Abstract
A Hypo helmet is a fabric hood soaked with an aqueous solution of two sodium salts for the detoxication of chlorine that was developed during the First World War. Herein, we report on a lecture, developed for non-chemistry majors that used the Hypo helmet to highlight the physical and chemical properties of chlorine. The lecture consisted of the following three components: (1) a brief introduction to the First World War (the Chemists’ War), (2) video demonstrations of the generation of chlorine by mixing bleaching powder and hydrochloric acid and the detoxication of chlorine using a dummy Hypo helmet, and (3) students’ written impressions of the lecture. The students’ written impressions revealed two things: the lecture on chlorine and the Hypo helmet was well received, and most of the students (71 %, N = 82) did not know that chlorine was used as a poisonous gas during the First World War.
1 Introduction
One way to engage students in chemistry is for chemistry teachers to introduce old technology content into chemistry lectures, including ancient artifacts (Careaga et al., 2023; Giménez, 2015), Athenian vases (Vyhnal, 2022), carbide lanterns (Crawford & Kiefer, 2016), and diesel-electric powered submarines (Horikoshi et al., 2016). With this in mind, we developed a lecture with a focus on a century-old anti-chlorine gas mask from the First World War.
Chlorine is reduced by sodium thiosulfate to generate hydrogen chloride, and hydrogen chloride is neutralized by sodium bicarbonate to form sodium chloride, as shown in Scheme A and B. During the First World War, these two chemical reactions saved soldiers’ lives (Freemantle, 2014, 2015; Friedrich et al., 2017). In response to the use of chlorine as a poisonous gas on the battlefield, several anti-chlorine gas masks were developed to protect the soldiers (Figure 1) (Haber, 1986; Padley, 2016). An early version was the Hypo helmet (Figure 1a). The term “hypo” came from the sodium thiosulfate, formerly called hypo (Haber, 1986), and used to impregnate the helmet. Although the Hypo helmet is called a “helmet,” it is actually a hood, comprising mica glasses and fabric soaked with an aqueous solution of the two sodium salts: sodium thiosulfate and sodium bicarbonate.

Chemical reactions.

Replicas (a) Hypo helmet, (b) Phenate helmet, and (c) Small box respirator. They are available from the online store What Price Glory (https://onlinemilitaria.net/).
The aim of the lecture was to illustrate the physical and chemical properties of chlorine to non-chemistry majors. The lecture consisted of the following content: (1) a brief description of the First World War, also known as the Chemists’ War (Freemantle, 2015), (2) videos demonstrating how chlorine is generated by the addition of hydrochloric acid to bleaching powder and chlorine detoxification using a dummy Hypo helmet (Figure 2), and (3) students’ written impressions of the lecture filled in the questionnaire. The student’s written impressions indicated that the lecture was well received, and most of the students (71 %, N = 82) did not know that chlorine was used as a poisonous gas during the First World War.
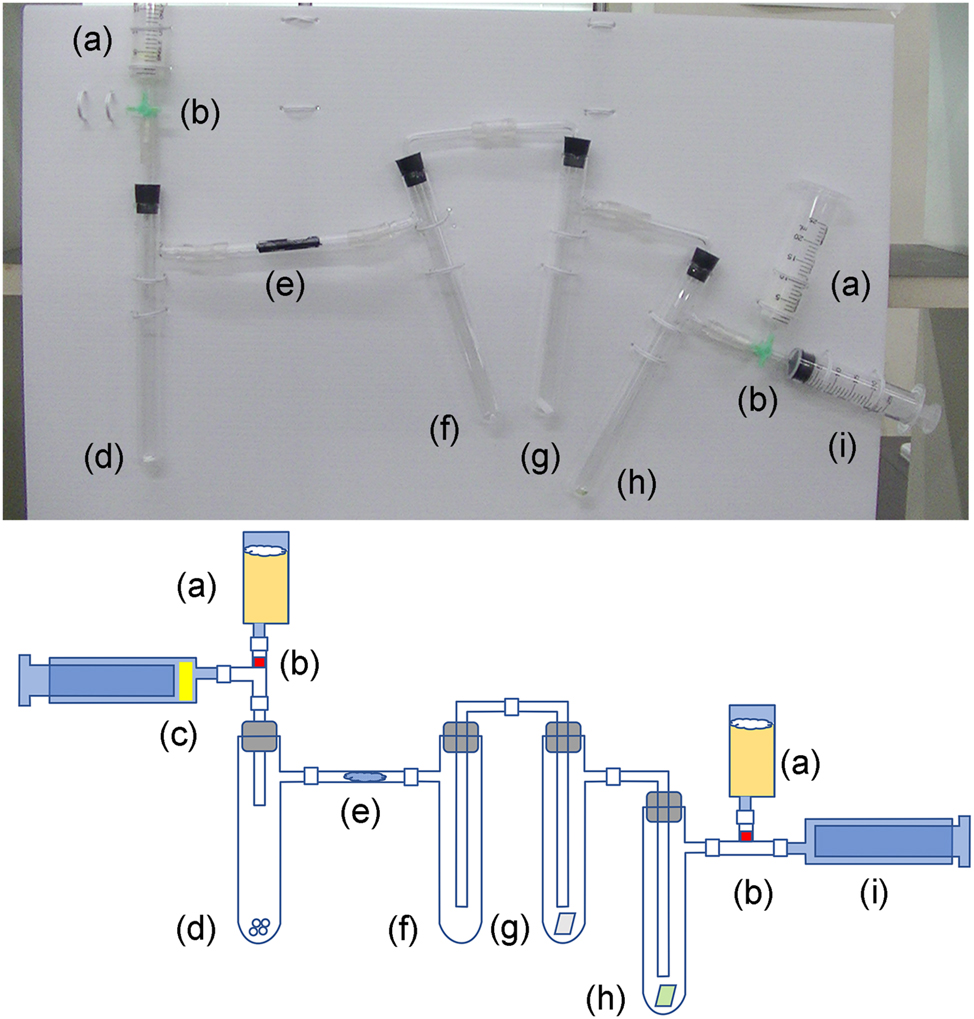
Photograph and schematic of the reaction apparatus: (a) Sodium thiosulfate, (b) three-way stopcock, (c) hydrochloric acid, (d) bleaching powder, (e) dummy Hypo helmet, (f) trap for cooling and spill prevention, (g) potassium iodide starch paper, (h) pH test paper, and (i) empty plastic syringe.
2 Lecture overview
The lecture contained aforementioned three main parts (1–3). The video, lecture slides, and questionnaires are summarized/presented in the Supplementary Material.
2.1 Brief description of First World War
Several striking photographs from Haber’s (1986) and Freemantle’s (2014, 2015 books were copied using a document camera and projected onto a screen (Figure S3). The First World War (1914–1918) was called the Chemists’ War because of the first use of several chemical munitions, namely, high explosives, poisonous gas, and gas masks (Haber, 1986; Freemantle, 2014; Freemantle, 2015). In addition, military hospitals used antiseptic solutions, anesthetics, and X-ray apparatus to treat the wounded (Freemantle, 2015).
In April 1915, the Imperial German Army released clouds of chlorine over the French and French Algerian armies; this is recognized as the first large-scale poisonous gas attack in history. Many soldiers died from this chlorine attack. In May 1915, the British Army was the first to issue a portable gas mask called the Hypo helmet.
The Hypo helmet was invented by Canadian physician Cluny MacPherson (Haber, 1986). Before the issue of Hypo helmets, Allied soldiers covered their noses and mouths with handkerchiefs and socks soaked with water or urine to protect them from chlorine (Freemantle, 2014, 2015). The Hypo helmet is a fabric hood (Figure 1a) soaked with a mixture of aqueous solutions of sodium thiosulfate and sodium bicarbonate. Although the Hypo helmet could effectively detoxify chlorine, it was eventually replaced by the improved Phenate helmet comprising two eyepieces, an exhaust valve, and a mouthpiece (Figure 1b). By the end of 1916, the Small box respirator comprising a rubber hood, round eyepieces, corrugated tube, and metal canister was introduced (Figure 1c) (Haber, 1986).
Chlorine was used as the poisonous gas for several reasons (Haber, 1986). High concentrations of chlorine gas inhalation can cause pulmonary edema, pulmonary inflammation, respiratory failure, and death (White & Martin, 2010). Chlorine is easy to produce and liquefy using compression and cooling, which made it easy to transport in large quantities to the front line. Because it is more than twice as dense as air (World Health Organization, 1982), it crept across the battlefields and lingered in trenches and shell holes. However, as a weapon, chlorine had a weakness (Haber, 1986). It was difficult to predict where the clouds of chlorine gas would travel to as they were dependent on weather conditions (i.e., wind direction, wind speed, temperature, and humidity) and topography. Moreover, because it has a pungent odor and a characteristic color (World Health Organization, 1982), it was easily noticed and could be avoided by the Allied forces when released.
In addition to chlorine, other chemicals, such as carbonyl dichloride (phosgene) and bis(2-chloroethyl)sulfide (mustard gas), were used as poisonous gases during the First World War (Haber, 1986; Freemantle, 2014, 2015). The use of poisonous gases was so widespread, disposal of dumped and unexploded shells filled with chlorine and other poisonous gases continues, even after more than 100 years after the end of the war (Freemantle, 2018).
2.2 Video demonstration
The following glassware and materials are required to demonstrate chlorine generation and detoxification using the dummy Hypo helmet. Detailed instructions are presented in the Supplementary Material. A photograph and schematic of the reaction apparatus are shown in Figure 2.
Reaction apparatus (Supplementary Material Figure S1): a glass tube threaded through a rubber stopper, three L-shaped glass tubes threaded through rubber stoppers, four glass test tubes with side arms, two plastic three-way stopcocks, two empty plastic syringes, and two plastic syringes filled with solid sodium thiosulfate (8 g).
Dummy Hypo helmet: glass tube (10 cm in length) filled with a small (4 × 2.5 cm) piece of fabric (50 % cotton; 50 % wool) soaked with a mixture of saturated aqueous solutions of sodium thiosulfate and sodium bicarbonate.
Bleaching powder: 0.2 g.
Hydrochloric acid: 1 mL (12 mol/L or 4 mol/L).
Saturated aqueous solution of sodium thiosulfate: 2 mL.
Saturated aqueous solution of sodium bicarbonate: 2 mL.
Potassium iodide starch paper: 5 mm square.
pH test paper: 5 mm square.
For the video demonstration, we prepared two short videos. One demonstrated the generation and identification of chlorine, and the other demonstrated chlorine detoxification using the dummy Hypo helmet. The video clips have no sound so that instructors can add their commentary to them. For the chlorine generation and identification experiments, 12 mol/L hydrochloric acid was adequate to generate visible chlorine. For the chlorine deactivation experiments, less chlorine was generated by using 4 mol/L hydrochloric acid to ensure detoxification by the dummy Hypo helmet.
The chlorine was generated in a test tube (d) by the reaction of 0.2 g bleaching powder with 1 mL 12 mol/L hydrochloric acid. Note that the chemical reaction makes the test tube (d) hot. This method produces a mixture of chlorine and hydrogen chloride (Scheme C). Chlorine accumulates in the bottom of the test tube, which passes through a glass tube (e) to an empty test tube (f). The empty test tube prevents water vapor from clouding the following test tubes (g and h) that contain potassium iodide starch paper and pH test paper, respectively. Chlorine changes the color of potassium iodide starch paper from white to violet (Figure 3, Scheme D); and hydrogen chloride, which is colorless with a pungent odor (World Health Organization, 1982), changes the color of pH test paper from green to orange (Figure 3). Excess chlorine further decolorizes the two test papers (Figure 3c). An empty syringe (i) is used to pass the generated chlorine through the glass tube (e) and test tubes (f–h). If there is sufficient sodium thiosulfate in the plastic syringe (a), the generated chlorine will not escape. The sodium thiosulfate in the plastic syringe (a) can be reused several times; however, as it gradually deteriorates, it becomes yellowish. The generated chlorine is somewhat difficult to see in the video; hence, instructors must tell the students to watch the video carefully.

Color changes in potassium iodide starch paper and pH test paper: (a) Before and (b) after exposure to chlorine and hydrogen chloride, and (c) after discoloration by chlorine.
Next, the generated chlorine is detoxified by the dummy Hypo helmet in the glass tube (e). It comprises a rolled piece of fabric soaked with a mixture of saturated aqueous solutions of sodium thiosulfate and sodium bicarbonate. If the amount of the generated chlorine is not high; that is, the concentration of the hydrochloric acid used is low (4 mol/L), the dummy Hypo helmet can deactivate chlorine for a while, and neither the potassium iodide starch paper nor the pH test paper is discolored. Note that the glass tube (e) becomes hot during the neutralization. Chlorine reacts with sodium thiosulfate to generate sodium sulfate, sulfur, and hydrogen chloride (Scheme A). The sulfur produced by the reaction can be observed as yellow staining in a glass tube (Figure 4). Hydrogen chloride is neutralized by sodium bicarbonate to produce harmless materials; sodium chloride, water, and carbon dioxide (Scheme B).
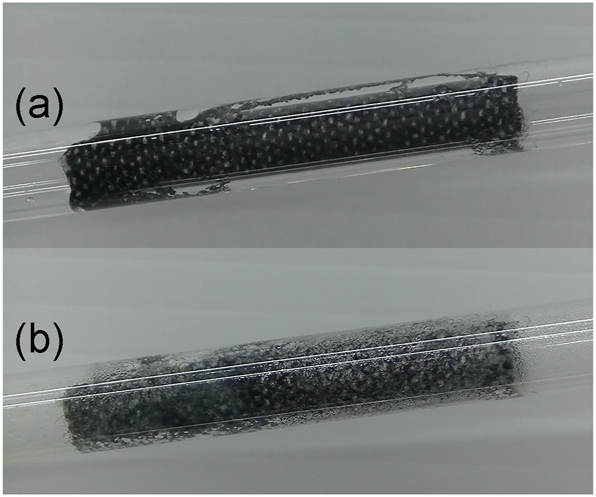
Dummy Hypo helmet (a) before and (b) after exposure to chlorine.
2.3 Students’ comments
In 2022, approximately one-third of a 90 min chemistry lecture was presented without the experimental videos mentioned in the main text. At the time, participating students told the authors that it would have been better to include a demonstration or video of chlorine generation. In 2023, a lecture was presented that included the experimental videos. In each year, the target students were undergraduates, including second-year environmental science majors and first-year civil engineering majors. The environmental science majors participated in the demonstration lecture as part of a class called General Chemistry. General Chemistry was an elective class; however, it was compulsory for students who wanted to obtain a science teacher’s certificate. On the other hand, the civil engineering majors took the demonstration lecture as part of the Basic Chemistry course, which was required for graduation. All the participants understood the basic properties of chlorine before the lecture, such that chlorine was a gas consisting of diatomic molecules of the halogen group. Many of the participants did not choose chemistry for their university entrance exams nor did they think they were good at chemistry. Students at our university who were not good at chemistry often did not listen to their chemistry lectures enthusiastically. Therefore, the author took to using some of the 90 min lecture time for creative presentation.
After the video demonstration, the students wrote down their impressions of the lecture. During this time, the instructor explained the duality of chlorine. Chlorine, as a poisonous gas, not only killed soldiers but also saved soldiers when used as a cleaning agent in drinking water and a disinfectant in the trenches (McGuire, 2013).
The following are some examples of the students’ impressions:
I was very surprised that the munition to protect against poison gas was so simple.
The use of poison gas has been and will continue to carry a great risk of retaliation.
The cat-and-mouse game … when a gas mask is developed, a more toxic gas is developed, is terrifying.
I learned in this lecture that chemicals can kill or help people, depending on how they are used.
3 Hazards
As the lecture explained, chlorine can be used as a poisonous gas; therefore, experiments to generate chlorine should be conducted in areas with good ventilation. Many of the chemicals used in the demonstration are dangerous and must be handled with great care. Rubber plugs and silicone tubes are degraded by chlorine and cannot withstand repeated use.
4 Results and discussion
Several books describing connections between historical episodes and chemistry have been published (Le Couteur & Burreson, 2003; Ponting, 2005). In particular, the chemistry of the First World War is described in detail in Freemantle’s (2014, 2015 books. This lecture and demonstration were based on these books.
Several methods for the laboratory-scale generation of chlorine have been reported (Alyea, 1969a, 1969b; Garman, 1969). However, each of these methods produces different products. The reaction of bleaching powder with hydrochloric acid (Scheme C) was selected for our video demonstration because it does not require heating, and this method can be performed in classrooms by simply using open windows for ventilation. However, for safety reasons, we decided to use videos. When undertaking the experiment, instructors should emphasize to students that they should not generate unnecessary chlorine or wear hoods soaked with chemicals, such as the Hypo helmet. If chlorine generation is the only experiment to be undertaken, a common instrument, such as an Erlenmeyer flask, can be used. However, if demonstrating both experiments simultaneously, the apparatus for both can be arranged in parallel on corrugated plastic board.
In addition, we speculated that the Hypo helmet was not highly effective against high concentrations of chlorine. Initially, when a clearly visible amount of chlorine was generated in the test tube (d), the dummy Hypo helmet failed to detoxify it, and the test paper was immediately discolored (g, h). Therefore, in the chlorine detoxification experiment, the amount of generated chlorine was deliberately reduced. We also imagined that it would have felt very stuffy when wearing the Hypo helmet and the wearer’s field of vision would have been narrowed.
Many students listened to this lecture with interest and eagerly filled in the questionnaire, partly because the war between Russia and Ukraine was being featured in the news almost daily when the lecture was conducted. Commonly, chemistry textbooks describe the use of chlorine as a disinfectant or fungicide; there is no mention of chlorine being used as a poisonous gas in the past. Furthermore, there is no mention in chemistry textbooks that chlorine leakage occurs when containers fall during transport (Hoyle & Svendsen, 2016).
As the authors expected, the lecture introducing old technology with chemistry became popular with the students. The authors attributed this to the ease of the lecture and the ghostly appearance of the Hypo helmet, which piqued student interest. It was an impressive lecture because participants had rarely seen chlorine generation experiments in their regular chemistry lectures, nor had they seen gas masks. Because the Hypo helmet had a simple structure, participants who were not good at chemistry could understand how it worked as well as the inherent chemistry.
5 Conclusions
The physical and chemical properties of chlorine were presented in a lecture in the context of chemical warfare. Chemistry textbooks in our country contain many color photographs and compared to past textbooks, the content has become more interesting to students. However, they still only include descriptions of the properties of chemical substances and lists of chemical reaction formulas; there is no story. In other words, they are not interesting to read because they are reference books for college entrance exams. Therefore, a narrative lecture like the lecture described here will be popular with students. The authors would like to conduct lectures that students can listen to with interest.
Funding source: National Institute for Environmental Studies
Award Identifier / Grant number: Unassigned
Acknowledgments
R.H. would like to acknowledge Prof. E. Koike (National Institute for Environmental Studies) for her constructive suggestions. The author is grateful to the lecture participants (Osaka Sangyo University). R.H. also thanks Enago (https://www.enago.jp) for the English language review.
-
Research ethics: Not applicable.
-
Author contributions: The authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Competing interests: The authors state no conflict of interest.
-
Research funding: None declared.
-
Data availability: Not applicable.
References
Alyea, H. A. (1969a). Chlorine by electrolysis (liquefaction). Journal of Chemical Education, 46(3), A218. https://doi.org/10.1021/ed046pa218.2 Search in Google Scholar
Alyea, H. A. (1969b). Chlorine from KMnO4 + HCl. Journal of Chemical Education, 46(3), A218. https://doi.org/10.1021/ed046pa218.6 Search in Google Scholar
Careaga, V. P., Guerrero, A. B., Siracusano, G., & Maier, M. S. (2023). High performance liquid chromatography as a micro-destructive technique for the identification of anthraquinone red dyestuffs in cultural heritage objects. Chemistry Teacher International, 5(1), 1–9. https://doi.org/10.1515/cti-2022-0018 Search in Google Scholar
Crawford, G. L., & Kiefer, A. M. (2016). Using a carbide lantern to illustrate general chemistry concepts and introduce students to artisanal and small-scale gold mining. Journal of Chemical Education, 93(4), 687–690. https://doi.org/10.1021/acs.jchemed.5b00604 Search in Google Scholar
Freemantle, M. (2014). Gas! Gas! Quick, Boys!: How Chemistry Changed the First World War. History Press.Search in Google Scholar
Freemantle, M. (2015). The Chemists’ War 1914–1918. Royal Society of Chemistry.10.1039/9781839168284Search in Google Scholar
Freemantle, M. (2018). The great war clean-up. Chemistry World. Retrieved June 2023 from https://www.chemistryworld.com/features/the-great-war-clean-up/3009456.article Search in Google Scholar
Friedrich, B., Hoffmann, D., Renn, J., Schmaltz, F., & Wolf, M. (Eds). (2017). In One Hundred Years of Chemical Warfare: Research, Deployment, Consequences. Springer International Publishing AG.10.1007/978-3-319-51664-6Search in Google Scholar
Garman, R. P. (1969). Chlorine from bleaching powder. Journal of Chemical Education, 46(3), A218. https://doi.org/10.1021/ed046pa218.4 Search in Google Scholar
Giménez, J. (2015). Finding hidden chemistry in ancient Egyptian artifacts: Pigment degradation taught in a chemical engineering course. Journal of Chemical Education, 92(3), 456–462. https://doi.org/10.1021/ed500327j Search in Google Scholar
Haber, L. F. (1986). The Poisonous Cloud: Chemical Weapons in the First World War. Clarendon Press.Search in Google Scholar
Horikoshi, R., Takeiri, F., Kobayashi, Y., & Kageyama, H. (2016). Exploring the gas chemistry of old submarine technologies using plastic bottles as reaction vessels and models. Journal of Chemical Education, 93(8), 1411–1414. https://doi.org/10.1021/acs.jchemed.5b00732 Search in Google Scholar
Hoyle, G. W., & Svendsen, E. R. (2016). Persistent effects of chlorine inhalation on respiratory health. Annals of the New York Academy of Sciences, 1378(1), 33–40. https://doi.org/10.1111/nyas.13139 Search in Google Scholar PubMed PubMed Central
Le Couteur, P., & Burreson, J. (2003). Napoleon’s buttons: 17 Molecules that changed history. Penguin Group.Search in Google Scholar
McGuire, M. J. (2013). The chlorine revolution: Water disinfection and the fight to save lives. Amer Water Works Association.Search in Google Scholar
Padley, A. J. (2016). Gas: The greatest terror of the great war. Anaesthesia & Intensive Care, 44, 24–30. https://doi.org/10.1177/0310057x1604401s05 Search in Google Scholar PubMed
Ponting, C. (2005). Gunpowder, The story. Random House.Search in Google Scholar
Vyhnal, C. R. (2022). Curricular materials on the chemistry of pottery, including thermodynamic calculations for redox reactions in the 3-stage firing process of Athenian black- and red-figure vases produced from the sixth–fourth centuries BCE. Journal of Chemical Education, 99(2), 768–776. https://doi.org/10.1021/acs.jchemed.1c00953 Search in Google Scholar
White, C. W., & Martin, J. G. (2010). Chlorine gas inhalation, human clinical evidence of toxicity and experience in animal models. Proceedings of the American Thoracic Society, 7(4), 257–263. https://doi.org/10.1513/pats.201001-008sm.Search in Google Scholar PubMed PubMed Central
World Health Organization. (1982). Chlorine and hydrogen chloride. Retrieved June 2023 from https://www.who.int/publications/i/item/9241540818 Search in Google Scholar
Supplementary Material
This article contains supplementary material (https://doi.org/10.1515/cti-2023-0046).
© 2023 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Articles in the same Issue
- Frontmatter
- Review Article
- Relativistic effects on the chemistry of heavier elements: why not given proper importance in chemistry education at the undergraduate and postgraduate level?
- Research Articles
- Designing a learning environment based on the spiral of skills to overcome the didactic obstacles associated with teaching the Daniell cell
- Analysis of the relationship between students’ argumentation and chemical representational ability: a case study of hybrid learning oriented in the environmental chemistry course
- Good Practice Report
- What makes representations good representations for science education? A teacher-oriented summary of significant findings and a practical guideline for the transfer into teaching
- Research Article
- Student exploration of the Henderson-Hasselbach equation and pH readings to determine the pK a value of 4,4′-trimethylenedipiperidine (TMDP)
- Good Practice Report
- Chemistry saving lives: using First World War Hypo helmets to avoid chlorine poisoning
- Research Article
- An exploration of the proton NMR problem-solving approaches of undergraduate students
- Good Practice Reports
- Chemical Quest: general knowledge and popular culture quizzes about the elements in a board game for the class
- Lessons learned from a case study on teaching the socioscientific issue of ethanol, used as an ingredient of sanitizers, to promote students’ learning of and about chemistry during the COVID-19 pandemic
Articles in the same Issue
- Frontmatter
- Review Article
- Relativistic effects on the chemistry of heavier elements: why not given proper importance in chemistry education at the undergraduate and postgraduate level?
- Research Articles
- Designing a learning environment based on the spiral of skills to overcome the didactic obstacles associated with teaching the Daniell cell
- Analysis of the relationship between students’ argumentation and chemical representational ability: a case study of hybrid learning oriented in the environmental chemistry course
- Good Practice Report
- What makes representations good representations for science education? A teacher-oriented summary of significant findings and a practical guideline for the transfer into teaching
- Research Article
- Student exploration of the Henderson-Hasselbach equation and pH readings to determine the pK a value of 4,4′-trimethylenedipiperidine (TMDP)
- Good Practice Report
- Chemistry saving lives: using First World War Hypo helmets to avoid chlorine poisoning
- Research Article
- An exploration of the proton NMR problem-solving approaches of undergraduate students
- Good Practice Reports
- Chemical Quest: general knowledge and popular culture quizzes about the elements in a board game for the class
- Lessons learned from a case study on teaching the socioscientific issue of ethanol, used as an ingredient of sanitizers, to promote students’ learning of and about chemistry during the COVID-19 pandemic

