Abstract
A series of thermotropic liquid crystalline poly(amide imide)s (PAIs) with well-defined structure were prepared by the Yamazaki-Higashi phosphorylation method. To obtain the target polymers, several diimide diacid monomers (DIDAs) as mesogenic units were synthesized by the dehydration cyclization of aromatic anhydride with aliphatic 11-aminoundecanoic acid (AU). The chemical structure of these DIDAs and PAIs was confirmed via Fourier transform infrared (FTIR) and proton nuclear magnetic resonance (1H-NMR) spectroscopy. Thermotropic liquid crystalline characteristics of the DIDAs and PAIs were investigated by differential scanning calorimetry (DSC), polarizing light microscopy (PLM) and X-ray diffraction (XRD) analysis. Encouragingly, all of these liquid crystalline PAIs exhibited good thermal stability, in which the decomposition temperatures are much higher than the melting temperatures of PAIs. Furthermore, the liquid crystalline PAIs can be dissolved into some common solvents such as dimethyl sulfoxide (DMSO) and m-cresol, which indicates these liquid crystalline PAIs could be processed not only by melting-processing but also by solution spin-coating.
1 Introduction
In the past few years, thermotropic liquid crystalline polymers (TLCPs) have been attracting much attention due to their unique properties such as good thermal stability, high strength and modulus, excellent chemical resistance (1), (2), (3), (4), (5), (6). For TLCPs, they consist of two basic units, rigid and flexible units, and the liquid crystalline features arise from rigidity present in the polymer backbone and/or pendant to the main chain from symmetric rigid sequences classified as mesogenic units. On the other hand, the flexible part contributes to the fluidity of the liquid crystal. In other words, there is a tradeoff between solubility and mechanical properties to TLCPs.
Aromatic poly(amide imide)s (PAIs) are considered as one kind of high performance polymers (7), (8), (9), (10), (11), (12), (13), (14), (15). The presence of an amide group in this polymer can increase the solubility, while the aromatic imide groups can provide thermal resistance and mechanical properties. As a result, PAIs combine thermal resistance and mechanical properties of polyimides as well as ease of processability of polyamides. Conventionally, PAIs have been investigated as engineering thermoplastic materials, and there were only few reports on the phase behavior of liquid crystalline PAIs (16), (17), (18), (19). Liu reported the phase transition and transition kinetics of a liquid crystalline copoly(amide-imide) (PAI37), which was prepared from 70 mol% pyromellitic dianhydride (PMDA), 30 mol% terephthaloyl chloride (TPC), and 1,3-bis[4-(4′-aminophenoxy) cumyl]benzene (BACB) (16). It should be noted that although the solubility of PAI37 was improved compared with PMDA-BACB, it is soluble only in warm N-methyl-2-pyrrolidone (NMP). Furthermore, this polymer was synthesized through random copolymerization, in other words, the properties of this copolymer would change depending on the ratio of PMDA, TPC and BACB in the main chain of the polymer. Therefore, it is well worth developing liquid crystalline PAIs with well-defined structures and good processability.
In this study, a series of liquid crystalline PAIs with well-defined structures have been prepared by the Yamazaki-Higashi phosphorylation of diimide diacid monomer as a mesogenic unit with p-phenylenediamine. To improve the solubility, a long alky chain (n-C10H21) is incorporated in the polymer main chain. The chemical structure of diimide diacid monomers and PAIs is characterized with Fourier transform infrared (FTIR) and proton nuclear magnetic resonance (1H-NMR). The phase transition of monomers and corresponding polymers are investigated by differential scanning calorimetry (DSC), polarizing light microscopy (PLM), and X-ray diffraction (XRD) analysis. The thermal stability and solubility of PAIs are also discussed.
2 Experimental
2.1 Materials
All starting reactants and solvents are purchased from the Aladdin Industrial Corporation (Shanghai, China). NMP was distilled over calcium hydride (CaH2). Other chemicals, 1,2,4,5-benzenetetracarboxylic anhydride (PMDA), benzophen-one-3, 3′,4,4′-tetracarboxylic-dianhydride (BTDA), 4,4′-oxy-diphthalic anhydride (ODPA), 3,3′,4,4′-biphenyltetracarboxylic di-anhydride (BPDA), 11-aminoundecanoic acid (AU), p-phenylenediamine (PPDA), d-trifluoroacetic acid (d-TFA), potassium bromide (KBr), triphenyl phosphite (TPP), pyridine (Py), m-cresol and methanol, are used without further purification.
2.2 Measurements
FTIR spectra were acquired with an Avatar 370 spectrometer (Thermo Nicolet, USA). Spectra were collected in the region of 4000–500 cm−1 with a spectral resolution of 0.1 cm−1. 1H-NMR spectra were acquired at room temperature for DIDAs and PAIs on an Avance Ш400 M NMR (Bruker, Rheinstetten, Germany) in trifluoroacetic acid (TFA) containing tetramethylsilane as the internal reference. The wide-angle X-ray diffraction (WXRD) measurement was obtained using a D/Max 2500 PC diffractometer (Rigaku Corporation, Japan) with a curved graphite crystal filter. The corresponding WXRD patterns were collected in the transmission mode at room temperature under a tube current of 300 mA and an acceleration voltage of 60 kV. The thermal properties of the sample were measured with a PE Pyris 1 DSC (Perkin Elmer, USA). About 7 mg of sample was sealed in an aluminum pan, an empty sealed pan was used as a reference. The sample was heated to 300°C at a rate of 10°C/min, the thermogram was recorded from the DSC first heating scanning. Decomposition characteristics of the samples were determined with a TG 209 F3 (Netzsch Tarsus, Germany). A total of 5 mg of sample was placed in the pan and heated from 50 to 750°C at a heating rate of 10°C/min under a nitrogen atmosphere. The morphological development of DIDAs and PAIs were observed with a Nikon 50I microscope coupled with a hot stage. The samples were sandwiched between two glass slides on a hot plate preheated to 300°C and then pressed, quenched to room temperature before morphology observation.
2.3 Synthesis of diimide diacid monomers (DIDAs)
The diimide diacid monomers (3a–3d) were synthesized through the dehydration cyclization method (20), (21). As shown in the Scheme 1, the monomers 3b, 3c and 3d were prepared by a procedure similar to that of 3a. The synthetic procedure of 3a is described as following. ODPA (1) (9.30 g, 0.03 mol), AU (12.08 g, 0.06 mol), and acetic acid (60 ml) were placed in a 100 ml three-neck round flask. The mixture was heated to reflux for 8 h under nitrogen atmosphere. And then, the mixture was cooled down to room temperature, poured into acetic acid and filtered. The crude product was washed with deionized water. Finally, the pure product was dried under vacuum of 80°C for 12 h.

Synthetic route of diimide diacid monomers (3a–3d).
2.4 Synthesis of poly(amide imide)s (PAIs)
The polymers (5a, 5b, 5c, and 5d) were prepared by a similar procedure of Yamazaki-Higashi phosphorylation reaction as shown in the Scheme 2 according to references (22), (23). The synthetic procedure of 5a is described in detail as following. CaCl2 (2 g, 0.018 mol) and LiCl (1 g, 0.024 mol) were added into the mixture of PPDA (0.541 g, 0.005 mol), 3a (3.384g, 0.005 mol), TPP (5 ml), and NMP (13 ml) in a 100 ml three-neck round flask under nitrogen protection. The reaction was gradually heated to reflux for 8–12 h. And then, the mixture was cooled to room temperature, poured into methanol and filtered. The crude product was washed with hot water and methanol alternatively, and dried under vacuum at 80°C overnight. After drying in vacuum, the pure product was obtained as a white-fabric solid.

Synthetic route of poly(amide imide)s (5a–5d).
3 Results and discussion
3.1 Structural characterization of diimide diacid monomers
The chemical structure of diimide diacid monomers (DIDAs) is proved by 1H-NMR and FTIR as shown in the Figures 1 and 2, respectively. In the FTIR spectra of ODPA-AU (3a) as an example, it shows a broad peak around 3467 cm−1, which is assigned to the COOH group. The formation of an imide ring between the anhydride and AUs is demonstrated by the characteristic absorption bands at about 1771 cm−1 (C=O asymmetric stretching, imide ring), 1694 cm−1 (C=O symmetric stretching, imide ring), 1393 cm−1 (C-N-C stretching, imide ring), and 745 cm−1 (imide ring deformation). Furthermore, the assignments of all the protons presented in 1H-NMR spectra are in good agreement with the proposed molecular structure as shown in the Scheme 1. In short, the results of FTIR and 1H-NMR spectra support the successful synthesis of diimide diacid monomers.
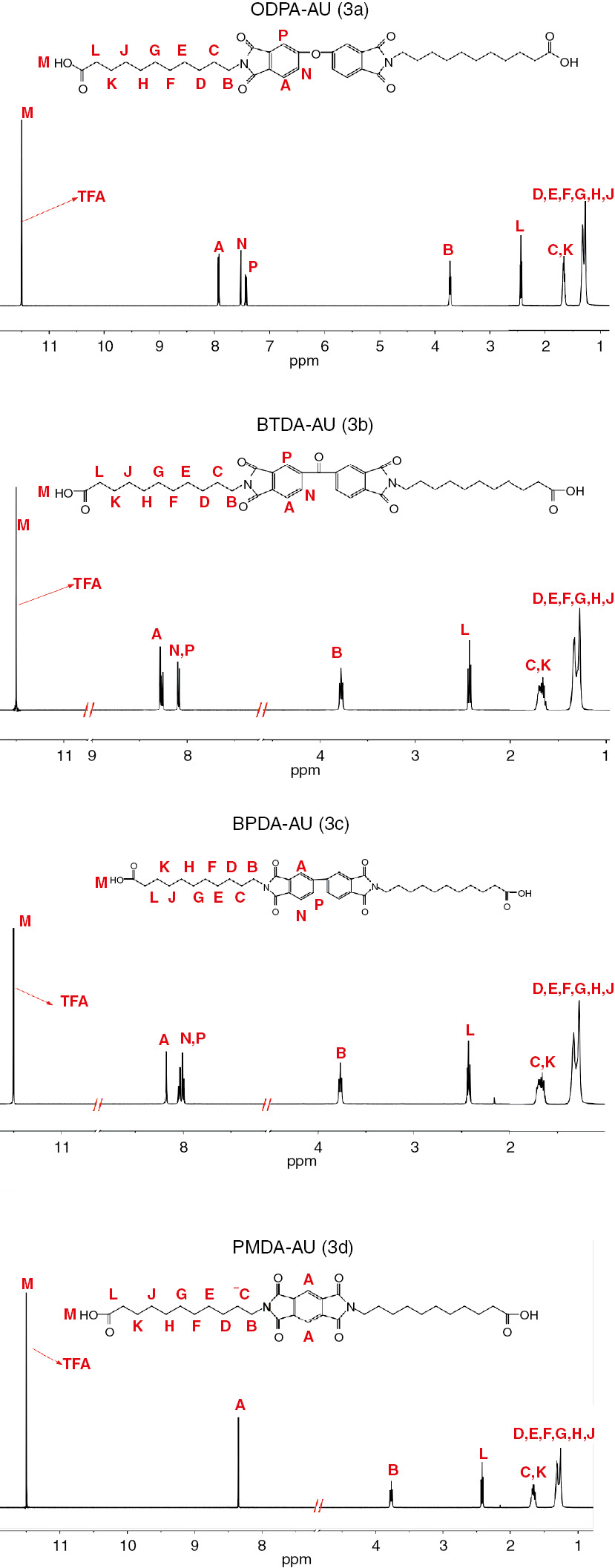
1H-NMR spectra of diimide diacid monomers (3a–3d).
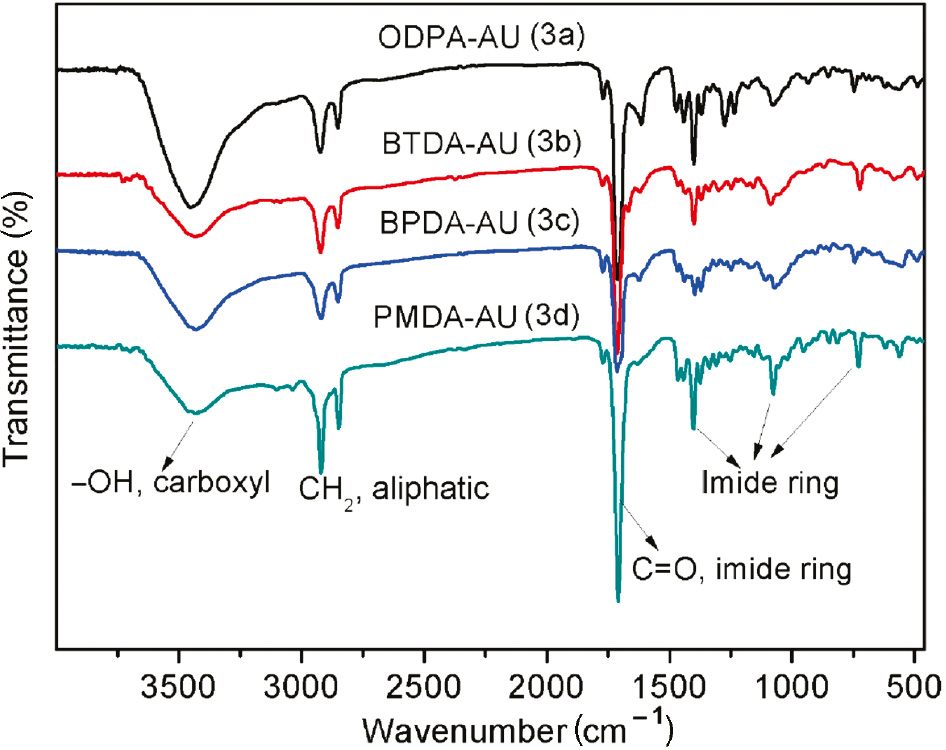
FTIR spectra of diimide diacid monomers (3a–3d).
3.2 Structural characterization of poly(amide imide)s
The PAIs (5a, 5b, 5c, and 5d) are synthesized by the Yamazaki-Higashi phosphorylation reaction of DIDAs and p-phenylenediamine. The chemical structure of the PAIs was confirmed by FTIR and 1H-NMR spectroscopy as shown in the Figures 3 and 4, respectively. A representative FTIR spectrum of PAI-ODPA (5a) in Figure 3 exhibits the N-H stretching vibration peak of amide group at 3294 cm−1, instead, the O-H stretching vibration peak of COOH group disappears, correspondingly. On the other hand, the assignment of all the protons presented in 1H-NMR spectra is also in good agreement with the proposed polymer structure as shown in the Scheme 2. Furthermore, in order to get the molecular weight information of PAIs, the inherent viscosity (η) of the PAIs is determined at a 0.5 g/dl polymer concentration in DMAc with an Ubbelohde viscometer at 30°C, and found to be in the range of 0.71–2.01 dl/g as summarized in Table 1, which indicates the molecular weight of PAIs is reasonably high and the polymerizations are successful.
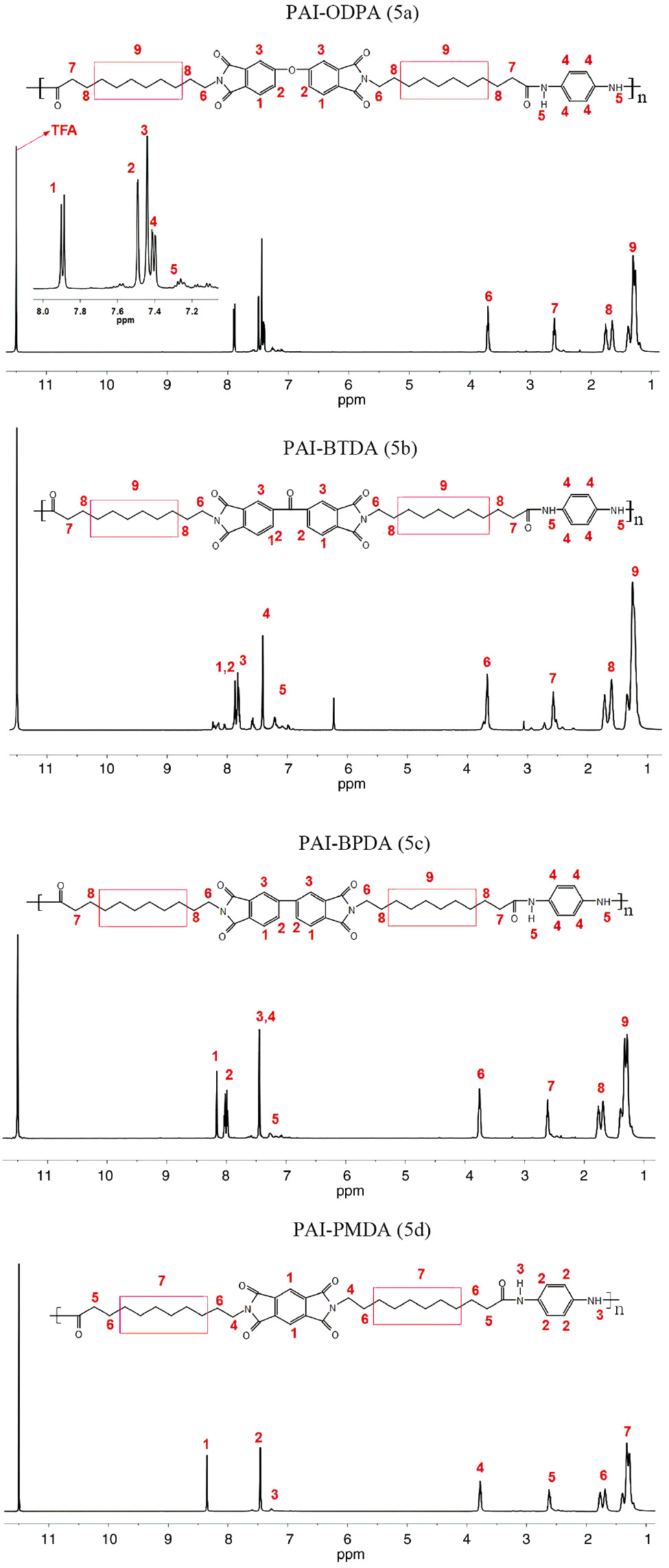
1H-NMR spectra of poly(amide imide)s (5a–5d).
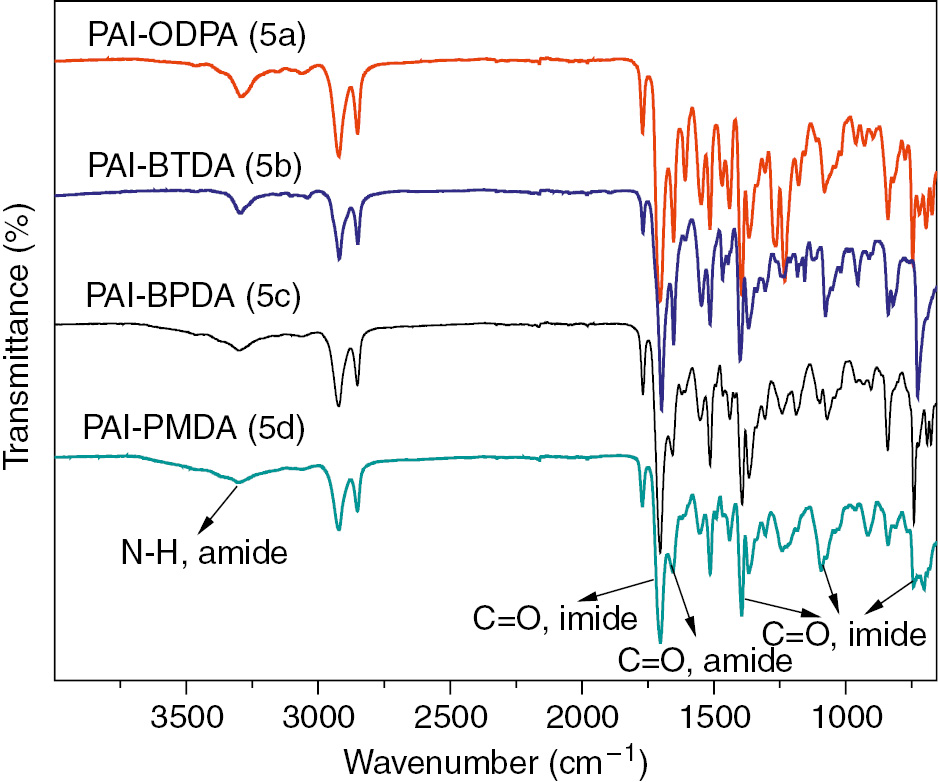
FTIR spectra of poly(amide imide)s (5a–5d).
The inherent viscosity (η) of poly(amide imide)s (5a–5d).
| PAIs | 5a | 5b | 5c | 5d |
|---|---|---|---|---|
| η (dl/g) | 2.01 | 0.96 | 0.71 | 0.80 |
3.3 Thermal properties and phase transition behavior of diimide diacid monomers
Firstly, the thermal properties and phase transition behavior of DIDAs are examined by DSC experiments. As illustrated in Figure 5, both monomers 3a and 3b display a cold crystallization temperature and a melting temperature, suggesting that monomers 3a and 3b are crystalline. However, monomers 3c and 3d exhibit a small exothermic transition rather than an obvious cold crystallization, which is a feature of liquid crystalline (24). These findings reveal that the molecular structure has significant effect on the thermal property and mesomorphic properties of the DIDAs.
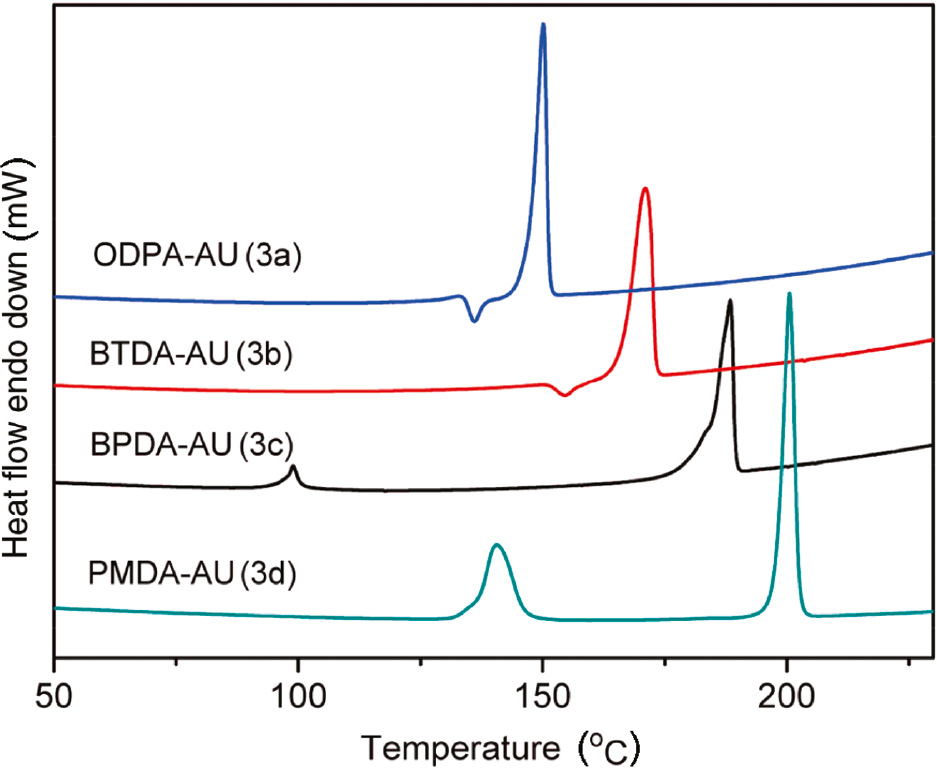
DSC traces of diimide diacid monomers (3a–3d).
Polarizing light microscopy is also used to observe the liquid crystalline phases and to complement the phase transitions observed by DSC. Samples on glass slides were heated to a molten state, covered with an upper cover slide and shear force was applied by the upper cover slide. They were then quenched in an ice-water bath. The quenched samples were heated and cooled at rate of 2°C per min, the optical microscopic textures for the mesophase of the monomers at different temperatures are observed as shown in the Figure 6. Upon heating, in the case of monomer 3a, the crystal texture starts to appear at 129°C, and becomes perfect until 139°C. It is consistent with the occurrence of a cold crystallization peak at 136°C in the DSC trace of monomer 3a. The monomer 3b shows a similar behavior with monomer 3a except that the crystal texture becomes perfect at 158°C. The monomer 3c is a glass liquid crystal at room temperature, and a few black spots are found in Figure 6 (3c–1). Upon heating to 96°C, the black spots disappear and the whole microscopic view becomes vivid. It means that the phase transition from LC glass to LC occurs, which is demonstrated by the appearance of a jump of the heat capacity at around 99°C as shown in the Figure 5. Similarly, the monomer 3d is a blue-phase LC glass at room temperature. Upon heating to 144°C, the phase transition from LC glass to LC occurs, and the whole microscopic view becomes yellowy. Accordingly, the DSC thermogram of monomer 3d displays a step at around 142°C associated with this phase transition.
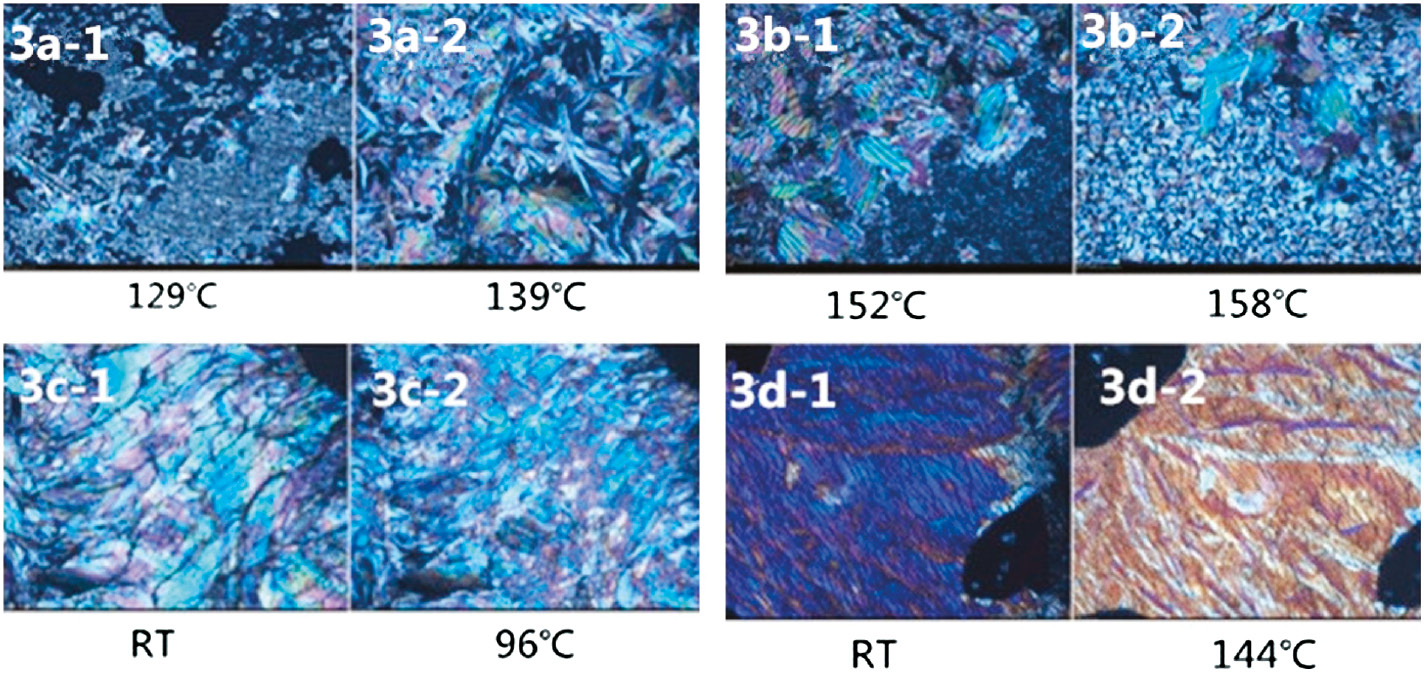
Polarized optical microscopic texture observed for diimide diacid monomers (3a–3d) at different temperatures.
The internal structure of liquid crystalline phases of monomers 3c and 3d is further verified using a WAXD instrument at room temperature. It is well known that the scattering factor ratio is a characteristic of the liquid crystalline phase. As shown in Figure 7, there are two peaks at 4.42° and 8.70° for monomer 3c, and three peaks at 3.07°, 5.97° and 8.87° for monomer 3d. By calculation, the scattering factor ratios of monomer 3c and 3d are 1:2 and 1:2:3, respectively, which is characteristic of lamellar liquid crystalline phase (25).
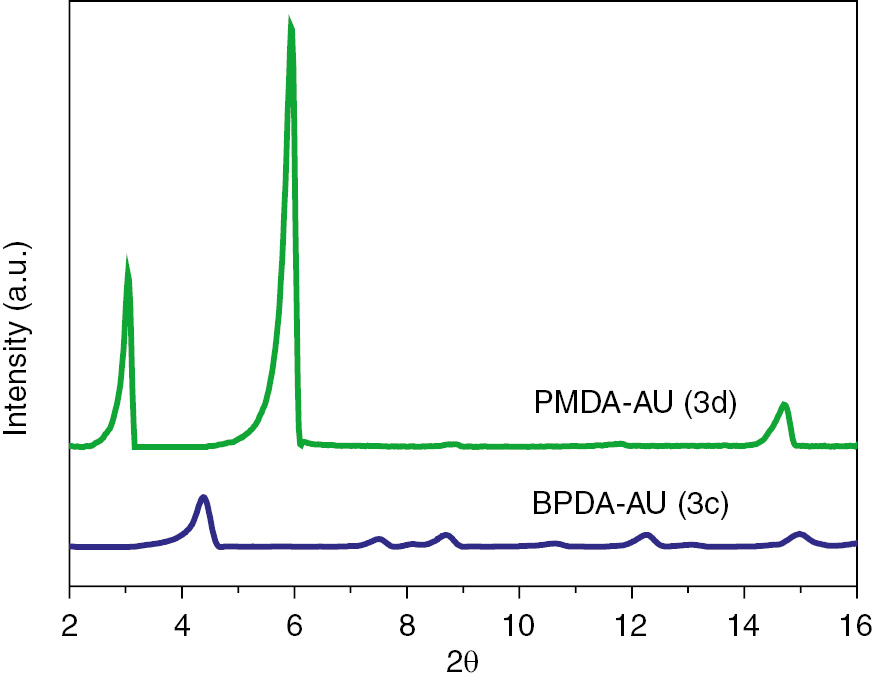
WAXD patterns of diimide diacid monomers 3c and 3d.
3.4 Phase transition of poly(amide imide)s
PLM is used to identify the phase transition temperatures of the polymers, and the obtained optical micrographs are shown in Figure 8. Liquid crystal phenomena are found for polymers 5b–d but not for polymer 5a. As can be seen, polymer 5b is isotropic and amorphous at the room temperature. Upon heating, liquid crystal appears at 120°C and develops gradually to a nematic banded texture. When the temperature approaches 223°C, the phase transition from nematic to isotropic occurs. During the cooling process followed, the liquid crystal reappears at 214°C, after which there is no further morphological change. The polymers 5c and 5d are liquid crystal glass at room temperature, and display nematic texture, and the liquid crystalline phenomenon could be observed during the heating and cooling process. The polymer 5d is liquid crystal glass at room temperature, and displays a nematic mosaic texture. Upon heating, the isotropic spots start to appear at 216°C, and the LC → I transition temperature (Ti) is around 322°C.
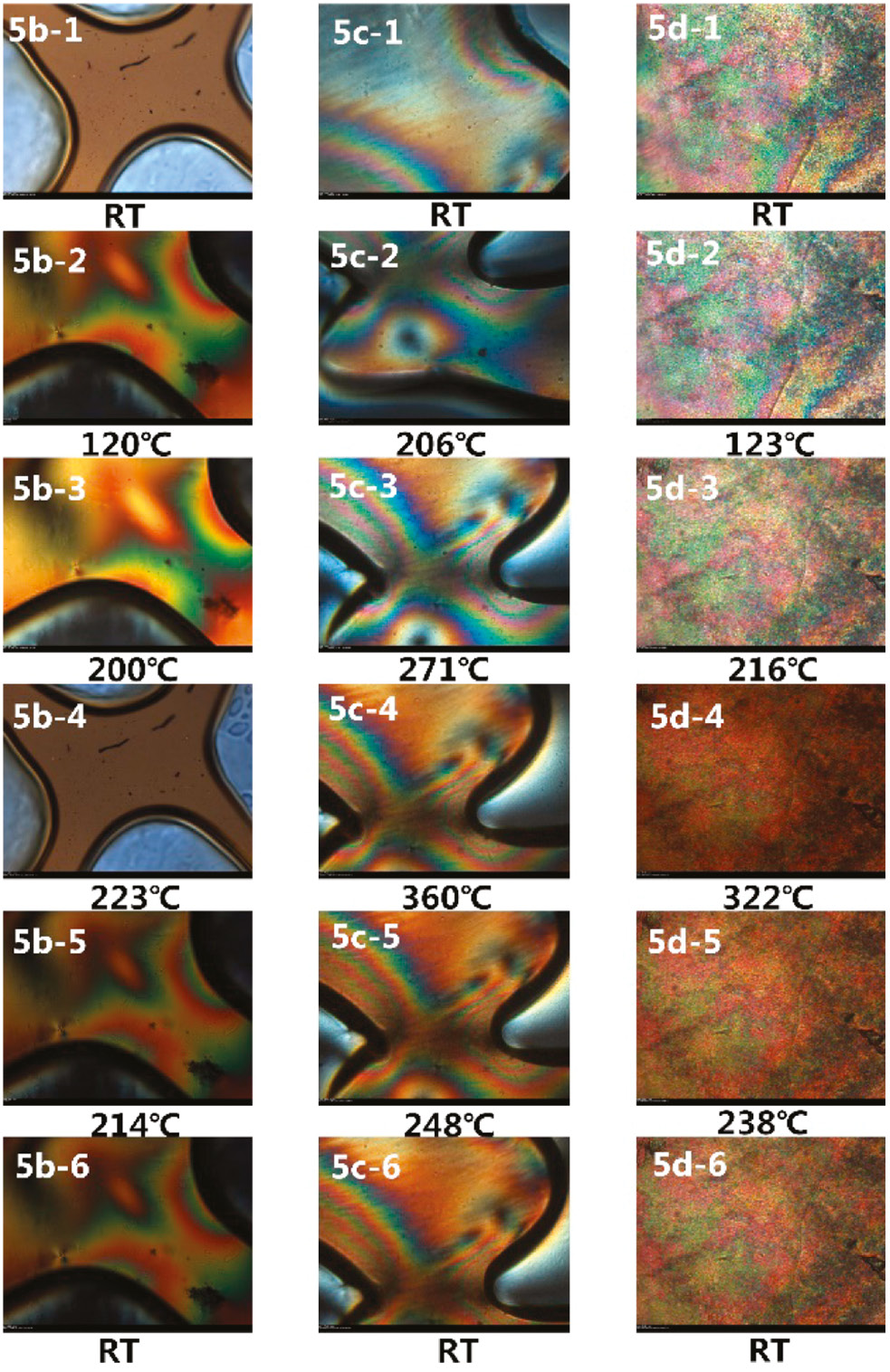
Photomicrographs of texture change of poly(amide imide)s (5b–5d) with procedure temperature elevation.
3.5 Solubility of poly(amide imide)s
One of the main objectives of this work is to synthesize liquid crystalline PAIs with improved solubility. Due to the incorporation of aliphatic units and amide groups in the polymer backbone, these liquid crystalline PAIs (5a, 5b, 5c, and 5d) exhibit good solubility in some common organic solvents. The solubility of PAIs was determined at 1% (g/ml) concentration, and the results are summarized in Table 2. Encouragingly, all of these PAIs can be dissolved at room temperature in aprotic polar solvents such as m-cresol and trifluoroacetic acid (TFA). With increasing temperature, these polymers can be also soluble in NMP, and dimethyl acetamide (DMAC). Actually, the polymer films are prepared by solution-casting method from solvent of m-cresol at room temperature. The films of PAIs 5c and 5d are too fragile to be stripped from the glassware whilst, and tough films can be achieved for the other two PAIs. The tensile strength of the PAI 5a and 5b films are determined to be 55 MPa and 102 MPa, respectively, which indicates the prepared liquid crystalline PAIs have a suitable mechanical property.
Solubility of poly(amide imide)s (5a–5d).
| PAIs | m-cresol | NMP | DMAC | DMF | TFA | CHCl3 |
|---|---|---|---|---|---|---|
| PAI-ODPA (5a) | ++ | ++ | +− | += | ++ | −− |
| PAI-BTDA (5b) | ++ | ++ | +− | +− | ++ | −− |
| PAI-BPDA (5c) | +− | += | += | += | ++ | −− |
| PAI-PMDA (5d) | +− | += | += | += | +− | −− |
++, Soluble at room temperature; +−, soluble on heating; +=, partly soluble on heating; −−, insoluble.
3.6 Thermal stability of poly(amide imide)s
The thermal properties of liquid crystalline PAIs (5a, 5b, 5c, and 5d) were determined by means of DSC and thermogravimetric analysis (TGA/DTG), and the data is summarized in Table 3. Depending on the structure of aromatic diimide diacid component as shown in Figure 9, the DSC thermograms of the polymers as shown in Figure 10 shows glass-transition temperatures (Tg) in the range of 112–184°C, and the increasing order of Tg generally corresponded to an increase in the rigidity and bulkiness of the diimide diacid monomer moiety. The thermal stability evaluation of the resulting PAIs was also carried out by their TGA/DTG in nitrogen atmosphere at a heating rate of 10°C/min. As shown in Figure 11, all the PAIs show one-step degradation process, and the initial decomposition temperatures of 5% weight losses range from 389.2 to 421.3°C, and then the PAIs decompose gradually above that temperature, in other words, the liquid crystalline PAIs exhibit good resistance to thermal decomposition. More importantly, as shown in Figure 12, it should be noticeable that the melting temperatures of PAIs are estimated to be 228°C, 263°C and 316°C for 5a, 5c and 5d, respectively, which are much lower than their decomposition temperatures, in other words, these polymers could be processed by the melting-process.
Thermal properties of poly(amide imide)s (5a–5d).
| Polymers | T5a, °C | T10b, °C | Tmax, °C | Tm, °C | Tic, °C | Char Yield at 700°C, % |
|---|---|---|---|---|---|---|
| PAI-ODPA (5a) | 421.3 | 438.9 | 468.7 | 228.2 | 33.3 | |
| PAI-BTDA (5b) | 414.2 | 433.6 | 458.6 | – | 223.4 | 58.0 |
| PAI-BPDA (5c) | 403.7 | 427.9 | 463.7 | 215.3 | 360.2 | 41.5 |
| PAI-PMDA (5d) | 389.2 | 407.4 | 449.0 | 316.1 | 322.2 | 47.4 |
aT5, Temperature at 5% weight loss in N2 atmosphere; bT10, temperature at 10% weight loss in N2 atmosphere; Tmax, temperature at the maximum degradation; cTi is obtained from the PLM test.
Chemical 3D structure of poly(amide imide)s (5a–5d).
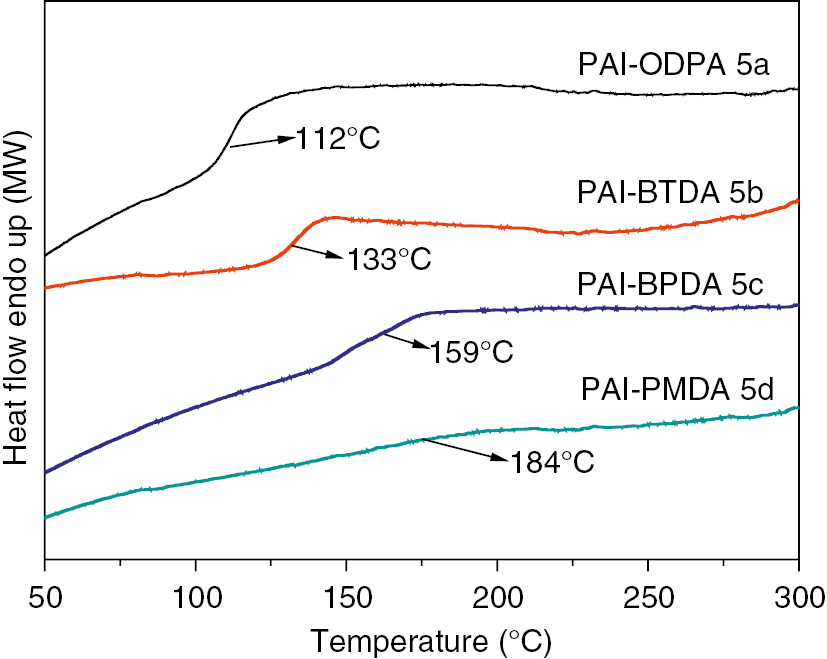
The DSC curves of poly(amide imide)s (5a–5d) on the second heating scan in N2 at a rate of 30°C/min.
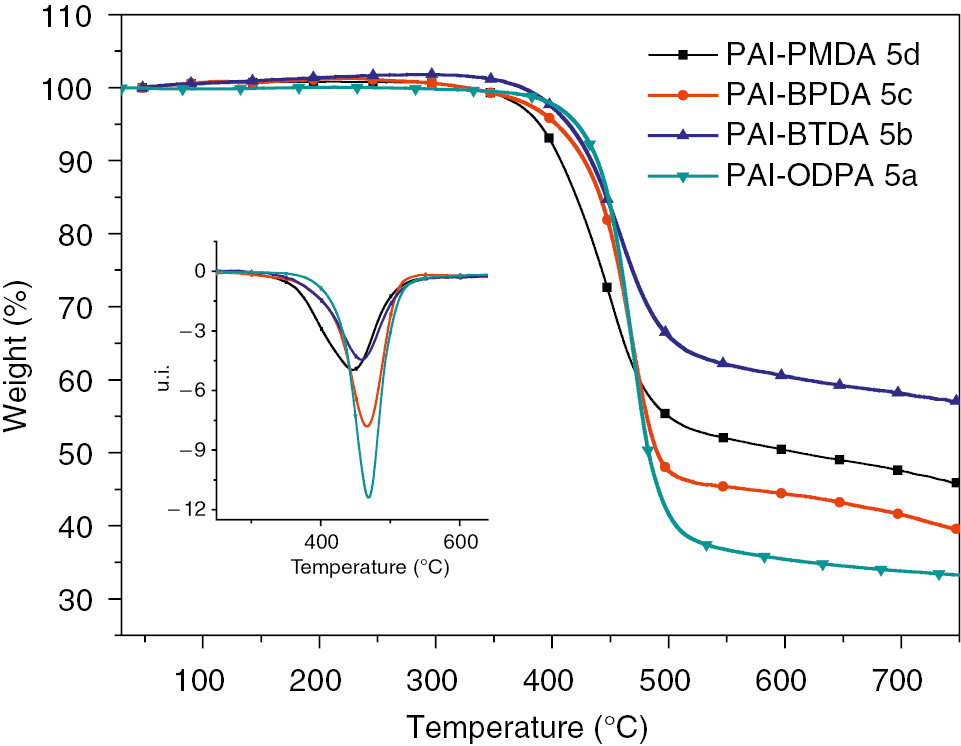
TGA and DTG traces of poly(amide imide)s (5a–5d) at a heating rate of 10°C/min.
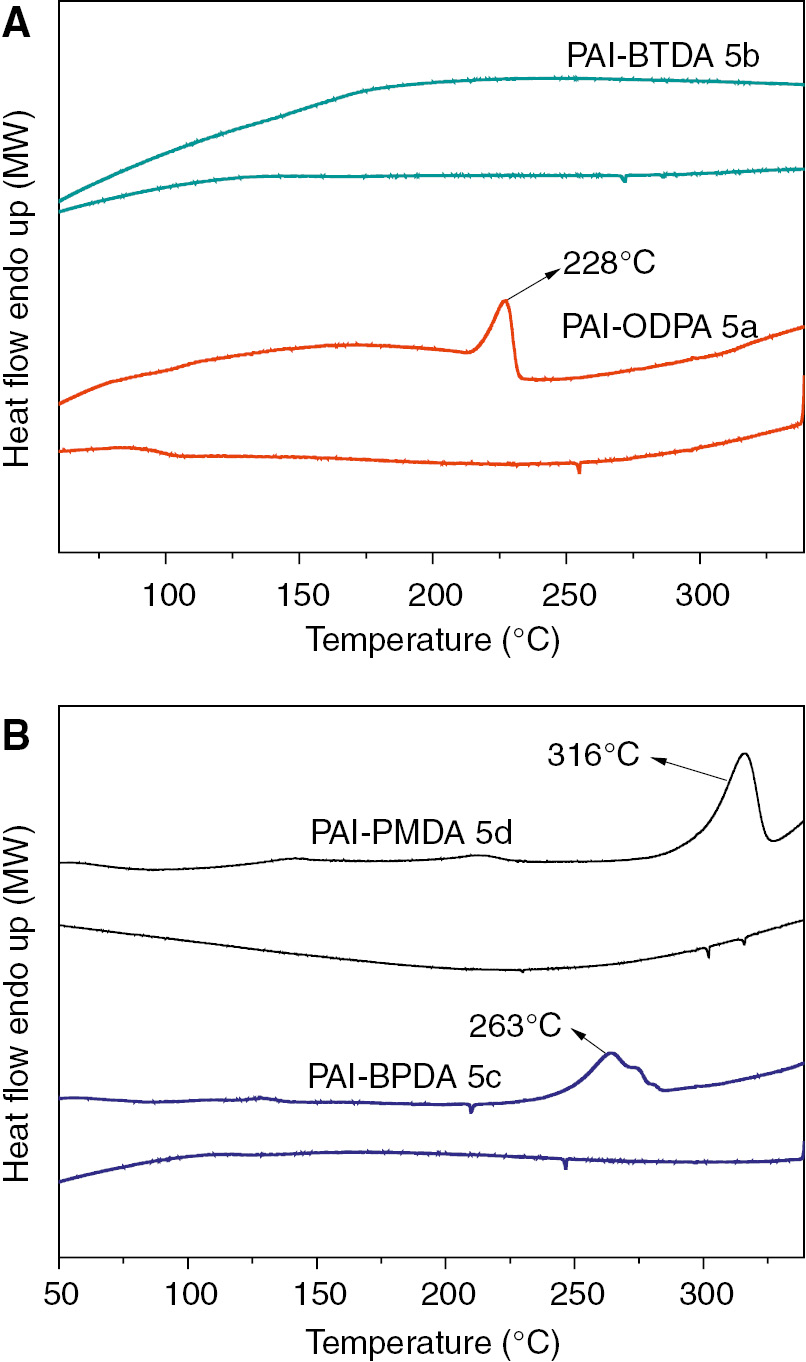
DSC traces of poly(amide imide)s (A: 5a, 5b; B: 5c, 5d) on their heating and cooling processes at a rate of 10°C/min.
The char yield can be a decisive factor to estimate the limited oxygen index (LOI) of polymers according to the Van Krevelen-Hoftyzer equation (26).
where CR is the char yield. The LOI values of PAIs are calculated to be 30.8 for 5a, 40.7 for 5b, 33.9 for 5c, and 36.5 for 5d, respectively. It means that the synthesized liquid crystalline PAIs can be classified as self-extinguishing polymers.
4 Conclusions
We have successfully synthesized several liquid crystalline diimide diacid monomers and PAIs. The thermal behaviors and thermotropic liquid crystalline properties of the monomers and polymers were investigated using DSC, PLM and WAXD measurements. With the help of aliphatic units and amide groups in the main chain, the liquid crystalline polymers exhibited good solubility. Meanwhile, the soluble liquid crystalline polymer also revealed high thermal stability and mechanical property.
Funding source: Natural Science Foundation of Jiangsu Province
Award Identifier / Grant number: BK20160280
Funding statement: This work was financially funded by the Priority Academic Program Development of Jiangsu Higher Education Institution, Jiangsu Joint Research Project of Industry, Education and Academy (KYZ13020168), Qing Lan project of Jiangsu, Natural Science Foundation of Jiangsu Province (BK20160280), Science and Technology Project of Changzhou University (ZMF15020116).
Acknowledgement
This work was financially funded by the Priority Academic Program Development of Jiangsu Higher Education Institution, Jiangsu Joint Research Project of Industry, Education and Academy (KYZ13020168), Qing Lan project of Jiangsu, Natural Science Foundation of Jiangsu Province (BK20160280), Science and Technology Project of Changzhou University (ZMF15020116).
References
1. Wilsens CHRM, Pepels MPF, Spoelstra AB, Portale G, Auhl D, Deshmukh YS, and Harings JAW. Improving stiffness, strength, and toughness of poly(ω-pentadecalactone) fibers through in situ reinforcement with a vanillic acid-based thermotropic liquid crystalline polyester. Macromolecules 2016;49(6):2228–37.10.1021/acs.macromol.5b02419Search in Google Scholar
2. Nelson AM, Fahs GB, Moore RB, Long TE. High-performance segmented liquid crystalline copolyesters. Macromol Chem Phys. 2015;216(16):1754–63.10.1002/macp.201500181Search in Google Scholar
3. Pathiranage TMSK, Magurudeniya HD, Bhatt MP, Rainbolt EA, Biewer MC, Stefan MC. Synthesis and characterization of side-chain thermotropic liquid crystalline copolymers containing regioregular poly(3-hexylthiophene). Polymer 2015;72:317–26.10.1016/j.polymer.2015.04.005Search in Google Scholar
4. Samui AB, Pandey S, Mishra SP. Main chain photoresponsive liquid crystalline polymer synthesized through hydrosilylation. Rsc Adv. 2015;5(84):68351–5.10.1039/C5RA14818ASearch in Google Scholar
5. Mulanl K, Momin M, Ganjave N, Chavan N. Thermotropic liquid crystalline polyesters derived from bis-(4-hydroxybenzoyloxy)-2-methyl-1,4-benzene and aliphatic dicarboxylic acid chlorides. Bull Mater Sci. 2015;38(5):1301–8.10.1007/s12034-015-1014-9Search in Google Scholar
6. Yang R, Chen L, Jin R, Wang Y. Main-chain liquid crystalline copolyesters with a phosphorus-containing non-coplanar moiety. Polym Chem. 2013;4:329–6.10.1039/C2PY20579CSearch in Google Scholar
7. Wu Q, Yang Z, Yao J, Yu D. Synthesis and biodegradation studies of optically active poly(amide-imide)s based on N,N′-(pyromellitoyl)-bis-L-amino acid. High Perform Polym. 2016;28:34–6.10.1177/0954008315569251Search in Google Scholar
8. Jalalian E, Ataei SM, Babanzadeh S, Khodabakhshi F. Silicon-containing poly(amide-imide)s: preparation, characterization, and properties. Des Monomers Polym. 2015;18(8):714–22.10.1080/15685551.2015.1070502Search in Google Scholar
9. Kowsari E, Ansari V, Moradi A, Zare A, Mortezaei M. Poly(amide-imide) bearing imidazole groups/sulfonated polyimide blends for low humidity and medium temperature proton exchange membranes. J Polym Res. 2015;22(5):1–15.10.1007/s10965-015-0729-zSearch in Google Scholar
10. Agrawal S, Narula AK. Synthesis and characterization of heat-resistant and soluble poly(amide-imide)s from unsymmetrical dicarboxylic acid containing 2-(triphenyl phosphoranylidene) moiety and various aromatic diamines. J Chem Sci. 2015;127:737–49.10.1007/s12039-015-0830-1Search in Google Scholar
11. Javadi A, Shockravi A, Rafieimanesh A, Malekb A, Ando S. Synthesis and structure–property relationships of novel thiazole-containing poly(amide imide)s with high refractive indices and low birefringences. Polym Int. 2015;64(4):486–95.10.1002/pi.4815Search in Google Scholar
12. Mallakpour S, Khani M, Sabzalian MR. Synthesis and biodegradability assessment of poly(amide-imide)s containing N-trimellitylimido-lamino acid and 5-(2-benzimidazole)- 1,3-phenylenediamine. Polym Bull. 2014;71(8):2159–72.10.1007/s00289-014-1179-1Search in Google Scholar
13. Hsiao SH, Guo W, Tsai TH, Chiu YT. Synthesis of soluble and thermally stable triptycene-based poly(amide-imide)s. J Polym Res. 2014;21(3):1–10.10.1007/s10965-014-0391-xSearch in Google Scholar
14. Parhami A, Nasab SS, Abbasi M, Jafarazad R, Bahmani R. Synthesis and characterization of novel nanostructured, organosoluble, thermally stable and wholly aromatic poly(amide-imide)s from a new diimide-diacid monomer by direct polycondensation. Des Monomers Polym. 2015;17(8):736–45.10.1080/15685551.2014.918012Search in Google Scholar
15. Mansoori Y, Zargar BK, Shekaari H, Zamanloo MR, Imanzadeh G. Synthesis of thermally stable polyamides with pendant 1,3,4-oxadiazole units via direct polycondensation in ionic liquids. Polym Bull. 2012;68(1):113–39.10.1007/s00289-011-0535-7Search in Google Scholar
16. Liu SL, Chung TS, Geng JX, Zhou EL, Tamai S. Phase transition and transition kinetics of a thermotropic poly(amide-imide) derived from 70% pyromellitic dianhydride, 30% terephthaloyl chloride, and 1,3-bis[4-(4′-aminophenoxy)cumyl]benzene. Macromolecules 2001;34(25):8710–9.10.1021/ma010895fSearch in Google Scholar
17. Sarkar A, More AS, Wadgaonkar PP, Shin GJ, Jung JC. Synthesis and liquid-crystal-aligning properties of novel aromatic poly(amide imide)s bearing n-alkyloxy side chains. J Appl Polym Sci. 2007;105(4):1793–801.10.1002/app.26163Search in Google Scholar
18. Hans RK, Sven AT. Liquid-crystalline polyimides, lyotropic poly(imide-amide)s and poly(benzoxazole-amide)s. Macromol Chem Rapid Commun. 1993;14(7):395–400.10.1002/marc.1993.030140705Search in Google Scholar
19. Mallakpour S, Zeraatpisheh F. Molten salt ionic liquid assisted synthesis of nano-structured poly(amide imide)s based on 4,4′-methylenebis(3-chloro-2,6-diethyl trimellit imidobenzene) via microwave process as an environmentally friendly methodology. Polym Sci Ser B. 2013;55(5):271–9.10.1134/S1560090413050096Search in Google Scholar
20. Yamazaki N, Higashi F, Matsumoto M. Studies on reactions of the n-phosphonium salts of pyridines. XIV. wholly aromatic polyamides by the direct polycondensation reaction by using phosphites in the presence of metal salts. J Polym Sci Polym Chem. 1975;13(6):1373–80.10.1002/pol.1975.170130609Search in Google Scholar
21. Ghaemy M, Qasemi S, Ghassemi K, Bazzar M. Nanostructured composites of poly(triazole-amide-imide)s and reactive titanium oxide by epoxide functionalization: thermal, mechanical, photophysical and metal ions adsorption properties. J Polym Res. 2013;20(10):1–15.10.1007/s10965-013-0278-2Search in Google Scholar
22. Hsiao SH, Yang CP. Preparation of poly( amide-Imide)s by direct polycondensation with triphenylphosphite IV. aliphatic-aromatic poly(amide-Imide)s based on N,N′-bis(o-carboxy- alkyl) pyromellitimides. J Polym Sci Polym Chem. 1990;28(8):2169–78.10.1002/pola.1990.080280812Search in Google Scholar
23. Hsiao SH, Yang CP. Preparation of polyamide-lmides by direct polycondensation with triphenyl phosphite. V. aliphatic-aromatic polyamide-lmides based on N,N,-bis(ω- carboxyalkyl) benzophenone-3,3′,4,4′-tetracarboxylic Diimides. J Polym Sci Polym Chem. 1991;29(3):447–52.10.1002/pola.1991.080290318Search in Google Scholar
24. Jones SM, Meehan SJ, Sankey SW, MacDonald WA, Colquhoun HM. Mesomorphic behaviour in copoly(ester-imide)s of poly(butylene-2,6-naphthalate) (PBN). Polymer 2015;51(1):66–72.10.1016/j.polymer.2015.05.025Search in Google Scholar
25. Zhang J, Dong B, Zheng LQ, Li N, Li XW. Lyotropic liquid crystalline phases formed in ternary mixtures of 1-cetyl-3-methylimidazolium bromide/p-xylene/water: a SAXS, POM, and rheology study. J Colloid Interf Sci. 2008;321(1):159–65.10.1016/j.jcis.2008.01.020Search in Google Scholar PubMed
26. Mallakpour S, Zadehnazari A. Synthesis of novel nanostructured chiral poly(amide-imide)s containing dopamine and natural amino acids. J Chem Sci. 2013;125(1):203–11.10.1007/s12039-012-0351-0Search in Google Scholar
©2017 Walter de Gruyter GmbH, Berlin/Boston
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Articles in the same Issue
- Frontmatter
- In this Issue
- Full length articles
- Hexafluoroisopropylidene based sulfonated new copolytriazoles: investigation of proton exchange membrane properties
- Effect of various formulation ingredients on thermal characteristics of PVC/clay nanocomposite foams: experimental and modeling
- TFC polyamide NF membrane: characterization, application and evaluation of MTPs and MTC for simultaneous removal of hexavalent chromium and fluoride
- Functionalization of epoxy resin and the performance of the resultant magnesium-rich primer
- Preparation of a poly(DMAEMA-co-HEMA) self-supporting microfiltration membrane with high anionic permselectivity by electrospinning
- Mechanical properties of norbornene-based silane treated glass fiber reinforced polydicyclopentadiene composites manufactured by the S-RIM process
- Effect of dip time on the electrochemical behavior of PPy-Cu(OH)2 hybrid electrodes synthesized using pyrrole and CuSO4
- Interfacial interaction exploration and oxygen barrier potential of polyethylene/poly(ethylene-co-vinyl alcohol)/clay hybrid nanocomposites
- Prediction of tensile modulus of PA-6 nanocomposites using adaptive neuro-fuzzy inference system learned by the shuffled frog leaping algorithm
- Molecular design and synthesis of thermotropic liquid crystalline poly(amide imide)s with high thermal stability and solubility
Articles in the same Issue
- Frontmatter
- In this Issue
- Full length articles
- Hexafluoroisopropylidene based sulfonated new copolytriazoles: investigation of proton exchange membrane properties
- Effect of various formulation ingredients on thermal characteristics of PVC/clay nanocomposite foams: experimental and modeling
- TFC polyamide NF membrane: characterization, application and evaluation of MTPs and MTC for simultaneous removal of hexavalent chromium and fluoride
- Functionalization of epoxy resin and the performance of the resultant magnesium-rich primer
- Preparation of a poly(DMAEMA-co-HEMA) self-supporting microfiltration membrane with high anionic permselectivity by electrospinning
- Mechanical properties of norbornene-based silane treated glass fiber reinforced polydicyclopentadiene composites manufactured by the S-RIM process
- Effect of dip time on the electrochemical behavior of PPy-Cu(OH)2 hybrid electrodes synthesized using pyrrole and CuSO4
- Interfacial interaction exploration and oxygen barrier potential of polyethylene/poly(ethylene-co-vinyl alcohol)/clay hybrid nanocomposites
- Prediction of tensile modulus of PA-6 nanocomposites using adaptive neuro-fuzzy inference system learned by the shuffled frog leaping algorithm
- Molecular design and synthesis of thermotropic liquid crystalline poly(amide imide)s with high thermal stability and solubility

