenosine monophosphate accumulation by combination of phosphodiesterase inhibitors and prostanoids. Eur. J. Immunol. 1995, 25, 147–153.10.1002/eji.1830250125Search in Google Scholar PubMed
[23] Śladowska, H.; Filipek, B.; Szkatuła, D.; Sapa, J.; Bednarski, M.; Ciołkowska, M. Investigations on the synthesis and pharmacological properties of N-substituted derivatives of 4-alkoxy-6-methyl-1H-pyrrolo[3,4-c]pyridine-1,3(2H)-diones. Il Farmaco2005, 60, 53–59.10.1016/j.farmac.2004.08.011Search in Google Scholar PubMed
Supplemental Material:
The online version of this article (DOI: 10.1515/hc-2015-0100) offers supplementary material, available to authorized users.
©2015 by De Gruyter
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Articles in the same Issue
- Frontmatter
- Guest Editorial
- Dedication to Kyoichi A. Watanabe
- Review
- From ribavirin to NAD analogues and back to ribavirin in search for anticancer agents
- Preliminary Communications
- 5′-Norcarbocyclic analogues of furano[2,3-d]pyrimidine nucleosides
- Fluorescent 1,2,3-triazole derivative of 3′-deoxy-3-azidothymidine: synthesis and absorption/emission spectra
- Research Articles
- Synthesis and characterization of N-glucosylated dithiadiazepine derivatives through carbon-sulfur bond formation
- Synthesis of 8-alkoxy-1,3-dimethyl-2, 6-dioxopurin-7-yl-substituted acetohydrazides and butanehydrazides as analgesic and anti-inflammatory agents
- 13C NMR spectroscopy of heterocycles: 3,5-diaryl-4-bromoisoxazoles
- Synthesis and anti-proliferative activity of pyridine O-galactosides and 4-fluorobenzoyl analogues
- Optimized synthesis of 3′-O-aminothymidine and evaluation of its oxime derivative as an anti-HIV agent
- Synthesis and antimicrobial properties of 5,5′-modified 2′,5′-dideoxyuridines
- Acyclic analogs of nucleosides based on tris(hydroxymethyl)phosphine oxide: synthesis and incorporation into short DNA oligomers
- Synthesis and antiviral evaluation of 2′,3′-dideoxy-2′,3′-difluoro-D-arabinofuranosyl 2,6-disubstituted purine nucleosides
Articles in the same Issue
- Frontmatter
- Guest Editorial
- Dedication to Kyoichi A. Watanabe
- Review
- From ribavirin to NAD analogues and back to ribavirin in search for anticancer agents
- Preliminary Communications
- 5′-Norcarbocyclic analogues of furano[2,3-d]pyrimidine nucleosides
- Fluorescent 1,2,3-triazole derivative of 3′-deoxy-3-azidothymidine: synthesis and absorption/emission spectra
- Research Articles
- Synthesis and characterization of N-glucosylated dithiadiazepine derivatives through carbon-sulfur bond formation
- Synthesis of 8-alkoxy-1,3-dimethyl-2, 6-dioxopurin-7-yl-substituted acetohydrazides and butanehydrazides as analgesic and anti-inflammatory agents
- 13C NMR spectroscopy of heterocycles: 3,5-diaryl-4-bromoisoxazoles
- Synthesis and anti-proliferative activity of pyridine O-galactosides and 4-fluorobenzoyl analogues
- Optimized synthesis of 3′-O-aminothymidine and evaluation of its oxime derivative as an anti-HIV agent
- Synthesis and antimicrobial properties of 5,5′-modified 2′,5′-dideoxyuridines
- Acyclic analogs of nucleosides based on tris(hydroxymethyl)phosphine oxide: synthesis and incorporation into short DNA oligomers
- Synthesis and antiviral evaluation of 2′,3′-dideoxy-2′,3′-difluoro-D-arabinofuranosyl 2,6-disubstituted purine nucleosides

