Abstract
Background: There are some problematic results in determination of differential renal function (DRF) by means of Tc-99m DMSA renal scintigraphy in hydronephrotic kidneys. In this study the classical method (CM) and unit area methods (UAM) in the estimation of DRF in unilateral hydronephrosis before and after intervention were compared.
Methods: Twenty patients (12 M, 8 F; mean: 42.6±18.5 years old) who were candidates for surgery or intervention because of unilateral hydronephrosis were the subjects of this study. All the patients were evaluated by Tc-99m DMSA scintigraphy before and 3–6 months after the intervention. In order to estimate DRF both CM and UAM (obtained by division of the counts including the kidney ROI to pixel of the same ROI) were performed. Bland-Altman analyses were performed in order to compare the DRF values obtained from both methods.
Results: The agreement between CM and UAM was poor in the preoperative estimation, however, the agreement was good after the operation or intervention.
Conclusions: In this study it seems that DRF estimation with CM in unilateral hydronephrosis might be problematic in the determination of surgery thus UAM might be introduced as the method of choice in the determination of DRF in unilateral hyronephrosis.
Introduction
Differential renal function estimation by means of various radionuclides has been the determining factor in decision to do renal interventions. Thus reliability of the method is essential since salvage of the functioning kidney tissue depends on the differential renal function (DRF) estimation. Static renal scintigraphy with Tc-99m DMSA has been accepted as the gold standard method in the estimation of renal cortical tissue regarding renal scarring and the best method to calculate the DRF. DRF evaluation by means of the renal nuclides like Tc-99m DTPA, Tc-99m MAG3 and Tc-99m EC with similar results is possible. Additionally these radionuclides provide information about the excretion and concentration functions and diuretic response of the obstruction of the kidney. However, DRF calculation by both dynamic and static scintigraphy has some drawbacks especially in hydronephrosis [1]. In fact DRF estimation by means of radionuclide methods is problematic and the problem is the enlargement of the kidney which influences the reliability of the results. The hydronephrotic kidney seems to have increased function in comparison to the other healthy kidney which is called ‘supranormal function’. The supranormal function were firstly determined to be a problem related to Tc-99m DTPA studies and are not present in Tc-99m DMSA studies [2]. However, experimental studies in rats have not confirmed the discrepancy of the DRF estimation reported in miniature pigs; the discrepant results in these two studies may be related to differences in the kidneys of the rat and the miniature pig [3]. In humans the deficiency of DRF calculation cannot be attributed to only one radionuclide and should not be underestimated. Gungor et al. clearly documented the influence of kidney size to the DRF estimation [4]. Recently, for the first time, as far as we know, Nam et al. introduced unit area estimation with DRF calculation [5]. However, Nam et al. choose the unit area estimation with nonradionuclide methods (volume calculation with BT or MRI) and performed their study in Tc-99m DTPA renal studies. The aim of this study is to compare the CM and UAM in a prospective study in hydronephrotic kidneys before and after intervention.
Materials and methods
Patients
Twenty patients (12 male, 8 female; mean: 42.6±18.5 years old) with diagnosis of unilateral hydronephrosis who are candidates for either surgery or intervention were included to the study. The patients under the age of 18 years old, pregnant or lactating women were excluded from the study population. After obtaining anamnesis and a physical examination the patients were subjected to further tests. The diagnosis of hydronephosis was based on ultrasonography or intravenous pyelography results. The patients with unilateral grade 1–4 hydronephrosis with any etiology were included to the study. All the patients were subjected to renal ultrasonography and additional intravenous pyelography, computed tomography and magnetic resonance imaging were performed if necessary. Additional urinary system pathologies were noted.
Static renal scintigraphy
The DMSA scintigraphies were performed before and 3–6 months after the surgery or intervention. The surgery or intervention was performed within 1 month after the first scintigraphy. The scintigraphy images were obtained by Infinia 2 model SPECT gamma camera (GE Medical Systems, Israel) equipped with a low energy high resolution parallel hole collimator. The imaging was performed in supine position with previous intravenous administration of approximately 5 mCi (187.5 MBq) (according to the body weight) of Tc-99m DMSA 2–3 h prior to the imaging. Diuretic administration was not performed on the patients. The parallel hole images were obtained for 5 min in anteroposterior projection and additional pin hole images were obtained in posterior and oblique projections for the right and left kidneys for 5 min each.
Quantification
Quantification was performed in parallel hole posterior images with the Xeleris workstations Renal DMSA Uptake Analysis program. The age, weight and height of the patients were recorded to the system. Renal regions of interest were drawn manually from the borders of the kidney and perirenal regions of interest were used for background substraction (Figure 1). The Taylor method was performed for depth correction. The relative renal functions of the kidneys were obtained as % percentage.
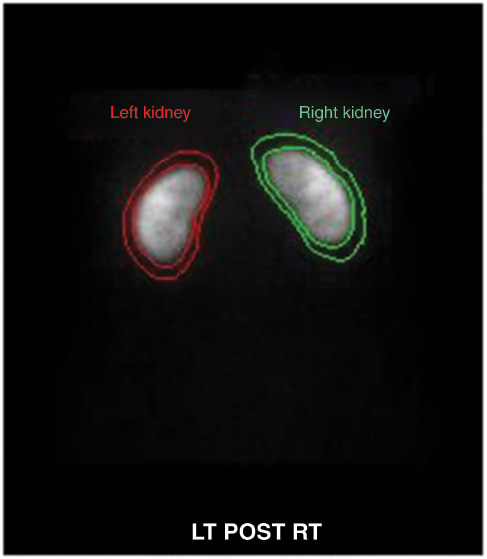
The image analysis and kidney and background region of interests.
Two methods were employed for relative renal function estimation:
Classical method: % ratio was obtained by subtracting the average cts/pixel in the background ROI from the average cts/pixel in each renal ROI and then multiplying by the total pixels in the renal ROI.
Unit area method: % ratio was obtained by subtracting the average cts/pixel in the background ROI from the average cts/pixel in each renal ROI and then multiplying by the total pixels in the renal ROI. The area of the each kidney was calculated according to the pixel information in each kidney ROI. After background subtraction the obtained counts of the kidneys were divided into the area.
Statistical analysis
In order to compare the classical and unit area methods Pearson correlation analysis and Bland-Altman analysis were performed and Bland-Altman plots were obtained. Classification of DRF: The supranormal function is considered to be >55% DRF of the kidney with hydronephrosis, where normal function is 45%–55% and abnormal is <45%.
Interventions: Endoscopic ureteral litotomy operation in ten patients, nephrostomy operation in four patients and pyeloplasty operation in one patient were performed.
Results
All the patients had unilateral hydronephrosis and in 19 of the patients the etiology was kidney stone disease and in one patient the etiology was ureteropelvic junction obstruction. The unilateral hydronephrosis included the right kidney in nine (%45) and left in 11 (%55) patients. The degree of hydronephrosis was between grade 1 and 3. Twelve patients had grade 1, six had grade 2 and two had grade 3 hydronephrosis (Table 1). The preoperative and postoperative mean values of DRF estimations for both CM and UAM are summarized in Table 2.
Patient characteristics.
| Age | Mean:42.6±18.5 years | Range:18–76 years | |
|---|---|---|---|
| Grade | Grade 1: n=12 | Grade 2:n=6 | Grade 3:n=2 |
| wSide | Left:n=11 | Right:n=9 | |
| Etiology | Nephrolithiasis:n=19 | UPJ obstruction:n=1 | |
Mean±SD values of DRF estimation by CM and UAM before and after intervention for the kidney with hydronephrosis.
| Mean | SD | n | |
|---|---|---|---|
| Preoperative CM | 43.01 | 10.15 | 20 |
| Postoperative CM | 42.90 | 8.68 | 20 |
| Preoperative UAM | 41.41 | 9.14 | 20 |
| Postoperative UAM | 42.32 | 11.43 | 20 |
In the preoperative period according to CM there were ten patients with abnormal DRF, one with supranormal (55% for CM, 51% for UAM); according to UAM there were ten patients with abnormal DRF (all were also abnormal for UAM) and none with supranormal other patients’ DRF results were in normal range.
According to the Pearson correlation analysis relative renal functions calculated with both methods were in strong correlation in both the preoperative and postoperative period (r=0.8411–0.9775, p<0.0001 and r=0.9959–0.9997, p<0.0001, respectively).
Bland-Altman analysis showed that there was no good agreement between classical and unit area methods in the preoperative period (Figure 2A) but the agreement between methods were good in the postoperative period after correction of the hydronephrosis (Figure 2B).
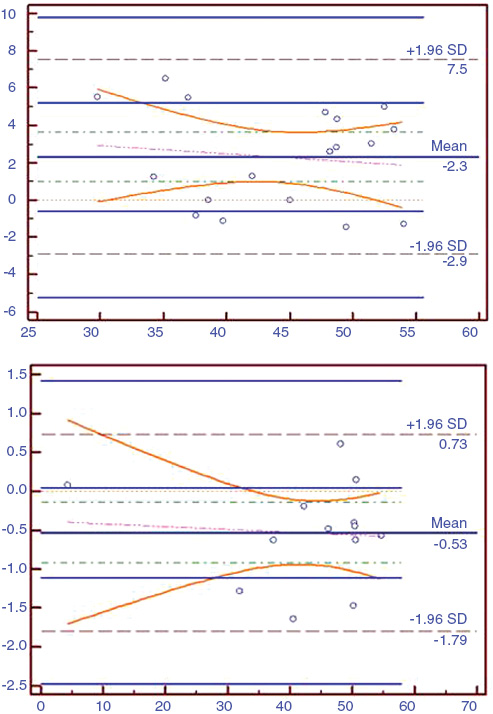
X axis: DRF values, Y axis: 95% CI (confidency interval). (A) Bland-Altman plots of the classical and unit area methods in preoperative period. (B) Bland-Altman plots of the classical and unit area methods in postoperative period.
Discussion
Supranormal function has been considered as an artifact or technical problem related to the increased size of kidney. Whether the origin of this problem is technical or something else it has to be considered as an important problem. In a previous study, Capolicchio et al. have described the larger size in supranormal functioning kidneys rather than other hydroneprotic kidneys [6]. DMSA renal scintigraphy has been considered as a less invasive tool and a better way of determining the DRF changes after an operation [7]. DMSA is a more reliable method in DRF estimation and to show subtle changes in DRF of kidneys after an operation or in the disease course [7]. Animal studies have confirmed DMSA scintigraphies may provide DRF estimation with low variation [8]. Thus we preferred to perform this study with this radiopharmaceutical.
Previously the best method to estimate the DRF has been determined as infrarenal background ROI and the Rutland-Patlak correction in dynamic renal studies [9]. In a previous study various factors that may influence the DRF estimation have been considered like the shape of background correction ROI, renal ROI and different operators. Their results showed that the difficulty appears in single or poorly functioning kidneys [10]. This was an expected result since in most of the analysis with radiopharmaceutics this is what happens with better results in normal and variations in abnormal subjects. These researchers also have suggested the use of the Rutland-Patlak plot and perirenal background ROI.
The incidence of supranormal function is between 9% and 22% [11]. A previous comparative study with DMSA and MAG 3 in patients with unilateral hydronephrosis has pointed out that two modalities have significant correlation and good reproducibility [12]. However, performance of MAG 3 instead of DMSA in DRF analysis is not recommended by the authors especially for high grade hydronephrosis in infants [12]. There are a limited number of studies in the literature about supranormal function estimation of DMSA [2, 4, 6] however, all these studies point out the problem especially in high grade hydronephrosis. Another solution suggestion has been diuretic administration during DMSA studies, however, recently this application has been proven to be unnecessary [13, 14].
In a previous study researchers defined the effect of the size of kidney ROI on the estimation of DRF and they have suggested surface area background subtraction to prevent the effect of the increased size of kidney ROI in the calculation of DRF [4]. Nam et al. proposed a novel method in the estimation of DRF associated with the modification of the classical method according to the renal cross sectional area and have demonstrated significant differences between the methods [5].
The limitations of our study were the relatively small number of patients, nonuniformity of the correction method (surgery and other methods) and the lower degree of hydronephrosis of the patients. It has previously been suggested to perform 24 h imaging or SPECT imaging in order to improve estimation of renal parenchyma in hydronephrosis, however, we did not perform any of those images, the aim was to analyze UAM in routine application. Additionally, not all subjects necessarily underwent USG estimation in post operative follow-up thus we cannot perform a comparison of pre- and postoperative degree of hydronephrosis. We unfortunately did not compare the size of the ROIs, however, standardization of the ROIs was provided by analysis of the images by the same interpreter. We had intended to perform the analysis in the patients with supranormal results, however, the patient groups consisted of patients with normal and abnormal DRF. This study might be more informative if it is repeated in only supranormal patients. There was only one patient with supranormal result (DRF=55) in our study population and it was corrected with UAM (DRF=50). However, the significant difference in this kind of patient in our study with mild hidronephrosis is promising for future studies with UAM.
UAM seems to be a more reliable method in the estimation of DRF in the hydronephrotic kidney. Although the grade of hydronephrosis in our patients was not advanced we could identify that CM might be problematic in hydronephrosis and UAM corrects this misinterpretation. If the degree was higher in our study population this discrepancy might be higher. Further future studies are warranted in this area with a higher degree of hydronephrosis and higher number of patients and maybe the addition of analysis of the 24 h images.
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
Financial Support: The study was approved by Fırat University ethics committee.
Employment or leadership: None declared.
Honorarium: None declared.
Competing interests: The funding organization(s) played no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the report for publication.
References
1. Kraft O, Kuba J, Ullmann V. Determination of differential renal function using dynamic scintigraphy]. Vnitr Lek 1995;41:699–703.Search in Google Scholar
2. Fung LC, McLorie GA, Khoury AE, Ash JM, Gilday DL, Churchill BM. Contradictory supranormal nuclear renographic differential renal function: fact or artifact? J Urol 1995;154: 667–70.10.1016/S0022-5347(01)67129-5Search in Google Scholar
3. van de Wiele C, Everaert K, van der Eecken H, van Haelst JP, Simons M, Dierckx RA. Differential renal function measured by 99Tcm-DTPA and 99Tcm-DMSA in a complete unilateral renal obstruction rat model. Nucl Med Commun 1997;18:1036–39.10.1097/00006231-199711000-00006Search in Google Scholar
4. Gungor F, Anderson P, Gordon I. Effect of the size of regions of interest on the estimation of differential renal function in children with congenital hydronephrosis. Nucl Med Commun 2002;23:147–51.10.1097/00006231-200202000-00006Search in Google Scholar
5. Nam JK, Lee SD, Chung MK. Modified differential renal function measurement revised by renal cross sectional area in children with ureteropelvic junction obstruction. Korean J Urol 2010;51:271–5.10.4111/kju.2010.51.4.271Search in Google Scholar
6. Capolicchio G, Jednak R, Dinh L, Salle JL, Brzezinski A, Houle AM. Supranormal renographic differential renal function in congenital hydronephrosis: fact, not artifact. J Urol 1999;161:1290–4.10.1016/S0022-5347(01)61671-9Search in Google Scholar
7. Konda R, Sakai K, Ota S, Abe Y, Hatakeyama T, Orikasa S. Ultrasound grade of hydronephrosis and severity of renal cortical damage on 99m technetium dimercaptosuccinic acid renal scan in infants with unilateral hydronephrosis during followup and after pyeloplasty. J Urol 2002;167: 2159–63.10.1016/S0022-5347(05)65118-XSearch in Google Scholar
8. Dissing TH, Eskild-Jensen A, Pedersen M, Anderson PJ, Rittig N, Frøkiaer J, et al. Reproducibility and repeatability of differential renal function in 4-week-old piglets. Nucl Med Commun 2008;29:76–82.10.1097/MNM.0b013e3282f20e1fSearch in Google Scholar
9. Monsieurs MA, Thierens HM, van de Wiele CV, Vral AM, Meirlaen IA, de Winter HA, et al. Estimation of risk based on biological dosimetry for patients treated with radioiodine. Nucl Med Commun 1999;20:911–7.10.1097/00006231-199910000-00008Search in Google Scholar
10. Lythgoe MF, Gordon I, Khader Z, Smith T, Anderson PJ. Assessment of various parameters in the estimation of differential renal function using technetium-99m mercaptoacetyltriglycine. Eur J Nucl Med 1999;26:155–62.10.1007/s002590050372Search in Google Scholar
11. Oh SJ, Moon DH, Kang W, Park YS, Park T, Kim KS. Supranormal differential renal function is real but may be pathological: assessment by Tc-99m mercaptoacetylglycine renal scan of congenital unilateral hydronephrosis. J Urol 2001;165:2300.10.1016/S0022-5347(05)66189-7Search in Google Scholar
12. Aktaş GE, Inanir S. Relative renal function with MAG-3 and DMSA in children with unilateral hydronephrosis. Ann Nucl Med 2010;24:691–5.10.1007/s12149-010-0397-3Search in Google Scholar PubMed
13. Buyukdereli G, Guney IB, Seydaoglu G. Effectiveness of diuretic injection on the measurement of differential renal function using Tc-99m DMSA in patients with a dilated renal pelvis. Clin Nucl Med 2005;30:721–4.10.1097/01.rlu.0000183614.76106.4dSearch in Google Scholar PubMed
14. Kabasakal L, Turkmen C, Ozmen O, Alan N, Onsel C, Uslu I. Is furosemide administration effective in improving the accuracy of determination of differential renal function by means of technetium-99m DMSA in patients with hydronephrosis. Eur J Nucl Med Mol Imaging 2002;29:1433–7.10.1007/s00259-002-0923-1Search in Google Scholar PubMed
Article note
Abstract of this study was presented as a poster presentation at the 23th National Congress of Nuclear Medicinein Antalya, Turkey, in 6–10 April 2013.
©2014, Zehra Pınar Koç et al., published by De Gruyter.
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 3.0 License.
Articles in the same Issue
- Frontmatter
- Editorial
- Measuring diagnostic safety of inpatients: time to set sail in uncharted waters
- Review
- Clinical criteria to screen for inpatient diagnostic errors: a scoping review
- Original Articles
- Types of diagnostic errors in neurological emergencies in the emergency department
- Missed diagnoses of acute myocardial infarction in the emergency department: variation by patient and facility characteristics
- Assessment of machine-learning techniques on large pathology data sets to address assay redundancy in routine liver function test profiles
- Clinical benefit of measuring both haemoglobin and transferrin concentrations in faeces: demonstration during a large-scale colorectal cancer screening trial in Japan
- Classical vs. unit area method in the evaluation of differential renal function in unilateral hydronephrosis
- Comparison of two methods for measuring methylmalonic acid as a marker for vitamin B12 deficiency
- Case Report
- A case of factitious hyponatremia and hypokalemia due to the presence of fibrin gel in serum
- Letter to the Editor
- Diagnostic difficulties in homozygous hemoglobin E disorders
- Erratum
- Benefits and limitations of laboratory diagnostic pathways
- DEM Congress Abstracts
- Diagnostic Error in Medicine 7th International Conference
Articles in the same Issue
- Frontmatter
- Editorial
- Measuring diagnostic safety of inpatients: time to set sail in uncharted waters
- Review
- Clinical criteria to screen for inpatient diagnostic errors: a scoping review
- Original Articles
- Types of diagnostic errors in neurological emergencies in the emergency department
- Missed diagnoses of acute myocardial infarction in the emergency department: variation by patient and facility characteristics
- Assessment of machine-learning techniques on large pathology data sets to address assay redundancy in routine liver function test profiles
- Clinical benefit of measuring both haemoglobin and transferrin concentrations in faeces: demonstration during a large-scale colorectal cancer screening trial in Japan
- Classical vs. unit area method in the evaluation of differential renal function in unilateral hydronephrosis
- Comparison of two methods for measuring methylmalonic acid as a marker for vitamin B12 deficiency
- Case Report
- A case of factitious hyponatremia and hypokalemia due to the presence of fibrin gel in serum
- Letter to the Editor
- Diagnostic difficulties in homozygous hemoglobin E disorders
- Erratum
- Benefits and limitations of laboratory diagnostic pathways
- DEM Congress Abstracts
- Diagnostic Error in Medicine 7th International Conference

