Abstract
A green and simple method for the synthesis of the title compounds 4 by the reaction of 5-chloroacetyl-8-hydroxyquinoline (1), pentane-2,4-dione (2), and amines 3 in the presence of a catalytic amount of 1,4-diazabicyclo[2.2.2]octane at 60°C is described. The procedure is amenable for the synthesis of other substituted pyrroles. Short reaction time, environmentally friendly procedure, and excellent yields are the main advantages. The structures of products 4a–n were characterized by 1H NMR, IR, and MS spectra.
Introduction
Multicomponent reactions have emerged as a valuable tool in modern combinatorial synthesis. Moreover, one-pot multicomponent reactions, because of their productivity, facile execution, and simple reaction profile, are an important strategy in multicomponent reactions [1–4]. The pyrrole ring is widely distributed in many natural and biologically important molecules such as porphyrins, coenzymes, and alkaloids [5]. There has been an enhanced interest in the synthesis of pyrrole and its oligomers due to their potential application as conducting materials [6]. Owing to their diverse biological and pharmaceutical applications [7–9], there is a continuous interest in the synthesis of pyrroles by simple methods [10, 11]. Recently, a synthesis of pyrroles was accomplished by the multicomponent reaction of phenacyl bromide, amine, and acetylacetone in the presence of β-cyclodextrin in water [12]. However, the reported method is limited to the use of phenacyl bromide and aryl amines. The use of an aqueous medium has attracted considerable interest because of environmental and economic issues [13]. Very recently, 1,4-diazabicyclo[2.2.2]octane (DABCO) has emerged as a promoter for various organic reactions [14–18]. DABCO is an organic base that can act as nucleophile and is soluble in water. In continuation of our research program to develop environmentally friendly reactions [19–22], we report herein a simple, practical, and general three-component reaction for the construction of pyrrole derivatives by the reaction of 5-chloroacetyl-8-hydroxyquinoline, acetylacetone, and aryl-, heteroaryl-, and alkylamines in aqueous medium in the presence of DABCO.
Results and discussion
The one-pot three-component reaction between 5-chloroacetyl-8-hydroxyquninoline (1, 1 mmol), acetylacetone (2, 1 mmol), and 4-methoxyaniline (3a, 1 mmol) (Scheme 1) in the presence of different catalysts and in different solvents under reflux conditions was investigated as a model reaction. The results showed that the yield of the desired product 4a was enhanced by using DABCO as a catalyst. The product was formed in low yields of 21%, 30%, and 18% in the presence of Et3N, l-proline, and pyridine, respectively. The use of Na2CO3 as a basic catalyst in water failed to give the target compound 4a. Thus, DABCO was chosen as an optimal catalyst for further investigations. After extensive screening of the molar ratio (5, 10, 15, and 20 mol%) of DABCO to the substrates, it was found that the amount of 10 mol% promoted the maximum conversion to the product, in a yield of up to 93%. It was also evident that an increase in the molar ratio above 10 mol% did not increase the yield and did not shorten the reaction time. After conducting the reaction in a variety of solvents including 1,2-dichloroethane, tetrahydrofuran, acetonitrile, and methanol, it was concluded that water was the most suitable solvent for this transformation. Higher yields were obtained when the reaction was conducted at 60°C compared to reflux conditions.
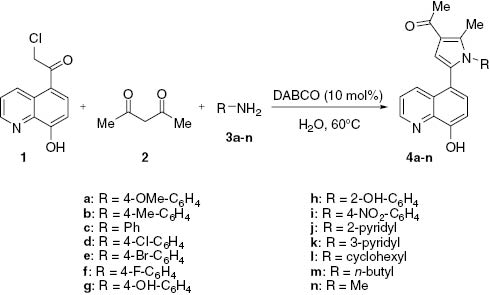
Synthesis of 5-(N-substituted pyrrol-2-yl)-8-hydroxyquinolines 4a–n.
Then, yields of the reactions conducted with various amines were compared. Under the optimized conditions as discussed above, the results showed that aromatic amines, heteroaromatic amines, and aliphatic amines successfully reacted with 5-chloroacetyl-8-hydroxyquinoline (1) and acetylacetone (2) to give the desired products 4a–n in good to excellent yields (60–93%).
The structures of products 4a–n were confirmed on the basis of their spectra and elemental analysis. In particular, the IR spectra of 4a–n show characteristic absorption bands of a conjugated acetyl group at 1665–1679 cm-1. In the 1H NMR spectra, the methyl protons of the pyrrole-methyl moiety give a singlet at 2.06–2.30 ppm and the acetyl protons resonate at 2.45–2.75 ppm, also as a singlet. A characteristic singlet at 6.40–6.6.72 ppm is ascribed to pyrrole H-3.
A suggested mechanism for the formation of pyrroles 4a–n is shown in Scheme 2. It can be presumed that initially pentane-2,4-dione 2 undergoes a reaction with amine 3 to form the unsaturated amino ketone 5, which is in equilibrium with its tautomer 6.The quaternization reaction of substrate 1 with DABCO generates the quaternary salt 7, which subsequently undergoes a reaction with 6 to form the intermediate product 8, which is the suggested precursor to the final product 4.
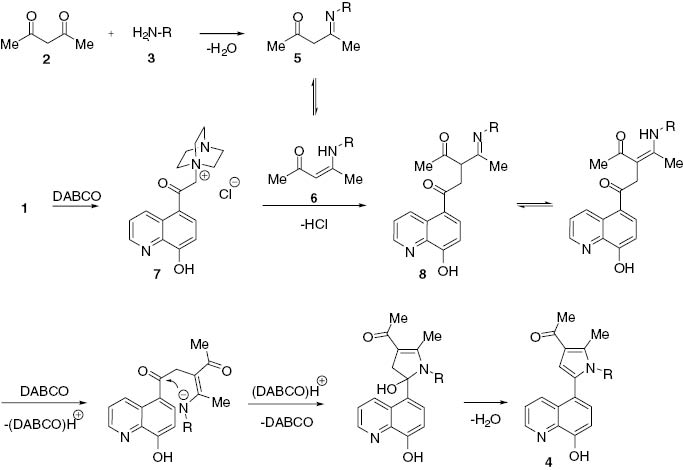
Mechanistic pathway to compounds 4a–n.
Conclusion
A novel, green, rapid, and efficient protocol for the synthesis of 5-(N-substituted pyrrol-2-yl)-8-hydroxyquinolines in water in the presence of DABCO was developed.
Experimental
Melting points were determined on a Kofler melting point apparatus and are not corrected. IR spectra were recorded on a Pye Unicam SP3-100 spectrophotometer using KBr pellets. The 1H NMR (400 MHz) and 13C NMR (100 MHz) spectra were recorded on a Jeol LA 400 instrument. Electron-impact mass spectra were taken on a JEOL JMS 600 spectrometer at an ionizing potential of 70 eV. Elemental analyses were carried out using a Perkin-Elmer 240 C Micro analyzer at Assiut University.
General procedure for the synthesis of 5-[1-substituted 4-acetyl-5-methyl-1H-pyrrol-2-yl)]-8-hydroxyquinolines 4a–n
A mixture of 5-chloroacetyl chloride (1, 1 mmol), acetylacetone (2, 1 mmol), amine (3a–n, 1 mmol), and DABCO (10 mol%) in water (5 mL) was stirred at 60°C for the period of time indicated below. The progress of the reaction was monitored by TLC. The solid obtained was washed with distilled water (3×10 mL) for removal of the catalyst and extracted with dichloromethane. The extract was filtered and concentrated under reduced pressure. Pure product 4a–n was obtained by crystallization from ethanol.
5-[4-Acetyl-1-(4-methoxyphenyl)-5-methyl-1H-pyrrol-2-yl]-8-hydroxyquinoline (4a)
Reaction time 1.5 h; yield 93%; yellow crystals; mp 231–233°C; IR: ν 3040 (CH, aromatic), 2950, 2850 (CH, aliphatic), 1655 (C=O), 1630 cm-1(C=C); 1H NMR: δ 2.25 (s, 3H, CH3), 2.45 (s, 3H, COCH3), 3.55 (s, 3H, OCH3), 6.60 (s, 1H, pyrrole H-3), 6.85–8.40 (m, 10H, aromatic H); 13C NMR: δ 9.5, 23.2 (2CH3), 60.3 (OCH3), 111.4 (C), 114.3 (CH), 115.7 (CH), 116.3 (2CH), 118.8 (C), 119.3 (C), 122.6 (CH), 123.3 (2CH), 124.1 (CH), 126.7 (C), 128.3 (C), 132.4 (C), 136.1 (CH), 139.4 (C), 150.7 (CH), 154.6 (C), 158.5 (C), 199.6 (C=O); MS: m/e 372.11 (M+, 62%). Anal. Calcd for C23H20N2O3(372.42): C, 74.18; H, 5.41; N, 7.52. Found: C, 74.51; H, 5.73; N, 7.89.
5-[4-Acetyl-1-(4-tolyl)-5-methyl-1H-pyrrol-2-yl]-8-hydroxyquinoline (4b)
Reaction time 2 h; yield 90%; yellow crystals; mp 191–193°C; IR: ν 3055 (CH, aromatic), 2965, 2850 (CH, aliphatic), 1666 (C=O), 1637 cm-1 (C=C); 1H NMR: δ 2.15 (s, 3H, CH3), 2.30 (s, 3H, CH3), 2.57 (s, 3H, COCH3), 6.60 (s, 1H, pyrrole H-3), 6.80–8.45 (m, 10H, aromatic H). Anal. Calcd for C23H20N2O2(356.42): C, 77.51; H, 5.66; N, 7.86. Found: C, 77.88; H, 5.94; N, 8.15.
5-(4-Acetyl-1-phenyl-5-methyl-1H-pyrrol-2-yl)-8-hydroxyquinoline (4c)
Reaction time 2.5 h; yield 88%; light yellow crystals; mp 150–152°C; IR: ν 3015 (CH, aromatic), 2975, 2850 (CH, aliphatic), 1665 (C=O), 1630 cm-1(C=C); 1H NMR: δ 2.10 (s, 3H, CH3), 2.55 (s, 3H, COCH3), 6.70 (s, 1H, pyrrole H-3), 6.95–7.90 (m, 11H, aromatic H); MS: m/e 342.51 (M+, 17%). Anal. Calcd for C22H18N2O2(342.39): C, 77.17; H, 5.30; N, 8.18. Found: C, 77.52; H, 5.61; N, 8.45.
5-(4-Acetyl-1-(4-chlorophenyl)-5-methyl-1H-pyrrol-2-yl)-8-hydroxyquinoline (4d)
Reaction time 2.5 h; yield 80%; yellow crystals; mp 213–215°C; IR: ν 3110 (CH, aromatic), 2980 (CH, aliphatic), 1675 (C=O), 1635 cm-1(C=C); 1H NMR: δ 2.25 (s, 3H, CH3), 2.50 (s, 3H, COCH3), 6.66 (s, 1H, pyrrole H-3), 6.98–8.12 (m, 10H, aromatic H). Anal. Calcd for C22H17ClN2O2(376.48): C, 70.12; H, 4.55; Cl, 9.41; N, 7.43. Found: C, 70.43; H, 5.00; Cl, 9.68; N, 7.71.
5-(4-Acetyl-1-(4-bromophenyl)-5-methyl-1H-pyrrol-2-yl)-8-hydroxyquinoline (4e)
Reaction time 2.5 h; yield 72%; yellow crystals; mp 303–305°C; IR: ν 3095 (CH, aromatic), 2990 (CH, aliphatic), 1670 (C=O), 1627 cm-1 (C=C); 1H NMR: δ 2.30 (s, 3H, CH3), 2.55 (s, 3H, COCH3), 6.77 (s, 1H, pyrrole H-3), 6.95–8.15 (m, 10H, aromatic H); MS: m/e 421.13 (M+, 55%). Anal. Calcd for C22H17BrN2O2(421.29): C, 62.72; H, 4.07; Br, 18.97; N, 6.65. Found: C, 63.09; H, 4.41; Br, 19.16; N, 6.89.
5-(4-Acetyl-1-(4-fluorophenyl)-5-methyl-1H-pyrrol-2-yl)-8-hydroxyquinoline (4f)
Reaction time 3.5 h; yield 69%; yellow crystals; mp 258–260°C; IR: ν 3085 (CH, aromatic), 2985 (CH, aliphatic), 1666 (C=O), 1625 cm-1(C=C); 1H NMR: δ 2.11 (s, 3H, CH3), 2.65 (s, 3H, COCH3), 6.65 (s, 1H, pyrrole H-3), 6.80–7.80 (m, 10H, aromatic H). Anal. Calcd for C22H17FN2O2 (360.38): C, 73.32; H, 4.75; N, 7.77. Found: C, 73.58; H, 5.11; N, 8.13.
5-(4-Acetyl-1-(4-hydroxyphenyl)-5-methyl-1H-pyrrol-2-yl)-8-hydroxyquinoline (4g)
Reaction time 3.5 h; yield 70%; yellow crystals; mp 323–325°C; IR: ν 3455 (OH), 3100 (CH, aromatic), 2980 (CH, aliphatic), 1670 (C=O), 1633 cm-1 (C=C); 1H NMR: δ 2.10 (s, 3H, CH3), 2.70 (s, 3H, COCH3), 6.57 (s, 1H, pyrrole H-3), 7.12–8.15 (m, 10H, aromatic H), 9.85 (s, 1H, OH); 13C NMR: δ 11.1, 21.8 (2CH3), 109.3 (CH), 112.4 (C), 113.2 (CH), 115.4 (2CH), 118.6 (2CH), 120.1 (C), 122.3 (2CH), 122.9 (CH), 126.5 (CH), 126.8 (C), 128.6 (C), 131.9 (C), 134.3 (CH), 138.1 (C), 153.2 (C), 156.4 (C), 205.3 (C=O); MS: m/e 358.51 (M+, 17%). Anal. Calcd for C22H18N2O3(358.39): C, 73.73; H, 5.06; N, 7.82. Found: C, 74.05; H, 5.41; N, 8.07.
5-(4-Acetyl-1-(2-hydroxyphenyl)-5-methyl-1H-pyrrol-2-yl)-8-hydroxyquinoline (4h)
Reaction time 4 h; yield 75%; pale yellow crystals; mp 270–272°C; IR: ν 3440 (OH), 3090 (CH, aromatic), 2990 (CH, aliphatic), 1656 (C=O), 1616 cm-1(C=C); 1H NMR: δ 2.06 (s, 3H, CH3), 2.56 (s, 3H, COCH3), 5.90 (s, 1H, OH), 6.48 (s, 1H, pyrrole H-3), 6.99–8.02 (m, 10H, aromatic H). Anal. Calcd for C22H18N2O3(358.39): C, 73.73; H, 5.06; N, 7.82. Found: C, 73.99; H, 5.39; N, 8.19.
5-(4-Acetyl-1-(4-nitrophenyl)-5-methyl-1H-pyrrol-2-yl)-8-hydroxyquinoline (4i)
Reaction time 4 h; yield 60%; yellow crystals; mp 284–286°C; IR: ν 3095 (CH, aromatic), 2899 (CH, aliphatic), 1660 (C=O), 1626 cm-1 (C=C); 1H NMR: δ 2.12 (s, 3H, CH3), 2.55 (s, 3H, COCH3), 6.45 (s, 1H, pyrrole H-3), 6.95–8.12 (m, 10H, aromatic H); MS: m/e 378.03 (M+, 12%). Anal. Calcd for C22H17N3O4(378.39): C, 68.21; H, 4.42; N, 10.85. Found: C, 68.43; H, 4.75; N, 11.11.
5-(4-Acetyl-1-(pyridin-2-yl)-5-methyl-1H-pyrrol-2-yl)-8-hydroxyquinoline (4j)
Reaction time 2.5 h; yield 70%; buff crystals; mp 166–168°C; IR: ν 3097 (CH, aromatic), 2898 (CH, aliphatic), 1667 (C=O), 1633 cm-1 (C=C); 1H NMR: δ 2.09 (s, 3H, CH3), 2.48 (s, 3H, COCH3), 6.50 (s, 1H, pyrrole H-3), 6.95–8.39 (m, 10H, aromatic H); 13C NMR: δ 8.6, 22.4 (2CH3), 108.3 (C), 108.7 (CH), 116.1 (CH), 118.8 (CH), 119.4 (C), 122.6 (CH), 123.3 (CH), 124.1 (CH), 127.6 (C), 128.8 (C), 130.7 (C), 134.3 (CH), 136.2 (CH), 139.6 (C), 150.1 (CH), 153.7 (CH), 155.2 (C), 160.3 (C), 202.4 (C=O). Anal. Calcd for C21H17N3O2(343.38): C, 73.45; H, 4.49; N, 12.24. Found: C, 73.71; H, 4.79; N, 12.53.
5-(4-Acetyl-1-(pyridin-3-yl)-5-methyl-1H-pyrrol-2-yl)-8-hydroxyquinoline (4k)
Reaction time 5.5 h; yield 75%; buff crystals; mp 229–231°C; IR: ν 3108 (CH, aromatic), 2895 (CH, aliphatic), 1672 (C=O), 1629 cm-1 (C=C); 1H NMR: δ 2.25 (s, 3H, CH3), 2.65 (s, 3H, COCH3), 6.58 (s, 1H, pyrrole H-3), 6.90–8.51 (m, 10H, aromatic H); 13C NMR: δ 9.3, 22.6 (2CH3), 108.7 (C), 110.3 (CH), 115.2 (CH), 118.2 (CH), 120.5 (C), 123.1 (CH), 123.6 (CH), 124.4 (CH), 125.5 (C), 129.8 (C), 131.9 (C), 133.6 (CH), 135.8 (CH), 138.4 (C), 148.2 (CH), 151.2 (CH), 152.6 (CH), 155.1 (C), 196.2 (C=O); MS: m/e 343.27 (M+, 9 %). Anal. Calcd for C21H17N3O2(343.38): C, 73.45; H, 4.49; N, 12.24. Found: C, 73.66; H, 4.82; N, 12.50.
5-(4-Acetyl-1-cyclohexyl-5-methyl-1H-pyrrol-2-yl)-8-hydroxyquinoline (4l)
Reaction time 2 h; yield 69%; pale yellow crystals; mp 192–194°C; IR: ν 3009 (CH, aromatic), 2965 (CH, aliphatic), 1664 (C=O), 1623 cm-1 (C=C); 1H NMR: δ 0.99 (m, 2H, CH2), 1.39 (m, 4H, 2CH2), 1.58 (m, 4H, 2CH2), 2.25 (s, 3H, CH3), 2.65 (s, 3H, COCH3), 3.66 (m 1H, N-CH), 6.60 (s, 1H, pyrrole H-3), 6.85–7.99 (m, 6H, aromatic H). Anal. Calcd for C22H24N2O2(348.44): C, 75.83; H, 6.94; N, 8.04. Found: C, 76.08; H, 7.29; N, 8.39.
5-(4-Acetyl-1-butyl-5-methyl-1H-pyrrol-2-yl)-8-hydroxyquinoline (4m)
Reaction time 4.5 h; yield 65%; yellow crystals; mp 167–169°C; IR: ν 3088 (CH, aromatic), 2977 (CH, aliphatic), 1679 (C=O), 1639 cm-1 (C=C); 1H NMR: δ 1.15 (t, 3H, CH3), 1.40 (m, 2H, CH2), 1.55 (m, 2H, CH2), 2.17 (s, 3H, CH3), 2.55 (s, 3H, COCH3), 3.25 (t, 2H, CH2), 6.72 (s, 1H, pyrrole H-4), 6.90–8.09 (m, 6H, aromatic H); MS: m/e 322.31 (M+, 33%). Anal. Calcd for C20H22N2O2(322.40): C, 74.51; H, 6.88; N, 8.69. Found: C, 74.84; H, 7.15; N, 8.97.
5-(4-Acetyl-1,5-dimethyl-1H-pyrrol-2-yl)-8-hydroxyquinoline (4n)
Reaction time 2 h; yield 79%; yellow crystals; mp 110–112°C; IR: ν 3070 (CH, aromatic), 2975 (CH, aliphatic), 1675 (C=O), 1633 cm-1 (C=C); 1H NMR: δ 2.20 (s, 3H, CH3), 2.49 (s, 3H, COCH3), 3.50 (s, 3H, N-CH3), 6.40 (s, 1H, pyrrole H-3), 6.95–8.30 (m, 6H, aromatic H); MS: m/e 280.42 (M+, 16%). Anal. Calcd for C17H16N2O2(280.32): C, 72.84; H, 5.75; N, 9.99. Found: C, 73.10; H, 6.07; N, 10.23.
References
[1] Park, S. J.; Keum, G.; Kang, S. B.; Koh, H. Y.; Kim, Y.; Lee, D. H. A facile synthesis of N-carbamoylmethyl-α-aminobutyrolactones by the Ugi multicomponent condensation reaction. Tetrahedron Lett. 1998, 39, 7109–7112.10.1016/S0040-4039(98)01509-3Search in Google Scholar
[2] Deshmukh, M. B.; Salunkhe, S. M.; Patil, D. R.; Anbhule, P. V. A novel and efficient one step synthesis of 2-amino-5-cyano-6-hydroxy-4-aryl pyrimidines and their anti-bacterial activity. Eur. J. Med. Chem. 2009, 44, 2651–2654.10.1016/j.ejmech.2008.10.018Search in Google Scholar
[3] Hasaninejad, A.; Shekouhy, M.; Golzar, N.; Zare, A.; Doroodmand, M. M. Silica bonded n-propyl-4-aza-1-azoniabicyclo[2.2.2]octane chloride (SB-DABCO): a highly efficient, reusable and new heterogeneous catalyst for the synthesis of 4H-benzo[b]pyran derivatives. Appl. Catal. A: Gen. 2011, 402, 11–22.10.1016/j.apcata.2011.04.012Search in Google Scholar
[4] Tamaddon, F.; Farahi, M.; Karami, B. Molybdate sulfuric acid as a reusable solid catalyst in the synthesis of 2,3,4,5-tetrasubstituted pyrroles via a new one-pot [2+2+1] strategy. J. Mol. Catal. A: Chem. 2012, 356, 85–89.10.1016/j.molcata.2012.01.003Search in Google Scholar
[5] Boger, L.; Boyce, C. W.; Labroli, M. A.; Sehon, C. A.; Jin, Q. Total syntheses of ningalin A, lamellarin O, lukianol A, and permethyl storniamide A utilizing heterocyclic azadiene Diels-Alder reactions. J. Am. Chem. Soc. 1999, 121, 54–61.10.1021/ja982078+Search in Google Scholar
[6] Tietze, L. F.; Nordmann, G. Synthesis of a linear oligomeric styrylpyrrole using multiple Heck and Wittig reactions. Synlett 2001, 3, 337–340.10.1055/s-2001-11385Search in Google Scholar
[7] Kulagowski, J. J.; Broughton, H. B.; Curtis, N. R.; Mawer, I. M.; Ridgill, M. P.; Baker, R.; Emms, F.; Freed-Man, S. B.; Marwood, R.; Patel, S.; et al. 3-[[4-(4-Chlorophenyl)piperazin-1-yl]methyl]-1H-pyrrolo[2,3-b]pyridine:an antagonist with high affinity and selectivity for the human dopamine D4 receptor. J. Med. Chem. 1996, 39, 1941–1942.10.1021/jm9600712Search in Google Scholar
[8] Henry, J. R.; Rupert, K. C.; Dodd, J. H.; Turchi, I. J.; Wadsworth, S. A.; Cavender, D. E.; Fahmy, B.; Olini, G. C.; Davis, J. E.; Genesy, J. L. P.; et al. 6-Amino-2-(4-fluorophenyl)-4-methoxy-3-(4-pyridyl)-1H-pyrrolo[2,3-b]pyridine (RWJ 68354): a potent and selective p38 kinase inhibitor. J. Med. Chem. 1998, 41, 4196–4198.10.1021/jm980497bSearch in Google Scholar
[9] Miszke, A.; Foks, H.; Kedazia, A.; Kwapisz, E.; Zwolska, Z. The synthesis and microbiological activity of 2-mercapto-4-(pyrrolidin-1-yl)pyridine-3-carbonitrile derivatives. Heterocycles 2008, 75, 2251–2261.10.3987/COM-08-11390Search in Google Scholar
[10] Alexander, V. K.; Sromek, A. W.; Gevorgyan, V. A novel Cu-assisted cycloisomerization of alkynyl imines: efficient synthesis of pyrroles and pyrrole-containing heterocycles. J. Am. Chem. Soc. 2001, 123, 2074–2075.10.1021/ja0058684Search in Google Scholar
[11] Binder, J. T.; Kirsch, S. F. Synthesis of highly substituted pyrroles via a multimetal-catalyzed rearrangement-condensation-cyclization domino approach. Org. Lett. 2001, 8, 2151–2153.10.1021/ol060664zSearch in Google Scholar
[12] Murthy, S. N.; Madhav, B.; Kumar, A. V.; Rao, K. R.; Nageswar, Y. V. D. multicomponent approach towards the synthesis of substituted pyrroles under supramolecular catalysis using β-cyclodextrin as a catalyst in water under neutral conditions. Helv. Chim. Acta 2009, 92, 2118–2124.10.1002/hlca.200900098Search in Google Scholar
[13] Li, C. J. Organic reactions in aqueous media with a focus on carbon-carbon bond formations: a decade update. Chem. Rev. 2005, 105, 3095–3166.10.1021/cr030009uSearch in Google Scholar
[14] Zorn, C.; Gnad, F.; Salmen, S.; Herpin, T.; Reiser, O. Deprotection of N-alloc amines by Pd(0)/DABCO – an efficient method for in situ peptide coupling of labile amino acids. Tetrahedron Lett. 2001, 42, 7049–7053.10.1016/S0040-4039(01)01453-8Search in Google Scholar
[15] Krishna, P. R.; Sekhar, E. R.; Kannan, V. The use of acetylenic aldehydes in Baylis-Hillman reactions: synthesis of versatile allyl propargyl alcohols. Tetrahedron Lett. 2003, 44, 4973–4975.10.1016/S0040-4039(03)01169-9Search in Google Scholar
[16] Kumar, A.; Pawar, S. S. Salt effects on the Baylis-Hillman reaction. Tetrahedron 2003, 59, 5019–5026.10.1016/S0040-4020(03)00760-9Search in Google Scholar
[17] Cecchi, L.; Sarlo, F. D.; Machetti, F. 1,4-Diazabicyclo[2.2.2]octane (DABCO) as an efficient reagent for the synthesis of isoxazole derivatives from primary nitro compounds and dipolarophiles: the role of the base. Eur. J. Org. Chem. 2006, 21, 4852–4860.10.1002/ejoc.200600475Search in Google Scholar
[18] Yang, H.; Tian, R.; Li, Y. Organic reactions catalyzed by 1,4-diazabicyclo [2.2.2] octane (DABCO). Front. Chem. China 2008, 3, 279–287.10.1007/s11458-008-0049-5Search in Google Scholar
[19] Abdel-Mohsen, S. A. Synthesis, reactions and antimicrobial activity of 2-amino-4-(8-quinolinol-5-yl)-1-(p-tolyl)-pyrrole-3-carbonitrile. Bull. Korean Chem. Soc. 2005, 26, 719–728.10.5012/bkcs.2005.26.5.719Search in Google Scholar
[20] El-Emary, T. I.; Abdel-Mohsen, Sh. A. Multi-component one-pot synthesis and antimicrobial activities of 3-methyl-1,4-diphenyl-7-thioxo-4,6,8,9-tetrahydro-pyrazolo[5,4-b]pyrimidino [5,4-e]pyridine-5-one and related derivatives. Molecule 2012, 17, 14464–14483.10.3390/molecules171214464Search in Google Scholar
[21] Abdel-Mohsen, S. A.; Hussein, E. M. A Green synthetic approach to the synthesis of Schiff bases from 4-amino-2-thioxo-1,3-diazaspiro[5.5]undec-4-ene-5-carbonitrile as potential anti-inflammatory agents. Russ. J. Bioorg. Chem. 2104, 40, 343–349.10.1134/S1068162014030029Search in Google Scholar
[22] Abdel-Mohsen, S. A. A convenient Synthesis and preparation of the derivatives of ethyl-6-(8-hydroxyquinolin-5-yl)-3-methylpyridazine-4-carboxylate as antimicrobial agents. Eur. J. Chem. 2014, 5, 517–525.10.5155/eurjchem.5.3.517-525.1072Search in Google Scholar
©2015 by De Gruyter
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Articles in the same Issue
- Frontmatter
- Preliminary Communications
- Montmorillonite K10 catalyzed multi component reactions (MCR): synthesis of novel thiazolidinones as anticancer agents
- Synthesis, antibacterial, and antifungal activities of new pyrimidinone derivatives
- Research Articles
- Formation of 1-methyl[1,2,4]triazolo[4,3-a] quinazolin-5(4H)-ones by reaction of 2-hydrazinoquinazolin-4(3H)-ones with acetylacetone
- Synthesis of new 4′-(N-alkylpyrrol-2-yl)-2,2′: 6′,2″-terpyridines via N-alkylation of a pyrrole moiety
- Efficient synthesis of 3-(bromomethyl)-5-methylpyridine hydrobromide
- One-pot synthesis of 5-[1-substituted 4-acetyl-5-methyl-1H-pyrrol-2-yl)]-8-hydroxyquinolines using DABCO as green catalyst
- A new on-fluorescent sensor for Ag+ based on benzimidazole bearing bis(ethoxycarbonylmethyl)amino groups
- Synthesis of new derivatives of 10H-benzo[b]pyridazino[3,4-e][1,4]thiazines
- Efficient and convenient synthesis of pyrido [2,1-b]benzothiazole, pyrimidopyrido[2,1-b]benzothiazole and benzothiazolo[3,2-a][1,8]naphthyridine derivatives
- Synthesis of 3-benzylidene-dihydrofurochromen-2-ones: promising intermediates for biflavonoid synthesis
- Synthesis and antitumor activities of piperazine- and cyclen-conjugated dehydroabietylamine derivatives
- Synthesis, characterization, and antimicrobial evaluation of novel spiropiperidones
Articles in the same Issue
- Frontmatter
- Preliminary Communications
- Montmorillonite K10 catalyzed multi component reactions (MCR): synthesis of novel thiazolidinones as anticancer agents
- Synthesis, antibacterial, and antifungal activities of new pyrimidinone derivatives
- Research Articles
- Formation of 1-methyl[1,2,4]triazolo[4,3-a] quinazolin-5(4H)-ones by reaction of 2-hydrazinoquinazolin-4(3H)-ones with acetylacetone
- Synthesis of new 4′-(N-alkylpyrrol-2-yl)-2,2′: 6′,2″-terpyridines via N-alkylation of a pyrrole moiety
- Efficient synthesis of 3-(bromomethyl)-5-methylpyridine hydrobromide
- One-pot synthesis of 5-[1-substituted 4-acetyl-5-methyl-1H-pyrrol-2-yl)]-8-hydroxyquinolines using DABCO as green catalyst
- A new on-fluorescent sensor for Ag+ based on benzimidazole bearing bis(ethoxycarbonylmethyl)amino groups
- Synthesis of new derivatives of 10H-benzo[b]pyridazino[3,4-e][1,4]thiazines
- Efficient and convenient synthesis of pyrido [2,1-b]benzothiazole, pyrimidopyrido[2,1-b]benzothiazole and benzothiazolo[3,2-a][1,8]naphthyridine derivatives
- Synthesis of 3-benzylidene-dihydrofurochromen-2-ones: promising intermediates for biflavonoid synthesis
- Synthesis and antitumor activities of piperazine- and cyclen-conjugated dehydroabietylamine derivatives
- Synthesis, characterization, and antimicrobial evaluation of novel spiropiperidones

