Montmorillonite K10 catalyzed multi component reactions (MCR): synthesis of novel thiazolidinones as anticancer agents
-
Ganapavarapu Veera Raghava Sharma
, Bandaru Devi
Abstract
Montmorillonite K10 is a suitable catalyst in a multicomponent reaction involving an aldehyde, an amine, and thioglycolic acid in N,N-dimethylformamide as solvent at moderate (50°C) to reasonably high (120°C) temperatures to form thiazolidinones. The reaction involves easy workup and purification. Several thiazolidinones were prepared. In particular, campholenic aldehyde obtained from α-pinene was used to synthesize potentially bioactive thiazolidinones. All products were characterized by IR, 1H NMR, and mass spectra. Preliminary anticancer screening tests revealed that two compounds show anticancer activity and can be taken up for further screening.
Developing green synthetic routes to novel molecules for various applications in general and for drug discovery in particular is of interest in organic synthesis. In this regard, multi component reactions (MCRs) are becoming popular choice to achieve the synthesis of target molecules in a few steps thereby increasing efficiency [1–6]. In addition, suitable catalysts such as montmorillonite can provide eco-friendly reactions. Several reactions catalyzed by montmorillonite have been reported in the literature [7–12]. Thiazolidinones are an important class of compounds with potential biological activity [13–15]. In our pursuit to synthesize novel molecules containing thiazolidinone moiety for screening against certain anticancer targets, we have come across an easily scalable and environmently benign method for the synthesis of thiazolidinones. In the present communication, we wish to disclose the method involving a one pot MCR catalyzed by ecofriendly montmorillonite K10 catalyst, with a reasonable reaction time, easy workup, and purification procedure to furnish products in good to excellent yields. The method was found to be suitable to synthesize several thiazolidinones of medicinal interest.
In a typical reaction as shown in Scheme 1, an aldehyde, an amine, and thioglycolic acid were taken in 1:1:2 ratio, dissolved in N,N-dimethylformamide (DMF), and montmorillonite K10 was then added to this solution. The mixture was stirred at 50°C for 6–8 h based on monitoring the reaction by thin layer chromatography (TLC). Campholenic aldehyde (obtained from natural product α-pinene in a single step) was transformed into potentially bioactive thiazolidinones 4a–e by reacting with different amines and thioglycolic acid. After preliminary optimization of the conditions, as reported in this communication, the products were obtained in good to excellent yields. All new products were characterized by IR, 1H NMR, MS, and elemental analysis. It is worth noting an interesting observation that 4-fluoroaniline did not react with the aldehyde using earlier reported catalysts ([15] and references cited therein); however, it smoothly underwent a conversion to the corresponding thiazolidinone 4d in good yield using conditions described in this communication.

In another preparation shown in Scheme 2, the amine 5 was allowed to react with 3,4-dimethoxybenzaldehyde (6) and thioglycolic acid in the presence of montmorillonite K10 in dimethylsulfoxide (DMSO) at elevated temperatures (~120°C) to furnish the thiazolidinone 7a in good yield. The product separated as solid during workup. It is interesting to note an important observation that this reaction did not provide thiazolidinone 7 using the earlier reported catalysts ([15] and references cited therein). It was also observed that using the earlier reported catalysts, the reaction produced a Schiff base that is presumed to be the intermediate product in the synthesis of thiazolidinone 7. These results clearly demonstrate that montmorillonite K10 is a superior catalyst in many ways. Interestingly, the reaction seem to be proceeding smoothly even under neat conditions without any solvent. The detailed results will be communicated in due course.

Preliminary anticancer screening gave promising results. The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was performed as per standard protocol [16]. The reduction of tetrazolium salts is now widely accepted as a reliable way to examine cell proliferation. The yellow tetrazolium MTT is reduced by metabolically active cells, in part by the action of dehydrogenase enzymes, to generate reducing equivalents such as NADH and nicotinamide adenine dinucleotide phosphate (NADPH). The MTT cell proliferation assay measures the cell proliferation rate and, conversely, when metabolic events lead to apoptosis or necrosis, the reduction in cell viability. Doxorubucin was used as a standard reference drug. Cells (5000 cells) were plated in 96-well plates. The cells were treated with different concentrations of drug for 16 h. PBS was used as the control. After completion of the treatment, the media were replaced with fresh media containing 5 mg/ml MTT. The color intensity developed by intracellular formazan was measured at 570 nm using a microplate reader. The percentage of cell growth was calculated as the percentage of the absorption of treated cells to the absorption of non-treated cells. The data obtained are represented in the form of a bar diagram in Figure 1.
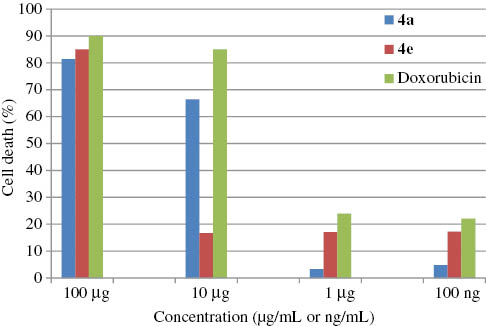
MTT assay results.
In conclusion, a montmorillonite K10-catalyzed MCR provides thiazolidinones in good to excellent yields. The preliminary screening of some of the new products using an MTT assay shows that several compounds are anticancer agents that can be proceeded for further screening. Additional details will be communicated in due course.
Experimental details
Reagents were obtained from Sigma-Aldrich or Acros and used without purification. Reactions were carriedout under inert atmosphere. Percentage yields refer to the isolated products after column chromatography. Silica gel (200 mesh) was used for column chromatography. Silica gel-coated aluminum sheets were used for TLC. Iodine or UV lamp was used to visualize the products on TLC plates. 1H NMR spectra (400 MHz, CDCl3) were recorded on a Bruker 400 instrument. Electron impact mass spectra (EI-MS) were obtained on an Apex spectrometer.
General experimental procedure for the preparation of thiazolidinones 4a–e
A mixture of campholenic aldehyde (1, 10 mmol), amine (10 mmol), thioglycolic acid (1.84 g, 20 mmol), and montmorillonite K10 (0.5 g) in DMF (15 mL) was heated at 50°C with stirring for 6 h. The reaction progress was monitored by TLC with dichloromethane and ethanol mobile phases. The reaction mixture was poured into water, extracted with dichloromethane (3×15 mL), and the extract was dried over silica, concentrated, and subjected to silica gel column chromatography using hexane/dichloromethane and dichloromethane/methanol as eluents to furnish the product.
2-[(2,2,3-Trimethylcyclopent-3-en-1-yl)methyl]-3-phenylthiazolidin-4-one (4a)
A viscous oil; yield 70%; purity 97%; 1H NMR: δ 7.42 (m, 2H, Ar), 7.30 (m, 3H, Ar), 5.22 (d, 1H, J = 10 Hz), 5.15 (m, 1H, olefinic), 3.70 (m, 2H), 1.8–2.3 (m, 5H), 1.67 (br s, 3H), 0.95 (s, 3H), 0.87 (s, 3H); IR (neat, cm-1): 3037, 1682; 13C NMR (100 MHz, CDCl3): δ 170.0, 150.0, 139.0, 130.0 (2C), 128.0, 125.0 (2C), 120.0, 65.0, 45.0, 42.0, 39.0, 35.0, 25.0, 19.4, 12.0. Anal. Calcd for C18H23NOS: C, 71.72; H, 7.69; N, 4.65; O, 5.31; S, 10.64. Found: C, 71.718; H, 7.69; N, 4.64; O, 5.31; S, 10.65.
3-(3-Chlorophenyl)-2-[(2,2,3-trimethylcyclopent-3-en-1-yl)methyl]thiazolidin-4-one (4b)
A viscous oil; yield 69%; purity 96%; 1H NMR: δ 7.19–7.39 (m, 4H), l (br s, 1H, olefinic), 5.11 (d, 1H, J = 10 Hz), 3.70 (m, 2H), 1.69–2.89 (m, 5H), 1.67 (br s, 3H), 0.95 (s, 3H), 0.87 (s, 3H); EI-MS: m/z 336 (M++1); IR (neat, cm-1): 2956, 1706. Anal. Calcd for C18H22ClNOS: C, 64.36; H, 6.60; Cl, 10.55; N, 4.17; O, 4.76; S, 9.55. Found: C, 64.34; H, 6.59; Cl, 10.55; N, 4.17; O, 4.75; S, 9.54.
3-(4-Chlorophenyl)-2-[(2,2,3-trimethylcyclopent-3-en-1-yl)methyl]thiazolidin-4-one (4c)
A viscous oil; yield 70%; purity 98%; 1H NMR: δ 7.4 (d, 2H, J=8 Hz) 7.2 (d, 2H, 8 Hz), 5.19–5.25 (dd, 1H, J = 10 Hz), 3.70–3.81 (m, 2H), 1.70–2.40 (m, 5H), 1.67 (br s, 3H), 0.95 (s, 3H), 0.87 (s, 3H); EI-MS: m/z 336 (M++1); IR (neat, cm-1): 2929, 1712. Anal. Calcd for C18H22ClNOS: C, 64.36; H, 6.60; Cl, 10.55; N, 4.17; O, 4.76; S, 9.55. Found: C, 64.35; H, 6.59; Cl, 10.54; N, 4.17; O, 4.76; S, 9.54.
3-(4-Fluorophenyl)-2-[(2,2,3-trimethylcyclopent-3-en-1-yl)methyl]thiazolidin-4-one (4d)
A viscous oil; yield 72%; purity 95%; 1H NMR: δ 7.25 (m, 2H), 7.15 (m, 2H), 5.2 (b s, 1H, olefinic), 5.05 (d, 1H, J = 10 Hz), 3.70–4.00 (m, 2H), 1.60–2.3 (m, 5H), 1.67 (b s, 3H), 0.95 (s, 3H), 0.87 (s, 3H); IR (neat, cm-1): 3037, 1682; EI-MS: m/z 320 (M+). Anal. Calcd for C18H22FNOS: C, 67.68; H, 6.94; F, 5.95; N, 4.38; O, 5.01, S, 10.04. Found: C, 67.67; H, 6.95; F, 5.95; N, 4.38; O, 5.02, S, 10.03.
3-(2-Hydroxyphenyl)-2-[(2,2,3-trimethylcyclopent-3-en-1-yl)methyl]thiazolidin-4-one (4e)
A viscous oil; yield 65%; purity 95%; 1H NMR: δ 7.24–6.94 (m, 4H), 5.22 (d, 1H, J = 10 Hz), 5.16 (br s, 1H, olefininic), 3.70 (br s, 2H), 2.26 (m, 1H), 1.88 (m, 1H), 1.79 (m, 2H), 1.69 (m, 2H), 1.67 (br s, 3H), 1.57 (m, 1H), 0.93 (s, 3H), 0.86 (s, 3H); IR (neat, cm-1): 3292.7, 3037.0, 2953.2, 1682; EI-MS: m/z 318 (M+). Anal. Calcd for C18H23NO2S: C, 68.10; H, 7.30; N, 4.41; O, 10.08; S, 10.10. Found: C, 68.08; H, 7.31; N, 4.42; O, 10.09; S, 10.11
Synthesis of N-{(R)-4,5,6,7-tetrahydro-2-[2-(3,4-dimethoxyphenyl)-4-oxothiazolidin-3-yl]benzo[d]thiazol-6-yl}propionamide (7)
A mixture of 3,4-dimethoxybenzaldehyde (1.66 g, 10 mmol), the amine 5 (2.25 g, 10 mmol), thioglycolic acid (1.84 g, 20 mmol), and montmorillonite K10 (0.5 g) in DMSO (15 mL) was heated at 120°C with stirring for 6 h. The reaction progress was monitored by TLC with dichloromethane and methanol as mobile phases. The reaction mixture was poured into water, extracted with dichloromethane (3×15 mL), and the extract was dried over silica, concentrated and subjected to silica gel column chromatography using hexane/dichloromethane and dichloromethane/methanol eluents to furnish the product 7 as a viscous material: yield 2.7 g (60%). 1H NMR: δ 6.8 (d, 1H, aromatic), 6.75 (s, 1H, aromatic), 6. 6 (d, 1H, aromatic), 5.5 (m, 1H), 4.4 (m, 1H), 3.8–4.1 (dd, 2H), 3.9 (s, 6H, OMe), 3.0 (m, 1H), 2.6 (m, 2H), 2.4 (q, 2H), 1.8–2.0 (m, 2H), 1.1 (t, 3H, Me); IR (neat, cm-1): 2900, 1690; EI-MS: m/z 448 (M++1). Anal. Calcd for C21H25N3O4S2: C, 56.35; H, 5.63; N, 9.39; O, 14.30; S, 14.33. Found: C, 56.38; H, 5.62; N, 9.38; O, 14.30; S, 14.31.
Acknowledgments
We thank Laila Impex and Insitute of Life Sciences (ILS) for the spectral data and Prof. K. R. Prasad of IISc for useful discussions.
References
[1] Childers, K. K.; Haidle, A. M.; Machacek, M. R.; Rogers, J. P.; Romeo, E. A one-step, multi-component reaction for the synthesis of fully substituted 5-amino-4-carboxamidethiazoles. Tetrahedron Lett. 2013, 54, 2506–2510.10.1016/j.tetlet.2013.03.014Search in Google Scholar
[2] Balan, B.; Bahulayan, D. A novel green synthesis of α/β-amino acid functionalized pyrimidinone peptidomimetics using triazole ligation through click-multi-component reactions. Tetrahedron Lett. 2014, 55, 227–231.10.1016/j.tetlet.2013.11.002Search in Google Scholar
[3] Hasaninejad, A.; Firoozi, S.; Mandegani, F. An efficient synthesis of novel spiro[benzo[c]pyrano[3,2-a]phenazines] via domino multi-component reactions using l-proline as a bifunctional organocatalyst. Tetrahedron Lett. 2013, 54, 2791–2794.10.1016/j.tetlet.2013.03.073Search in Google Scholar
[4] Rajesh, U. C.; Kholiya, R.; Pavan, V. S.; Rawat, D. S. Catalyst-free, ethylene glycol promoted one-pot three component synthesis of 3-amino alkylated indoles via Mannich-type reaction. Tetrahedron Lett. 2014, 55, 2977–2981.10.1016/j.tetlet.2014.03.112Search in Google Scholar
[5] Maharani, S.; Kumar, R. R. Domino four-component synthesis of novel cycloocta[b]pyridines. Tetrahedron Lett. 2015, 56, 179–181.10.1016/j.tetlet.2014.11.052Search in Google Scholar
[6] Kumar, B. S.; Dakhinamoorthy, A.; Pitchumani, K. K10 montmorillonite clays as environmentally benign catalysts for organic reactions. Catal. Sci. Technol. 2014, 4, 2378–2396.10.1039/C4CY00112ESearch in Google Scholar
[7] Bharate, S. B.; Padala, A. K.; Dar, B. A.; Yadav, R. R.; Singh, B.; Vishwakarma, R. A. Montmorillonite clay Cu(II) catalyzed domino one-pot multicomponent synthesis of 3,5-disubstituted isoxazoles. Tetrahedron Lett. 2013, 54, 3558–3561.10.1016/j.tetlet.2013.04.102Search in Google Scholar
[8] Muhammad, N.; Nawaz, C. M.; Salama, R.; Rana, A. One pot synthesis of potentially biologically active novel 4-thiazolidinone derivatives. Asian J. Chem. 2012, 24, 4317.Search in Google Scholar
[9] Dintzner, M. R.; Mondjinou, Y. A.; Pileggi, D. J. Montmorillonite clay-catalyzed cyclotrimerization and oxidation of aliphatic aldehydes. Tetrahedron Lett. 2010, 51, 826–827.10.1016/j.tetlet.2009.12.009Search in Google Scholar
[10] Dintzner, M. R.; Little, A. J.; Pacilli, M.; Pileggi, D. J.; Osner, Z. R.; Lyons, T. Montmorillonite clay-catalyzed hetero-Diels-Alder reaction of 2,3-dimethyl-1,3-butadiene with benzaldehydes. Tetrahedron Lett. 2007, 48, 1577–1579.10.1016/j.tetlet.2007.01.010Search in Google Scholar
[11] Motokura, K.; Matsunaga, S.; Noda, H.; Miyaji, A.; Baba, T. Water-accelerated allylsilylation of alkenes using a proton-exchanged montmorillonite catalyst. ACS Catal. 2012, 2, 1942–1946.10.1021/cs300261bSearch in Google Scholar
[12] Jacobine, A. M.; Posner, G. H. Three component, one flask synthesis of rhodanines. J. Org. Chem. 2011, 76, 8121–8125.10.1021/jo201561tSearch in Google Scholar PubMed PubMed Central
[13] Kumar, K. S. S.; Swaroop, T. R.; Harsha, K. B.; Narasimhamurthy, K. H.; Rangappa, K. S. T3P®-DMSO mediated one pot cascade protocol for the synthesis of 4-thiazolidinones from alcohols. Tetrahedron Lett. 2012, 53, 5619–5623.10.1016/j.tetlet.2012.08.020Search in Google Scholar
[14] Kaminskyy, D.; Khyluk, D.; Vasylenko, O.; Lesyk, R. An efficient method for the transformation of 5-ylidenerhodanines into 2,3,5-trisubstituted-4-thiazolidinones. Tetrahedron Lett. 2012, 53, 557–559.10.1016/j.tetlet.2011.11.095Search in Google Scholar
[15] Thakare, M. P.; Kumar, P.; Kumar, N.; Pandey, S. K. Silica gel promoted environment-friendly synthesis of 2,3-disubstituted 4-thiazolidinones. Tetrahedron Lett. 2014, 55, 2463–2466.10.1016/j.tetlet.2014.03.007Search in Google Scholar
[16] Kondapi, A. K.; Satyanarayana, N.; Saikrishna, A. D. A study of the topoisomerase II activity in HIV-1 replication using the ferrocene derivatives as probes. Arch. Biochem. Biophys. 2006, 450, 123–132.10.1016/j.abb.2006.04.003Search in Google Scholar PubMed
©2015 by De Gruyter
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Articles in the same Issue
- Frontmatter
- Preliminary Communications
- Montmorillonite K10 catalyzed multi component reactions (MCR): synthesis of novel thiazolidinones as anticancer agents
- Synthesis, antibacterial, and antifungal activities of new pyrimidinone derivatives
- Research Articles
- Formation of 1-methyl[1,2,4]triazolo[4,3-a] quinazolin-5(4H)-ones by reaction of 2-hydrazinoquinazolin-4(3H)-ones with acetylacetone
- Synthesis of new 4′-(N-alkylpyrrol-2-yl)-2,2′: 6′,2″-terpyridines via N-alkylation of a pyrrole moiety
- Efficient synthesis of 3-(bromomethyl)-5-methylpyridine hydrobromide
- One-pot synthesis of 5-[1-substituted 4-acetyl-5-methyl-1H-pyrrol-2-yl)]-8-hydroxyquinolines using DABCO as green catalyst
- A new on-fluorescent sensor for Ag+ based on benzimidazole bearing bis(ethoxycarbonylmethyl)amino groups
- Synthesis of new derivatives of 10H-benzo[b]pyridazino[3,4-e][1,4]thiazines
- Efficient and convenient synthesis of pyrido [2,1-b]benzothiazole, pyrimidopyrido[2,1-b]benzothiazole and benzothiazolo[3,2-a][1,8]naphthyridine derivatives
- Synthesis of 3-benzylidene-dihydrofurochromen-2-ones: promising intermediates for biflavonoid synthesis
- Synthesis and antitumor activities of piperazine- and cyclen-conjugated dehydroabietylamine derivatives
- Synthesis, characterization, and antimicrobial evaluation of novel spiropiperidones
Articles in the same Issue
- Frontmatter
- Preliminary Communications
- Montmorillonite K10 catalyzed multi component reactions (MCR): synthesis of novel thiazolidinones as anticancer agents
- Synthesis, antibacterial, and antifungal activities of new pyrimidinone derivatives
- Research Articles
- Formation of 1-methyl[1,2,4]triazolo[4,3-a] quinazolin-5(4H)-ones by reaction of 2-hydrazinoquinazolin-4(3H)-ones with acetylacetone
- Synthesis of new 4′-(N-alkylpyrrol-2-yl)-2,2′: 6′,2″-terpyridines via N-alkylation of a pyrrole moiety
- Efficient synthesis of 3-(bromomethyl)-5-methylpyridine hydrobromide
- One-pot synthesis of 5-[1-substituted 4-acetyl-5-methyl-1H-pyrrol-2-yl)]-8-hydroxyquinolines using DABCO as green catalyst
- A new on-fluorescent sensor for Ag+ based on benzimidazole bearing bis(ethoxycarbonylmethyl)amino groups
- Synthesis of new derivatives of 10H-benzo[b]pyridazino[3,4-e][1,4]thiazines
- Efficient and convenient synthesis of pyrido [2,1-b]benzothiazole, pyrimidopyrido[2,1-b]benzothiazole and benzothiazolo[3,2-a][1,8]naphthyridine derivatives
- Synthesis of 3-benzylidene-dihydrofurochromen-2-ones: promising intermediates for biflavonoid synthesis
- Synthesis and antitumor activities of piperazine- and cyclen-conjugated dehydroabietylamine derivatives
- Synthesis, characterization, and antimicrobial evaluation of novel spiropiperidones

