Abstract
In a teaching context based on the competency approach, the creation of an appropriate teaching-learning environment requires, among other things, teachers to master the meaning of the concepts taught and teaching-learning activities designed according to the constructivist approach and the investigative approach. In this article, we focused on the operating principle of the Daniell cell. The research study involved identifying the epistemological gaps of 58 future teachers in relation to the concepts describing the previous theme via an open questionnaire, as well as the degree of compliance of the activities proposed in the textbook with the curricular guidelines. The main results showed that the respondents had not mastered the meaning of the positive and negative poles of a cell or the concept of its electrical voltage. With regard to the textbook studied, we found that the design of the activities did not comply with the principles of the competency-based approach and the spiral progression of knowledge. To overcome these constraints, we propose pedagogical designs aimed at reinforcing and developing the skills of teachers and learners while progressing in the spiral of knowledge.
1 Introduction
Education in Morocco is organized into three cycles: primary education, secondary education and higher education. The secondary cycle includes middle school and high school. The three-year high school system leads to a certificate that gives access to higher education. As a result, the final year of high school is crucial and an effective learning environment is necessary. This environment should enable learners to have an active role in the teaching and learning process, supporting the acquisition of appropriate and meaningful knowledge.
The most appropriate environment should be based on activities that could improve learners’ problem-solving skills, enabling the development and application of chemical knowledge and practices, with the main aim of transforming the substance for everyday use (Sevian & Talanquer, 2014). It is an environment that requires effective learning, focused on how students learn, not just what they learn (Munna & Kalam, 2021; Taber, 2014). This requires a pedagogy based on constructivism to help learners construct a problem in accordance with the teaching-learning objectives and to reflect on the elements involved in solving it. Problem-solving based on innovative teaching methods stimulates their thinking and enables them to develop a profound understanding of the content studied (Cooper, 2018; Kwangmuang et al., 2021). This learning problem must also focus on bringing out learners’ conceptions in order to correct them (Taber, 2019a). Generally, learners come to class with certain conceptions relating to the concept under study, but these conceptions are often erroneous or incomplete. They tend to resist the construction of new knowledge, since its acquisition requires radically replacing or reorganizing learners’ knowledge (Lucariello & Naff, 2013; Novick & Catley, 2014). Thus, a conceptual change must occur during the learning process for the learner to be able to construct new knowledge correctly (Becker & Cooper, 2014; Butler et al., 2015; Novak, 2010; Soeharto & Csapó, 2021).
According to Cooper (2018), the best learning environment should be based on a competency-based approach that enables learners to master epistemological knowledge, practical skills and the process of constructing scientific knowledge. In addition to practical skills, experimentation is an essential didactic component of chemistry teaching, helping learners to understand and grasp the meaning of different theoretical concepts (Alkan, 2016; Campbell et al., 2022). The use of the problem-solving activity and inquiry-based learning contribute to and consolidate the acquisition of scientific process skills (Firman et al., 2019; Minner et al., 2010). From this perspective, the new status of the experimental activity has become a mean of testing learners’ hypotheses rather than verifying facts (Bell et al., 2010). This activity requires learners to design an experimental protocol (Pedaste et al., 2015). The scientific approach also relies on modelling, which involves developing a concrete representation of an abstract situation that is difficult to grasp. The aim is to make it easier to understand a phenomenon or concept, and to predict and argue certain facts (Chittleborough & Treagust, 2009). According to Christodoulou and Osborne (2014), modelling and argumentation activities are key elements of effective learning that enable learners to construct knowledge in accordance with the epistemic practices of science. Learners rely on abstraction and develop representations that relate to the underlying concepts of the teaching activities and problems they have solved. Therefore, the choice of these activities through a learning environment is crucial to enable the learners to better assimilate the concepts (McDaniel et al., 2018). On the other hand, the investigation method based on triggering situations must be based on a phenomenon or fact linked to everyday life and which poses a challenge that must be overcome. Its didactic role is to arouse learners’ attention and interest in science (Swarat et al., 2012). The problem-solving process requires a strategy rather than a direct response, during which the learners must use their prerequisites and representations to try to find answers (Frey et al., 2022). The learners must then mobilize new knowledge in order to correctly solve the problem of the triggering situation (Reid & Ali, 2020). The construction of any knowledge in a coherent and integrated way must be the response to a constructed problem, during which the learners are obliged to overcome an epistemological obstacle in relation to the teaching-learning objectives (Berland & McNeill, 2010; Mayer, 2011; Yeşiloğlu & Köseoğlu, 2020).
All these guidelines are taken into account in the Moroccan curriculum, which advocates a skills-based approach to teaching physical sciences based on problem-solving and the experimental investigation approach, while promoting modelling activities (MEN, 2006). The curriculum also stresses the importance of a spiral progression in the process of constructing the targeted concepts.
The spiral approach suggests that the same concept should be tackled several times during the learning process, in a progression from the simple to the complex and with increasing abstraction and nuance (Galloway & Bretz, 2016; Shavelson & Kurpius, 2012).
The aim of this curriculum is to ensure that learners acquire transferable disciplinary and transdisciplinary skills. Learners should not limit themselves to learning declarative and procedural knowledge, but are expected to mobilize this knowledge in contextualized situations that give meaning to the knowledge acquired. To achieve these learning objectives, teachers should help learners to reinforce the skills they have acquired and then to deepen them by “ascending the spiral of knowledge” while integrating this knowledge into an everyday context (Campbell et al., 2022).
Chemistry teachers should also reflect more on the content to be taught, the pedagogical methods employed and the appropriate sequencing of knowledge (Cooper et al., 2013). However, according to the study we carried out on a sample of 3rd year high school learners aimed at studying the effect of the structure of the chemistry syllabus on their reasoning, it was found that they only mastered some fragmented knowledge, and were therefore unable to mobilize it in an integrated way in everyday situations. The analysis also showed that the didactic transposition used for the conceptualization of concepts related to redox chemical transformations, favored only the memorization of a few declarative and procedural knowledges, and that the construction of knowledge proceeded in a linear rather than a spiral progression (Mennani et al., 2023a). According to another study we conducted, which focused on identifying conceptual difficulties in teachers when teaching redox concepts, revealed epistemological deficiencies related to the knowledge to be taught and they were unable to mobilize the appropriate knowledge to solve problems related to everyday life (Mennani et al., 2023b). Whereas, according to the curriculum, the teacher must play an important role in creating a teaching-learning environment conducive to the construction of concepts with physical or chemical meaning and to the development of transferable skills in learners. These observations led us, as teacher-researchers, to reflect on how to improve the training of our future teachers who will have to teach at high school, and on the activities proposed for the teaching of chemistry. Our concern was to find out whether these activities were structured according to the skills-based approach and problem solving, and whether they followed the spiral progression recommended in the official guidelines. For more targeted research, we chose the topic of electrochemical cells for the following reasons:
Teaching this topic requires the mobilization of redox concepts that pose problems for teachers and learners (Mennani et al., 2023a, 2023b);
As part of our guidance of future physics and chemistry teachers in the educational establishments, their course supervisors (CS), who are also teachers, have informed us that they and the trainees have found it difficult to answer their learners’ questions on a number of concepts linked to the subject of electrochemical cells, particularly on the physical meaning of the (+) and (−) poles.
This second consideration was like a catalyst that drove us to undertake this research, especially as the future teachers have at least a bachelor’s degree and have benefited from training to reinforce their knowledge of chemistry.
With regard to the initial training of physics and chemistry teachers, the qualification curriculum for trainee teachers is geared towards greater professionalization (MEN, 2012). It aims to train teaching professionals who are able to reflect and act autonomously and responsibly. With this in mind, the aim of the training plan is to enable trainee teachers to move from mastery of subject-specific knowledge and know-how to their application in teaching situations and their appropriation by learners. This presupposes a mastery of the content of physics and chemistry teaching in particular, followed by the appropriation of concepts, knowledge and know-how relating to the didactics of these subjects. Trainee teachers are qualified in training centers using a modular system that alternates with practical situations in the schools. Each module is structured around a professional skill that must be acquired by the end of the module. The training system includes several modules, in particular the chemistry training module. The objectives of this module are to develop the following skills: design and carry out experiments using teaching aids available in their professional environment, exploit the results of an experimental activity effectively in order to compare the predictions of a theoretical model with experimental results and use these results to refine a theoretical model, master the process of constructing the knowledge taught and promote the sense of observation which is at the origin of major discoveries and develop in learners a taste for experimentation and the concrete, recognize their role as mediators in the development of ideas in class. Regarding the topic of electrochemical cells, the program recommended for the training of future teachers is identical to that for the 1st year of university. This program recommends the use of the Nernst equation to express the voltage E of an electrochemical cell (UCDFS, 2013). According to this syllabus, an electrochemical cell is made up of two half-cells connected by an ion bridge allowing the flow of ions. In the Daniell cell, there is a potential difference between the two copper and zinc electrodes. If these two electrodes are joined by a metal cable, an electric current flow through them, enough to light a small ampoule. This current flows from copper to zinc, so the electrons are transferred from Zn to Cu. The intensity of the current decreases over time. When the potential difference becomes zero (E = 0) the system no longer evolves chemically. The anode (site of oxidation) is marked (−), the cathode (site of reduction) is marked (+). The greater the difference between the standard potentials of the two pairs, the greater the cell voltage and the greater the equilibrium constant.
Starting from the background mentioned above, we proceeded to analyze the official pedagogical guidelines relating to the teaching of chemistry in the high school (MEN, 2015). These guidelines introduce cells as systems enabling spontaneous transformations to produce electrical energy. The syllabus recommends experimenting with the Daniell cell, using an ammeter to show the direction of the current and a voltmeter to measure the electrical voltage E. Theoretically, the use of the spontaneous evolution criterion is useful for determining the direction of movement of charge carriers in the cell. This cell consists of two compartments connected by an ion bridge. The electrons leave the anode, which is the negative terminal of the cell, and enter the cathode, which is the positive terminal of the cell. In the schematic diagram describing the operation of the cell, it is necessary to show the movement of the charge carriers as well as the (+) and (−) poles. The spontaneous chemical transformation that produces the current in the cell is described by electronic half-equations.
This description seems to suggest an approach devoid of physical meaning for a number of electrical concepts and represents a didactic break with the approach which introduced the physical meaning of the positive and negative poles of a battery as part of the middle school syllabus. In fact, in the program for this cycle, the notions of the (+) and (−) poles of a battery were introduced on the basis of a similarity between the concepts that describe a hydraulic current and those that describe an electric current. However, there is no reference to this in the high school program. Nor does the syllabus on electrochemical cells mention the cause of the electric current in these cells. However, since middle school, learners have known that the production of a current requires the existence of an electrical potential difference, also known as an electrical voltage.
Furthermore, and as a first step, we sought to identify the epistemological and didactic gaps in our future teachers’ knowledge of electrochemical cell, particularly those they encountered during their training courses in the presence of their (CS). Secondly, we analyzed the teaching-learning activities in the textbook most frequently used by the (CS). These activities are the result of a didactic transposition carried out by the designers of this textbook on the basis of the syllabus described in the preceding paragraphs, and they should in principle give concrete form to the curriculum guidelines. The (CS) have informed us that they rely entirely on these activities to teach the topic of cell.
These findings led us to question the conceptions of future teachers regarding the concepts covering the functioning of the Daniell cell, as well as the activities adopted in the textbook most frequently used by the (CS).
2 Aim of the present study
The main objective of our research was to propose a more appropriate teaching-learning environment for the Daniell cell. This environment focuses on developing skills while progressing through the knowledge spiral, enabling learners to revisit increasingly complex concepts several times. Our focus was on the concept of cell voltage and the notion of positive and negative poles.
To achieve this main objective, we needed: (1) the recommended syllabus for the teaching of electrochemical cells at high school and university level; (2) the curricular guidelines for the teaching of physical sciences at high school level; (3) the results of the various articles cited in our research; (4) the reasoning used by trainee teachers to interpret the meaning of certain concepts in connection with the topic of study; (5) the teaching-learning activities proposed in the textbook most frequently used by the teachers (CS). Our concern was to find out whether: (i) the trainee teachers’ reasoning was based on a well-constructed knowledge base; (ii) these activities were structured according to the skills-based approach and problem solving, and whether they followed the spiral progression recommended in the curricular guidelines following were the research questions of the study:
What are the epistemological and didactic needs of trainee teachers in relation to the concepts that describe how the Daniell stack works?
How are these concepts constructed in the textbook?
3 Research methodology
In order to propose an appropriate learning environment for the Daniell cell to high school learners, we first looked at the knowledge of our future teachers in relation to the subject studied. These trainees have all studied in 1st year university that to express the potential of an electrode, they can use Nernst’s law and therefore should be able to give the physical meaning of the poles of a battery and also the notion of electrical voltage E (UCDFS, 2013).
3.1 The participants
The study sample consisted of 58 trainee teachers divided into two groups, 48 % of whom were women trainee teachers. 65 % have a bachelor’s degree in chemistry or physics (3 years’ training at university), while the others have a master’s degree in chemistry (5 years’ training). They come from the University of Casablanca and the University of El-Jadida. These trainee teachers will be teaching the operating principle of the Daniel cell from next year. We note that access to the training center is by pre-selection on the basis of the best university results and success in written and oral entrance examinations. The training lasts 6 months, including practical training (8 h per week).
3.2 Instruments and data collection- procedure
During the month of January 2023, the researchers met several times with 10 (CS). The discussions focused on the conceptual difficulties associated with the various concepts that describe how a cell functions and that create problems for teaching and learning. On the basis of these discussions, we formulated the research questions. The questionnaire consisted of 7 items, all of which were open-ended.
Two weeks before collecting the results of the questionnaire, the researchers met with the trainee teachers and their chemistry trainer. Discussions focused on how to effectively involve and motivate these participants, as well as the content of the questionnaire. We were informed that these respondents had just undergone theoretical and experimental training on the subject of electrochemical cells during their training at the training center, and that several of them had had the opportunity to teach this subject during their placements in school establishments.
The questionnaire was administered to the respondents on 7 March 2023, at their training center. They answered the test for about an hour. To answer the questions, the respondents were invited to use the following resources: The concepts of electric voltage and electric current, the analogy between an electric current and a hydraulic current, the analogy between altitude and electric voltage, Nernst’s law and the meaning of the standard potential of a redox couple, the notion of the reference associated with electrical potential, in particular the standard potential of the H+/H2 couple, the role of the ionic bridge in an electrochemical cell, anode, cathode, the electromotive force of an electrochemical cell and the criteria for the spontaneous evolution of a chemical transformation.
Questionnaire items:
Answer the following questions, give details of your reasoning and use diagrams to explain. Try to draw on all the knowledge electrical and chemical fields. Use the similarities.
Cu 2+ + 2e− ⇌ Cu At a given time t: are the electrons released by the zinc exactly the same as those captured by the Cu 2+ ions? Model this process on the electrodes.
|
The first four questions were posed by learners to (CS) and trainee teachers during the teaching of Daniell Cell content. All these teachers, according to their declarations, found it difficult to answer these questions. The other questions were asked in the same context: question (5) enabled us to identify the trainees’ conceptions of the concept of electrode voltage. In particular, the standard potential is a given value in relation to an electrical reference. We wanted to know whether the trainees understood this notion of electrical reference and whether they could evoke in their reasoning by analogy the reference adopted to determine the geographical altitude of a point. Question (6) enabled us to identify the students’ reasoning on the cause of the electric current in a battery and why its intensity decreases until it cancels out. We wanted to know whether the students understood this cause and whether they could use it as an analogy with hydraulic current. In particular, they should know that the potential of the electrode associated with the cathode decreases and the potential of the electrode associated with the anode increases. Question (7) enabled us to identify the trainees’ thermodynamic reasoning on the evolution of the reaction quotient associated with the reaction equation describing the chemical transformation within the cell. We wanted to find out whether the trainees were able to reason using thermodynamic knowledge.
In principle, the knowledge required to answer these questions is at the level of the third year of high school, with the exception of question (5) which requires knowledge at university level.
We believe that the knowledge required to answer the questions in the questionnaire correctly is essential and will help teachers to guide their learners so that they acquire a well-constructed and well-structured knowledge of the content of the Daniell cell.
In order to understand the respondents’ reasoning better and to refine their training requirements, we conducted a 15-min individual interview with each of them after the questionnaire had been analyzed, followed by focus groups (29 respondents per group) lasting 2 h. We asked them to comment on the questionnaire. In particularly if they were familiar with this type of task, and then to explain and discuss their reasoning and their expectations so that they could make up for their deficiencies and improve their skills to enhance their performance in class. The analysis of the respondents’ reasoning focused on answers that were inadequate in relation to the knowledge recommended in the high school and university syllabus. Our objective was essentially focused on the ability of these trainees to mobilize their knowledge in an integrated way, and whether they take into account in their teaching practice the spiral progression of knowledge relating to concepts related to the theme of Daniell’s cell. We compared their reasoning with that adopted by scientists. In other words, the reasoning that could be interpreted and analyzed in terms of canonical chemistry illustrated by the versions of concepts that chemists currently hold in university (Evagorou & Osborne, 2013; Taber, 2019b).
Following this study, we were interested in the didactic transposition of the content to be taught in the textbook most used by the (CS). The aim is to exploit the results of the analysis of content construction, in order to propose a more appropriate approach to the construction of the targeted concepts.
Morocco has chosen to adopt the skills-based approach and the investigative approach to teaching. However, the question facing us as trainers and researchers is the extent to which textbook designers are complying with these guidelines, especially as most teacher’s state that they adopt the activities in the textbook. The analysis of the manual’s content focused on the following axes:
The triggering activity and its relevance to the target objective, as well as its formulation on the basis of an everyday problem situation;
The proposed activities, in particular their relevance to the target objective, their structuring scope, the modelling process and the experimental protocol supposed to be in harmony with problem-solving and the spiral construction of concepts.
On the basis of the above, we drew up an analysis grid with a list of indicators relating to the proposed triggering situation, the experimental and modelling process and the compliance of the spiral approach in the construction of knowledge describing the theme studied.
4 Results and discussion
4.1 Results of the questionnaire
We note that the electrochemical cell program recommended in the 1st year of university constitutes a continuation of that recommended in high school. The progression of this new content is reflected in the introduction of Nernst’s law to express the potential difference between the electrodes. However, assigning the (−) sign of the cell to the anode and the (+) sign to the cathode without any electrical justification could lead to a superficial understanding of these concepts.
Analysis of the questionnaire revealed that most trainee teachers had little grasp of the epistemological aspects of the various concepts that describe how the Daniell cell operates. They were unable to mobilize their knowledge in an integrated way. Answering the questions requires the mobilization of several resources that are linked to epistemological aspects in the fields of electricity and chemistry. We note that none of the trainee teachers mentioned the similarity between hydraulic current and electric current.
With regard to the construction of new knowledge according to a spiral progression, we noted that during the interviews, the respondents stated that during their placements in educational establishments, they did not take into account the prerequisites of middle school learners, linked to the physical meaning of the (+) and (−) poles of a cell and its voltage. They limited their teaching to the fact that the anode corresponds to the (−) pole and the cathode to the (+) pole.
On the other hand, we found that the questionnaire stimulated their interest. They declared that the tasks in the questionnaire were interesting because they were structured around their skill gaps. We found no difference in level between those with a bachelor’s degree and those with a master’s degree in chemistry.
Below are the responses of these respondents:
To question Q1, In the Daniel cell, the copper electrode is the cathode (the + terminal) and the zinc electrode is the anode (the – terminal). What is the physical of these connections (+) and (−)? Justify this electrically, try not to reason on the definitions of anode and cathode: The answer should be based on the similarities between electric current and hydraulic current and on the knowledge developed at university, in particular on the potential of an electrode, which is expressed by Nernst’s law. Consequently, since the potential of the copper electrode is higher than that of the zinc electrode, by convention we assign the (+) pole to the electrode with the higher potential.
The reasoning of 58 trainee teachers
|
The reasoning of all the trainee teachers was based on the definitions of anode and cathode, even though they had been asked to draw on knowledge of electricity. Several answers were incorrect. These trainees do not have a well-organized knowledge base linking the chemical and electrical fields. During the interviews, they all stated that they had never had the opportunity either at high school or university to solve or discuss this type of question in our questionnaire. They added that during their school and university studies they had never used the similarity between hydraulic current and electric current.
To question Q2, what is the role of the ion bridge? Can it be replaced by a copper wire?
All trainee teachers answered as follows:
| The ionic bridge is used to ensure the electrical neutrality of the two solutions of Cu2+ and Zn2+. It cannot be replaced by a copper wire because this wire will displace electrons and will therefore not ensure the electrical neutrality of the two solutions. It cannot be replaced by a copper wire because there will be an excess of positive ions in the copper solution. |
During the discussion, we solicited those who had taught the Daniell cell during their courses to explain the teaching methods they had used. They confirmed that they had used the ion bridge directly without questioning their learners.
All 58 trainees interviewed defended the idea that the ion bridge serves to ensure the electrical neutrality of the solutions in the cell. For them, it’s like a law that shouldn’t be discussed. This observation would be the result of transmissive learning that they received during their school or university careers. Then, we asked them if it was possible to use a copper wire to close the circuit. They all refuted this idea. We suggested they go to the laboratory to test our suggestion. They were surprised to see that a current could be passed through the circuit by connecting the two solutions with a copper wire. Clearly, during their learning career, they did not have the opportunity to solve problems, propose and test hypotheses.
To question Q3, during operation of the cell, the copper electrode does not react. Can it be replaced by a carbon graphite electrode? We found several incorrect answers:
| According to 24 trainee teachers, carbon graphite is a conductor of electricity, it has no redox couple, i.e., chemically it does not react and since in this experiment the copper plate does not react, it can be replaced by carbon graphite. The copper formed will deposit on the carbon graphite electrode. According to 28 other trainee teachers, carbon graphite is a good conductor, so the current in the battery will increase. 6 trainee teachers reasoned correctly: According to Nernst’s law, a battery requires the use of two redox couples. The cell voltage represents the difference between the potentials of the two electrodes. The voltage of each electrode is given by Nernst’s formula, so the presence of the copper electrode is a necessary condition. |
According to the (CS), this question poses a serious problem for them with their students. The copper electrode does not react during cell function, so the learners could raise this issue during the course and suggest replacing the copper electrode with another metal such as carbon graphite. In principle, at university, all students should know that the operation of a cell requires the use of two redox couples in addition to Nernst’s law to express the potential difference between the electrodes of the cell.
To question Q4, the redox half-equations describing the chemical transformation in the cell are as follows:
Z n ⇌ Zn 2+ + 2e −
Cu 2+ + 2e − ⇌ Cu
At a given time t: are the electrons released by the zinc exactly the same as those captured by the Cu 2+ ions? Model this process on the electrodes.
All respondents answered as follows:
|
The zinc plate gives up its electrons spontaneously to Cu2+ ions in the following process: the zinc releases electrons: Zn ⇌ Zn2+ + 2e− and the copper ions capture these electrons: Cu2+ + 2e− ⇌ Cu |
However, none of the trainee teachers tried to model this process on the electrodes. During the discussion, we prompted them to draw the two electrodes and the two solutions and then to model the electrons released by the zinc and spontaneously transferred to the Cu2+ ions. It was clear that the task asked of the respondents was complicated. They were certainly not used to microscopically modelling the transfer of electrons between a reducing agent and an oxidizing agent. Given that the zinc electrode is not in direct contact with the Cu2+ ions, all the respondents were unable to offer an explanation for this electron transfer. They were unaware that the electric current is not the result of the individual movement of an electron, but rather like a fluid in movement. They were unaware of the similarities between electric current and hydraulic current.
To question Q5, what does the following value mean E 0 (Cu 2+ /Cu) = 0.34 V? Is this value absolute or relative? The trainee teachers should know that electrical potential is expressed in relation to a reference exactly as in the case of the altitude of a point. By analogy, the altitude of a point is determined in relation to sea level (altitude reference) and the potential of an electrode is determined in relation to the standard potential of the hydrogen electrode. Therefore, the value 0.34 V is a potential difference between the copper electrode and the hydrogen electrode under standard conditions.
|
To question Q6, Electrically, under what condition does the cell stop delivering a current? The respondents should reason as follows: during the transformation, the potential of the cathode decreases and that of the anode increases. When the two potentials become equal, the cell stops delivering current. In this context, the similarity with hydraulic current could be mentioned: no flow from one container to another if they have the same water level.
|
To question Q7, Thermodynamically, under what condition does the cell stop delivering a current?
The reasoning can be as follows: During the transformation, the reaction quotient changes and increases to reach the value of the equilibrium constant K and, as a result, the concentration of zn2+ ions increases while the concentration of Cu2+ ions decreases. This knowledge is recommended in the high school and university program.
To answer this question, knowledge of thermodynamics is needed, whereas the previous question required knowledge of electricity.
| The answer from all the trainee teachers is: From a thermodynamic point of view, the cell stops delivering a current when all the reactants have reacted. In other words, when the reaction reaches its maximum rate of advancement X max |
Their reasoning centered on the limiting reactant. They did not use the criteria for a spontaneous evolution of a chemical transformation. These trainee teachers were content with a fragmented disciplinary content devoid of a more holistic vision of chemistry and its integral link with thermodynamics and electricity.
To sum up, and to answer the first question in our research, the respondents have only a fragmented grasp of the principle of cell operation. They do not master the concepts in depth. In particular, they did not have a well-articulated knowledge base linking the fields of chemistry and electricity. They tended to rely on heuristics rather than scientific arguments. Most probably, they are not used to solving problems or practicing the investigative approach during their school or university career. They stated that they were used to carrying out tasks requiring only the mobilization of a few declarative and procedural knowledges. These respondents were unaware of the cause of the electric current produced by the cell. However, since middle school, the learners have known that an electric current requires a difference in electrical potential. Most of these trainees relied on misconceptions about the anode and cathode to explain how and why the electric current is produced by the cell.
It is therefore possible that these trainees are not guiding their learners to improve their skills and understanding of the various electrical concepts that describe the operation of the Daniell cell as they progress through the knowledge spiral.
4.2 Content analysis in the textbooks
We based our work on the textbook used by the teachers responsible for training future teachers. This textbook is written in French “L’archipel de chie. 2021, édition: Moynier”. In this textbook, the first paragraph of the chapter is presented in the form of an experimental activity.
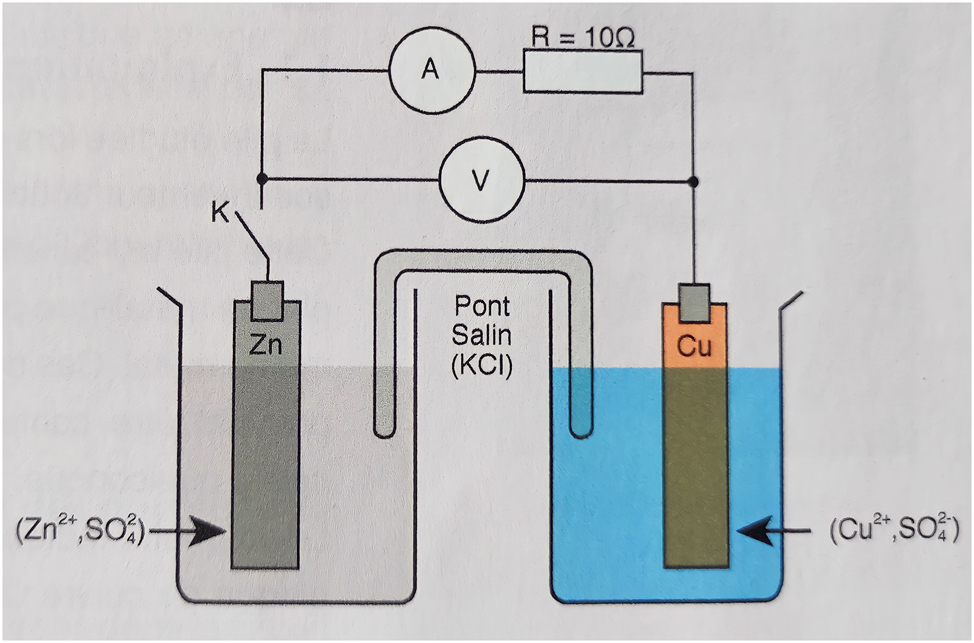
Schematic of the Daniell cell.
Below the translation of the content of this paragraph of the textbook:
Or Cu ⇌ Cu 2 + + 2e− et Zn 2+ + 2e− ⇌ Zn
|
During the focus groups we organized with the course supervisors stated that they based in a literal form the activities proposed in the textbook without making any effort to adapt them in order to promote the development of learners’ skills. Moreover, the role of the learners was limited to answering the guiding questions adopted in the textbook.
We have noted that the experimental protocol is proposed in the form of a recipe. The adapted method consists of a succession of predefined steps in an immutable order. This teaching approach does not allow learners to participate in the development of an experimental protocol and learning is focused on the teacher. In principle, this protocol should be the result of a problem solved by all the learners under the guidance of their teacher. However, according to the recipe in the textbook, learners are only expected to answer certain fragmented questions relating to experimental observation. The cognitive abilities mobilized by the learners to carry out the tasks proposed to them belong only to the lower taxonomic level, such as identifying the oxidant and the reductant, writing the redox half-equations, recognizing the anode and the cathode. We note the absence of higher-level skills such as: analyzing, interpreting, arguing, synthesizing, or proposing an experimental protocol to verify a hypothesis. With this pedagogical approach to presenting the cell in the textbook, the learner is not involved in the construction of knowledge as recommended by the curriculum, but rather plays a passive role. As a result, teachers who rely on the textbook do not find activities based on investigation and problem solving. The content to be taught, as proposed by the designers of the textbook, does not establish clear links between chemical and electrical knowledge.
From a didactic and epistemological point of view, this activity did not give any importance to the meaning of the concept of battery voltage and its (+) and (−) poles. These different concepts had been introduced since the 1st year of middle school. At this level, the study was made by similarity with a hydraulic current. However, at high school, this similarity is not reinvested with the aim of interpreting them differently and assimilating them in greater detail. Moreover, even the teachers (CS) declared that these concepts presented epistemological obstacles for them. Manipulating the Daniell cell could offer a way of overcoming this difficulty, but unfortunately this idea was not taken into account by the designers of the textbook. For example, the learner does not “observe” the (+) and (−) connections of the Daniell cell. Instead, they observe the electrodes, the solutions and the values of the current and the voltage between the terminals of this cell, whereas in middle school, the learner did not have the possibility of knowing the internal constituents of a cell. The last epistemological obstacle to be raised in this paragraph is vocabulary: in the textbook, no explanation has been suggested as to the origin of the names anode and cathode attributed to the electrodes. In fact, these two concepts are misrepresented in the textbook. In this way, the textbook transmits erroneous or partially constructed conceptions, which would be inculcated in the learners and could constitute obstacles to their subsequent learning. This activity should enable the learner to use the microscopic, macroscopic and symbol registers to describe the transformations at electrode level. However, these different levels are not illustrated in the textbook, especially the microscopic modelling of electron transfer from Zn to Cu2+. And in this context, the trainee teachers were unable to model microscopically the chemical transformation that describes the operation of the Daniell cell. On the other hand, the learner can rely on the criterion of spontaneous evolution to predict the real direction of the transformation in the cell. In this context, the teachers (CS) told us that using a criterion to predict the direction of a transformation is not an interesting tool for learning. Indeed, the learners are only required to compare the quotient of the reaction Q r,i with the equilibrium constant K, and then they can deduce the direction of the transformation. These are cognitive procedures that do not contribute to a good understanding of how a cell operates from an electrical point of view. In the earlier curriculum (before 2007), the prediction of this kind of transformation was based on the standard potentials of the redox couples. This approach is logical, since it is based on electrical rather than thermodynamic concepts. The didactic reasons for choosing to introduce the criterion of spontaneous evolution instead of standard potentials are not explained in the curriculum. The designers of the program seem to have adopted this idea from the French program recommended in 2003.
To sum up and answer the second research question, there is no problem-solving or investigative learning in the textbook. We can therefore conclude that the didactic transposition carried out in the conceptualization of the different concepts describing the functioning of the Daniell cell only promotes the memorization of some declarative and procedural knowledge. These concepts were constructed in a linear rather than spiral progression. The activities did not mention either the cause of the electric current created in the cell or the electrical significance of the cell’s two poles (+) and (−). This is not an appropriate approach for building skills, as the structuring and articulation of different types of knowledge are not appropriate. Experimental activities are presented as recipes to be followed in order to demonstrate facts. Yet the curriculum recommends that teachers adopt a scientific approach. This approach should be based on questioning, the formulation and validation of a hypothesis and then argumentation through modelling. Although the science curricula recommends that educational renovation should be based on the skills-based approach, the teaching scenario as presented in the textbook seems to reflect behavioral teaching and learning. Learners are asked to answer 20 fragmented questions, which are presented in the form of behavioral operational objectives. The knowledge acquired after studying the experimental activity is fragmented and difficult to mobilize in a complex situation. The cognitive abilities mobilized by the learner to carry out the tasks proposed belong only to the lower taxonomic level. As a result, learning may remain superficial. Consequently, teachers who rely on textbook activities cannot guide their learners to extend their understanding of the different electrical concepts that describe how the Daniell cell operates while progressing through the spiral of knowledge. These activities only encourage fragmented, non-interconnected learning.
5 Designing a learning environment based on the skills spiral. The case of the Daniell cell voltage
The aim is to develop activities that ensure the development and retention of skills, while ascending the spiral of skills and knowledge. This approach allows students to revisit the same concept several times throughout their learning process.
During the progression in learning about chemical transformations, the teacher must develop teaching activities based on problem-solving, enabling the learners to mobilize their pre-requisites by relying on scientific practice. Chemical transformations are generally interpreted from a cause-and-effect perspective: learners have to reason causally about how the reaction occurs. For example, an acid-base transformation in the Brönsted sense is modelled by an arrow curve, that describes the result of the electrostatic interaction between a free doublet of a base B and a less bound proton of an acid A (Cooper et al., 2016). If the cause-effect relationship is straightforward in the previous example, then in the case of Daniell cell operation, the cause-effect relationship would be less obvious, or even more complex since the two reactants Zn and Cu2+ are not in direct contact. Understanding this new knowledge requires a learning environment built around a problem, in which the learners are obliged to overcome this epistemological obstacle. To do this, the teacher must first check that the learners’ basic knowledge is already well acquired, and then intervenes to reinforce and extend it. We were interested on the concept of electrical voltage when teaching the Daniell cell. This choice is justified by the fact that the current produced by the Daniell cell is due to the existence of a potential difference between its two electrodes. Furthermore, this concept is very abstract and extremely complex, and depends entirely on models/analogies/metaphors. This difficulty of abstraction may be at the root of most other misconceptions (Mulhall et al., 2001).
Based on the results of this research and our experience as trainers and researchers, we recommend the following guidelines for teaching the Daniell cell principle:
The teacher must build on the pre-requisites of the learners, whereas this task has been neglected by trainee teachers and their (CS):
A redox transformation is the result of a transfer of electrons from a reducing agent to an oxidizing agent. The teacher can start with the direct transformation between the ions Zinc and Cu2+ and ask the learners to model the transfer of electrons between the reactants;
During the transformation, the quotient of the reaction changes to reach the value of the equilibrium constant K;
The similarity between the concepts that describe a hydraulic current and an electric current.
In a closed circuit, the production of an electric current requires a difference in potential. This current flows through the circuit from the highest to the lowest potential. We believe that as learners assimilate these electrical concepts, their understanding of cause-and-effect relationships also evolves. It should be mentioned that the learners have the opportunity to reuse the measurement equipment and to set up an experimental plan after having carried out the investigative approach based on a triggering situation.
The teacher must anticipate the learning difficulties that may hinder understanding of how the Daniell cell operates. In particular, certain concepts are complex and require special attention. For us, it is the difficulties encountered by trainee teachers and their (CS): significance of the (+) and (−) poles, role of the ion bridge, microscopic modelling of electron transfer from zinc to Cu2+ ions, role of the copper plate. It is therefore important for teachers to master the various concepts during their initial and in-service training. We suggest that it be emphasized that the (−) pole of the cell corresponds to the zinc electrode, since its electrical potential is lower than that of the copper electrode, which forms the (+) terminal. On the other hand, the concepts of anode and cathode are purely chemical: the anode is the electrode to which a reducing agent releases electrons and the cathode is the electrode to which an oxidizing agent fixes electrons;
Teachers need to construct a problem with their learners, which is not the case for the teachers in our research. The problem must be well-structured and the solution must not require a direct or evident answer, but rather a strategy that targets higher-order cognitive abilities: asking questions, proposing hypotheses, proposing an experimental protocol to verify these hypotheses, analyzing, interpreting, arguing using models, and then summarizing. The problem should concern the experimental way of transforming chemical energy into electrical energy by transferring electrons from zinc to Cu2+ ions. The teacher should stimulate the learners’ thinking by directing them to propose an electrical circuit, that allows the electrons released by the zinc to create a current.
As the transfer of electrons is indirect, the reactants must be placed separately in two beakers. The teacher has to gradually build up the key problem in the construction of the new knowledge by drawing on the learners’ prior knowledge: a current is created if the circuit is closed, using an ammeter to detect it; the learners have known since the 2nd year of high school that the movement of an electron in a metal requires an electric field, resulting from a potential difference. Learners could ask about the obligation to use a potential difference between the two beakers. And it is this last idea that constitutes the epistemological obstacle that the learners have to overcome. In general, the preceding situation is complicated, and could be outside the learner’s ZPD (Vygotsky, 1978). With the teacher’s scaffolding, the learners could suggest conductive substances that could connect the two beakers, and as they go along, the learners’ conceptions could appear. Once the set-up is properly closed and the ammeter indicates the existence of a current, the teacher should discuss with the learners the experimental observations and the cause of the current created. During these activities, learners are encouraged to mobilize chemical and electrical knowledge in an integrated way, thus ensuring the development and maintenance of skills, while progressing through the spiral of knowledge. During the discussion, the learners should try to model the chemical transformation on the two electrodes and should electrically identify the (+) and (−) terminals of the cell. Learners must associate the (+) terminal to the electrode with the highest potential. However, Nernst’s law, which gives the expression for this potential, is not recommended in the high school syllabus, so teachers may find it difficult to explain the chemical and physical functions of the copper electrode to their learners.
Through this participatory and reflective learning context, learners’ knowledge will be restructured from a naïve understanding to a deeper understanding of scientific content and practice, potentially leading to conceptual change.
In our view, microscopic modelling of the chemical transformation at the electrodes and reasoning based on potential difference form the basis for understanding how the Daniell cell operates.
6 The spiral describing the progression in the construction of the concept of the electrical voltage of a cell
Generally, a learner’s knowledge is in the form of an amalgam of knowledge: conceptions and scientific knowledge. The conceptions can be completely wrong or partially correct. In the course of teaching and learning, these conceptions should evolve towards the canonical scientific model (Duit & Treagust, 2003; Taber, 2019b). In this context, one of the specific concerns reported in chemistry education is the design of an effective pedagogy that can be based on these conceptions in order to help learners abandon them and understand concepts in a correct way (Taber, 2019a). It is important for teachers to adopt teaching approaches that can cause cognitive conflict and lead students to reveal their conceptions and discover their mistakes for themselves (Stroumpouli & Tsaparlis, 2022). Also, the vertical articulation of the spiral progression during teaching allows a thorough understanding and contributes to the retention of scientific concepts (Orbe, et al., 2018). In our study, we were interested in the concept of electrical voltage of a cell. We have described its spiral progression during teaching in the three cycles.
From the base of the spiral to Level-1 (Figure 2), the learner’s electrical knowledge is in the form of conceptions.
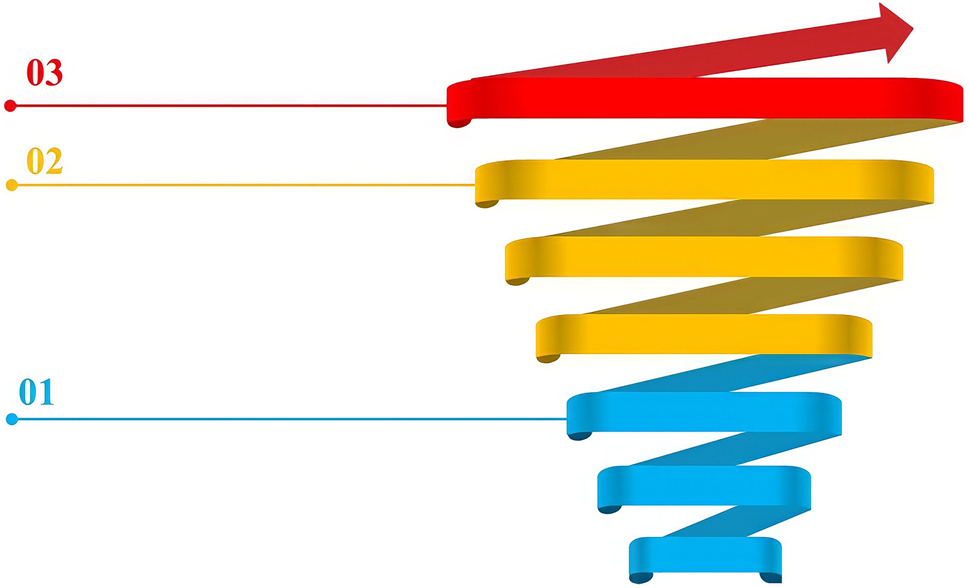
The spiral of electrical voltage skills.
The construction of the first scientific knowledge of electricity begins in the first year of middle school (13–14 years), which corresponds to the first level of the knowledge spiral. At this level, the process of abstraction in the construction of the concept of electrical voltage is based on reasoning by analogy. The program recommends drawing on the similarities between electric current and hydraulic current, and then between the difference in potential (or voltage) and the difference in altitude: electric current descends potentials in the same way that the flow of water descends altitudes (Figure 3). At this age, the learners understand the (+) sign as being the high electrical level and the (−) sign as being the low electrical level of the cell.
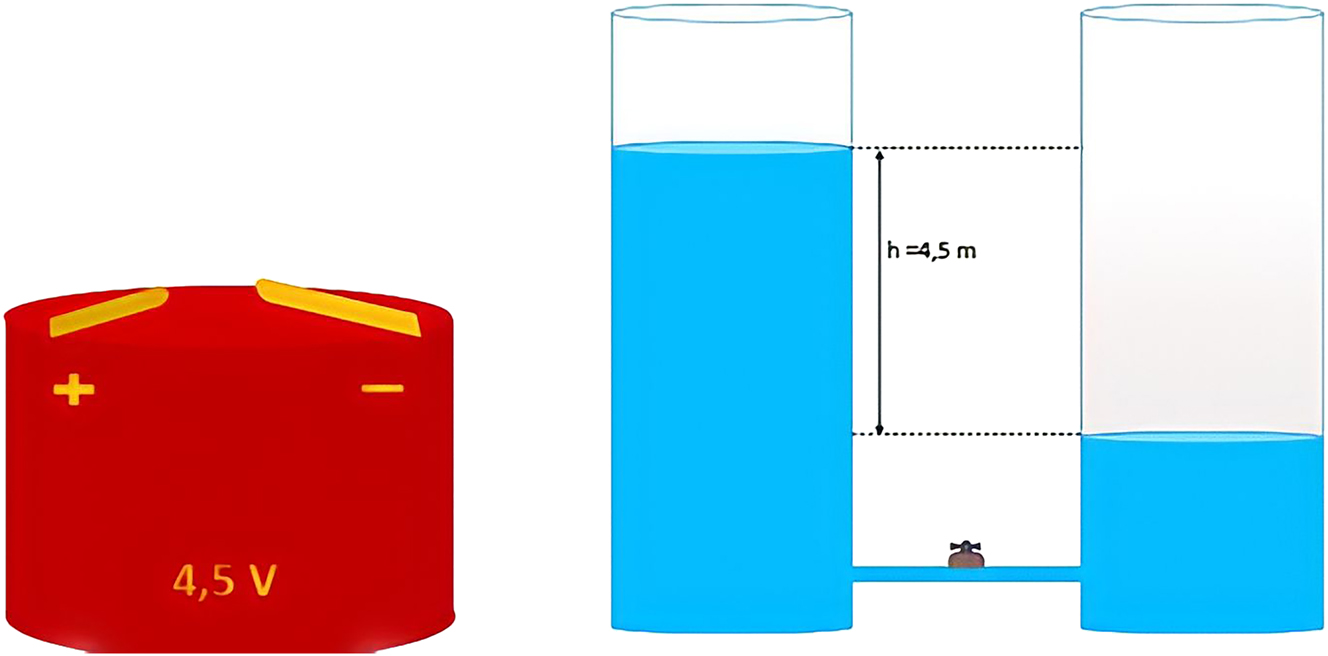
The similarity between the electrical voltage of a cell and the difference in altitude describing a hydraulic current.
Between Level 1 and Level 2 of the skills spiral, the learners consolidate the concept of electrical voltage through the study of Ohm’s law, series circuits and parallel circuits. The syllabus for the 2nd year of high school recommends teaching the concept of the electrical force responsible for the movement of electrons in a metal. This force is the result of an electric field created by a potential difference.
In the 3rd year of high school, learners are able to extend their knowledge in relation to the concept of electrical voltage by studying the internal constituents of the Daniell cell. This corresponds to level 2 of the skills spiral. At this level, the teaching-learning process is based on the diagram of the experiment describing how the cell operates (Figure 4). However, the learners do not “observe” the difference in heights between the two solutions and, what’s more, the two solutions have the same initial concentration. The learners can’t identify any parameter that differentiates the two compartments of the cell and so, for them, there is no potential reason that could create an electrical voltage. Consequently, learners may wonder how and why the phenomena observed occur in the Daniell cell: passage of current and gradual disappearance of reactants. This situation would tend to resist the construction of new knowledge, since the process of acquiring it requires learners to reorganize their knowledge structure (Lucariello & Naff, 2013).
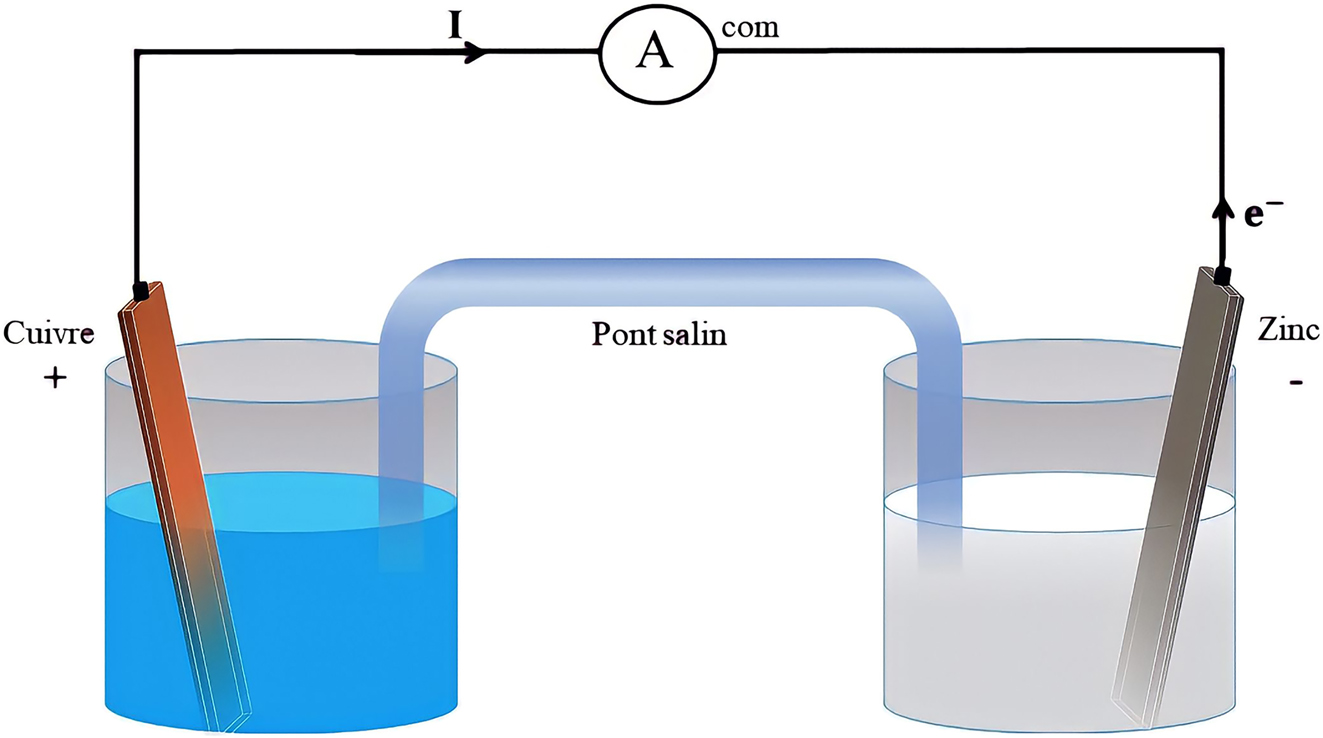
Daniell cell experimental set-up.
To ensure this progression without causing a major epistemological rupture, we recommend that the designers of the program reintroduce the notion of standard potentials for redox couples. At this level of study, the concept of “electrical altitude” is no longer linked to mechanical altitude, but rather to the nature of metals and their redox couples.
As for predicting the direction of transformation in the Daniell cell, we suggest using Gamma’s rule rather than the spontaneous evolution criterion recommended in the current program. This didactic choice would enable the learner to gain an in-depth understanding of the concept of electrical voltage and the notions of the (+) and (−) poles of a cell in general.
From a pedagogical point of view, learners have the opportunity to re-use the measuring instruments and to implement an experimental plan after carrying out the investigative approach. We suggest that the teacher builds on the basic knowledge corresponding to level −1 of the skills spiral, and then reinforces and extends the learners’ new knowledge and skills.
At university (level-3 of the skills spiral), the student is able to extend the previous skills in a practical and theoretical way based on Nernst’s law. At this level of study, they correctly understand the meaning of the value of the standard potential of a couple which is measured in relation to a reference based on the hydrogen electrode, and they can use modelling to explain the process that generates the current in the Daniell cell.
We suggest that this notion of electrical potential reference should preferably be evoked by analogy with that used in electrical circuits. In these circuits, the potential reference is taken in relation to an electrical earth, which is generally connected to the ground. In this way, the same concept is taught several times in different contexts.
7 Conclusion and outlook
In the first part of our research, we identified the difficulties and gaps in the skills of trainee teachers when they mobilized their knowledge to solve situations related to the operating principle of the Daniell cell. The results showed that these respondents had major epistemological difficulties relating to the physical meaning of several electrical concepts. On the other hand, analyses of the content of the same topic in the textbook showed that the experimental activities were presented in the form of recipes, their role being to show facts rather than to verify hypotheses. The cognitive capacities mobilized by the learners to carry out the tasks proposed belong only to the lower taxonomic level, and the knowledge is not constructed according to a spiral progression. According to trainee teachers and their (CS), the practices developed in the textbook are the reference model for them. However, these practices do not comply with the principles of the skills-based approach and the investigative approach advocated by the curriculum. On the basis of the results we have obtained, we have proposed guidelines to ensure the development and retention of knowledge, while moving up the skills spiral in line with the operating principle of the Daniell cell. The proposed approach can be used both in the training of future teachers and in teaching learners about the subject studied. In fact, our research prospects aim to present efficient teaching-learning activities based on the competency-based approach and the investigative approach in relation to the theme of Daniell cell. We will discuss the results of their implementation in the classroom by trainee teachers in the presence of their (CS), as well as by other practicing teachers.
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: None declared.
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
-
Data availability: Not applicable.
References
Alkan, F. (2016). Apprentissage expérientiel: Ses effets sur les résultats et les compétences en matière de processus scientifique. Journal de l’enseignement Scientifique Turc, 13(2), 15–26.Search in Google Scholar
Becker, N. M., & Cooper, M. M. (2014). College chemistry students’ understanding of potential energy in the context of atomic–molecular interactions. Journal of Research in Science Teaching, 51(6), 789–808. https://doi.org/10.1002/tea.21159.Search in Google Scholar
Bell, T., Urhahne, D., Schanze, S., & Ploetzner, R. (2010). Collaborative inquiry learning: Models, tools, and challenges. International Journal of Science Education, 32(3), 349–377. https://doi.org/10.1080/09500690802582241.Search in Google Scholar
Berland, L. K., & McNeill, K. L. (2010). A learning progression for scientific argumentation: Understanding student work and designing supportive instructional contexts. Science Education, 94(5), 765–793. https://doi.org/10.1002/sce.20402.Search in Google Scholar
Butler, J., Mooney Simmie, G., & O’Grady, A. (2015). An investigation into the prevalence of ecological misconceptions in upper secondary students and implications for pre-service teacher education. European Journal of Teacher Education, 38(3), 300–319. https://doi.org/10.1080/02619768.2014.943394.Search in Google Scholar
Campbell, C. D., Midson, M. O., Bergstrom Mann, P. E., Cahill, S. T., Green, N. J., Harris, M. T., Hibble, S. J., O’Sullivan, S. K. E., To, T., Rowlands, L. J., Smallwood, Z. M., Vallance, C., Worrall, A. F., & Stewart, M. I. (2022). Developing a skills-based practical chemistry programme: An integrated, spiral curriculum approach. Chemistry Teacher International, 4(3), 243–257. https://doi.org/10.1515/cti-2022-0003.Search in Google Scholar
Chittleborough, G. D., & Treagust, D. F. (2009). Why models are advantageous to learning science. Educación Química, 20(1), 12–17. https://doi.org/10.1016/s0187-893x(18)30003-x.Search in Google Scholar
Christodoulou, A., & Osborne, J. (2014). The science classroom as a site of epistemic talk: A case study of a teacher’s attempts to teach science based on argument. Journal of Research in Science Teaching, 51(10), 1275–1300. https://doi.org/10.1002/tea.21166.Search in Google Scholar
Cooper, M. M. (2018). Evidence-based approaches to curriculum reform ad assessment. Educació Química, (23), 24–31. https://doi.org/10.2436/20.2003.02.171.Search in Google Scholar
Cooper, M. M., Corley, L. M., & Underwood, S. M. (2013). An investigation of college chemistry students’ understanding of structure–property relationships. Journal of Research in Science Teaching, 50(6), 699–721. https://doi.org/10.1002/tea.21093.Search in Google Scholar
Cooper, M. M., Kouyoumdjian, H., & Underwood, S. M. (2016). Investigating students’ reasoning about acid–base reactions. Journal of Chemical Education, 93(10), 1703–1712. https://doi.org/10.1021/acs.jchemed.6b00417.Search in Google Scholar
Duit, R., & Treagust, D. F. (2003). Conceptual change: A powerful framework for improving science teaching and learning. International Journal of Science Education, 25(6), 671–688. https://doi.org/10.1080/09500690305016.Search in Google Scholar
Evagorou, M., & Osborne, J. (2013). Exploring young students’ collaborative argumentation within a socioscientific issue. Journal of Research in Science Teaching, 50(2), 209–237. https://doi.org/10.1002/tea.21076.Search in Google Scholar
Firman, M. A., Ertikanto, C., & Abdurrahman, A. (2019). Description of meta-analysis of inquiry-based learning of science in improving students’ inquiry skills. Journal of Physics: Conference Series, 1157(2), 022018. https://doi.org/10.1088/1742-6596/1157/2/022018.Search in Google Scholar
Frey, R. F., Brame, C. J., Fink, A., & Lemons, P. P. (2022). Teaching discipline-based problem solving. CBE-Life Sciences Education, 21(2), fe1. https://doi.org/10.1187/cbe.22-02-0030.Search in Google Scholar PubMed PubMed Central
Galloway, K. R., & Bretz, S. L. (2016). Video episodes and action cameras in the undergraduate chemistry laboratory: Eliciting student perceptions of meaningful learning. Chemistry Education: Research and Practice, 17(1), 139–155. https://doi.org/10.1039/c5rp00196j.Search in Google Scholar
Kwangmuang, P., Jarutkamolpong, S., Sangboonraung, W., & Daungtod, S. (2021). The development of learning innovation to enhance higher order thinking skills for students in Thailand junior high schools. Heliyon, 7(6), e07309. https://doi.org/10.1016/j.heliyon.2021.e07309.Search in Google Scholar PubMed PubMed Central
Lucariello, J., & Naff, D. (2013). How do I get my students over their alternative conceptions (misconceptions) for learning. http://www.apa.org/education/k12/misconceptions.aspx?item=1.Search in Google Scholar
Mayer, R. E. (2011). Problem solving and reasoning. Learning and cognition in education, 112–117. https://doi.org/10.1016/B978-0-08-044894-7.00487-5.Search in Google Scholar
McDaniel, M. A., Cahill, M. J., Frey, R. F., Rauch, M., Doele, J., Ruvolo, D., & Daschbach, M. M. (2018). Individual differences in learning exemplars versus abstracting rules: Associations with exam performance in college science. Journal of Applied Research in Memory and Cognition, 7, 241–251. https://doi.org/10.1016/j.jarmac.2017.11.004.Search in Google Scholar
MEN. (2006). Specific syllabus and guidelines for teaching physical sciences at high school. Ministry of National Education. https://p0.storage.canalblog.com/01/84/651696/43779810.pdf.Search in Google Scholar
MEN. (2012). Specific program and guidelines for the training of physical science teachers. Ministry of National Education. https://www.chimiephysique.net/Pdf/sc-phisiques-qualif.pdf.Search in Google Scholar
MEN. (2015). Specific syllabus and guidelines for teaching physical sciences at high school. Ministry of National Education. https://www.men.gov.ma/Ar/Documents/N118151104.pdf.Search in Google Scholar
Mennani, M., Raouf, K., & Khyati, A. (2023a). Investigation the association between the Moroccan high school chemistry program and students’ reasoning. Journal of Educational and Social Research, 13(5), 278. https://doi.org/10.36941/jesr-2023-0137.Search in Google Scholar
Mennani, M., Raouf, K., & Khyati, A. (2023b). Epistemological and didactic difficulties of teaching chemistry in Moroccan high schools. Journal of Educational and Social Research, 13(3), 61–69.10.36941/jesr-2023-0057Search in Google Scholar
Minner, D. D., Levy, A. J., & Century, J. (2010). Inquiry‐based science instruction—what is it and does it matter? Results from a research synthesis years 1984 to 2002. Journal of Research in Science Teaching: The Official Journal of the National Association for Research in Science Teaching, 47(4), 474–496. https://doi.org/10.1002/tea.20347.Search in Google Scholar
Mulhall, P., McKittrick, B., & Gunstone, R. (2001). A perspective on the resolution of confusions in the teaching of electricity. Research in Science Education, 31(4), 575–587. https://doi.org/10.1023/a:1013154125379.10.1023/A:1013154125379Search in Google Scholar
Munna, A. S., & Kalam, M. A. (2021). Impact of active learning strategy on the student engagement. GNOSI: an interdisciplinary journal of human theory and praxis, 4(2), 96–114.Search in Google Scholar
Novak, J. D. (2010). Learning, creating, and using knowledge: Concept maps as facilitative tools in schools and corporations. Routledge.Search in Google Scholar
Novick, L. R., & Catley, K. M. (2014). When relationships depicted diagrammatically conflict with prior knowledge: An investigation of students’ interpretations of evolutionary trees. Science Education, 98(2), 269–304. https://doi.org/10.1002/sce.21097.Search in Google Scholar
Orbe, J. R., Espinosa, A. A., & Datukan, J. T. (2018). Teaching chemistry in a spiral progression approach: Lessons from science teachers in the Philippines. Australian Journal of Teacher Education (Online), 43(4), 17–30. https://doi.org/10.14221/ajte.2018v43n4.2.Search in Google Scholar
Pedaste, M., Mäeots, M., Siiman, L. A., de Jong, T., van Riesen, S. A. N., Kamp, E. T., Manoli, C. C., Zacharia, Z. C., & Tsourlidaki, E. (2015). Phases of inquiry-based learning: Definitions and the inquiry cycle. Educational Research and Reviews, 14(1), 47–61. https://doi.org/10.1016/j.edurev.2015.02.003.Search in Google Scholar
Reid, N., & Ali, A. A. (2020). Making sense of learning. A research-based approach. Evidence to guide policy and practice, with an emphasis on secondary stages. Springer.10.1007/978-3-030-53677-0Search in Google Scholar
Sevian, H., & Talanquer, V. (2014). Rethinking chemistry: A learning progression on chemical thinking. Chemistry Education: Research and Practice, 15(1), 10–23. https://doi.org/10.1039/c3rp00111c.Search in Google Scholar
Shavelson, R. J., & Kurpius, A. (2012). Reflections on learning progressions. Learning progressions in science (pp. 13–26). Brill.10.1007/978-94-6091-824-7_2Search in Google Scholar
Soeharto, S., & Csapó, B. (2021). Evaluating item difficulty patterns for assessing student misconceptions in science across physics, chemistry, and biology concepts. Heliyon, 7(11), 1–10. https://doi.org/10.1016/j.heliyon.2021.e08352.Search in Google Scholar PubMed PubMed Central
Stroumpouli, C., & Tsaparlis, G. (2022). Chemistry students’ conceptual difficulties and problem-solving behavior in chemical kinetics, as a component of an introductory physical chemistry course. Chemistry Teacher International, 4(3), 279–296. https://doi.org/10.1515/cti-2022-0005.Search in Google Scholar
Swarat, S., Ortony, A., & Revelle, W. (2012). Activity matters: Understanding student interest in school science. Journal of Research in Science Teaching, 49(4), 515–537. https://doi.org/10.1002/tea.21010.Search in Google Scholar
Taber, K. S. (2014). Constructing active learning in chemistry: Concepts, cognition and conceptions. In Learning with understanding in the chemistry classroom (pp. 5–23). Springer.10.1007/978-94-007-4366-3_1Search in Google Scholar
Taber, K. S. (2019a). Alternative conceptions and the learning of chemistry. Israel Journal of Chemistry, 59(6–7), 450–469. https://doi.org/10.1002/ijch.201800046.Search in Google Scholar
Taber, K. S. (2019b). The nature of the chemical concept: Re-constructing chemical knowledge in teaching and learning (Vol. 3). Royal Society of Chemistry.Search in Google Scholar
UCDFS. (2013). Specific program for teaching chemistry at the university. University Chouaib Doukkali, Faculty of Sciences. https://www.studocu.com/row/document/universite-sidi-mohamed-ben-abdellah-de-fes/chimie/cours-chimie-des-solutions-smpc-s2-universite-chouaib-doukkali-faculte-des-sciences-el-jadida-maroc/45986878.Search in Google Scholar
Vygotsky, L. S. (1978). Interaction between learning and development. In M. Cole, V. John-Steiner, S. Scribner, & E. Souberman (Eds.), Mind and society: The development of higher psychological processes (pp. 79–91). Harvard University Press.Search in Google Scholar
Yeşiloğlu, S. N., & Köseoğlu, F. (2020). Epistemological problems underlying pre-service chemistry teachers’ aims to use practical work in school science. Chemistry Education: Research and Practice, 21(1), 154–167. https://doi.org/10.1039/c8rp00212f.Search in Google Scholar
© 2023 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Articles in the same Issue
- Frontmatter
- Review Article
- Relativistic effects on the chemistry of heavier elements: why not given proper importance in chemistry education at the undergraduate and postgraduate level?
- Research Articles
- Designing a learning environment based on the spiral of skills to overcome the didactic obstacles associated with teaching the Daniell cell
- Analysis of the relationship between students’ argumentation and chemical representational ability: a case study of hybrid learning oriented in the environmental chemistry course
- Good Practice Report
- What makes representations good representations for science education? A teacher-oriented summary of significant findings and a practical guideline for the transfer into teaching
- Research Article
- Student exploration of the Henderson-Hasselbach equation and pH readings to determine the pK a value of 4,4′-trimethylenedipiperidine (TMDP)
- Good Practice Report
- Chemistry saving lives: using First World War Hypo helmets to avoid chlorine poisoning
- Research Article
- An exploration of the proton NMR problem-solving approaches of undergraduate students
- Good Practice Reports
- Chemical Quest: general knowledge and popular culture quizzes about the elements in a board game for the class
- Lessons learned from a case study on teaching the socioscientific issue of ethanol, used as an ingredient of sanitizers, to promote students’ learning of and about chemistry during the COVID-19 pandemic
Articles in the same Issue
- Frontmatter
- Review Article
- Relativistic effects on the chemistry of heavier elements: why not given proper importance in chemistry education at the undergraduate and postgraduate level?
- Research Articles
- Designing a learning environment based on the spiral of skills to overcome the didactic obstacles associated with teaching the Daniell cell
- Analysis of the relationship between students’ argumentation and chemical representational ability: a case study of hybrid learning oriented in the environmental chemistry course
- Good Practice Report
- What makes representations good representations for science education? A teacher-oriented summary of significant findings and a practical guideline for the transfer into teaching
- Research Article
- Student exploration of the Henderson-Hasselbach equation and pH readings to determine the pK a value of 4,4′-trimethylenedipiperidine (TMDP)
- Good Practice Report
- Chemistry saving lives: using First World War Hypo helmets to avoid chlorine poisoning
- Research Article
- An exploration of the proton NMR problem-solving approaches of undergraduate students
- Good Practice Reports
- Chemical Quest: general knowledge and popular culture quizzes about the elements in a board game for the class
- Lessons learned from a case study on teaching the socioscientific issue of ethanol, used as an ingredient of sanitizers, to promote students’ learning of and about chemistry during the COVID-19 pandemic

