Abstract
Microwave systems have been used in organic chemistry since the late 1990s for applications including Microwave-Assisted Organic Synthesis (MAOS). The main advantages of microwave-assisted procedures compared to traditional synthesis methods are the 100- to 1000-fold increase in reaction speeds, higher yields, purer products, and less energy consumption. So far, only a few examples for integrating microwave-induced chemistry into high school chemistry classes have been proposed. This paper presents a set of experiments intended to provide insights into using microwave technology in the context of green, organic chemistry lessons in high school.
Introduction
Microwave technology like Microwave-Assisted Organic Synthesis (MAOS) has been used in industrial organic chemistry since the 1990s (Lindström et al. 2001). MAOS is seen as a contribution to green chemistry (De la Hoz et al. 2016). Microwave technology has become indispensable (Leadbeater 2010), especially in the areas of sustainable organic and polymer chemistry. It has also become very important for pharmaceutical chemistry applications (Wagner 2006). The main advantages of MAOS over traditional synthesis methods are the 100- to 1000-fold increase in reaction rates, higher product yields and purer end products. The quicker reaction speeds are based on the ability of molecules to more readily absorb microwave radiation and convert it into heat. One example in Table 1 given by Mitra et al. (2010) for the synthesis of aspirin, which begins with 18 mL of acetic anhydride and 10 g of salicylic acid, illustrates the potential efficiency of MAOS quite well.
Efficiency of microwave use in the synthesis of aspirin (Mitra et al. w.y.).
| Synthesis method | Time [min] | Energy turnover [kJ] | Yield [%] |
|---|---|---|---|
| Thermic | 5 | 180 | 90 |
| Microwave-assisted | 1 | 36 | 95 |
The efficient use of energy such as that found in MAOS is one of the main principles of green chemistry (Anastas & Warner 1998). Microwave-induced chemistry, however, also tends to save time. In organic chemistry, the spectrum of MAOS applications ranges from organic reactions in solution, organometallic reactions, oxidations, reductions, and polymer syntheses (Leadbeater 2010). Modern microwave-assisted synthesis carried out in industry or the laboratory usually occurs in closed vessels using temperatures above the boiling point of the reaction medium. In a laboratory microwave, integrated magnetic stirrers ensure continuous mixing and sensors allow technicians to control the reaction progress. Several examples using laboratory microwaves have already been published for educational purposes at the undergraduate level (e.g. McGiwan & Leadbeater 2006).
One decisive disadvantage of using laboratory microwaves in high schools is their high price. Other problems include the fact that a microwave can only be used by one individual at a time and the fact that a reaction cannot be observed due to the closed reaction vessel. However, the acquisition of laboratory microwaves for use in schools is desirable for the long term, especially in view of the very wide range of applications and the importance of MAOS techniques in modern (green) synthetic chemistry. In the meantime, kitchen microwave ovens can serve as a cheap alternative.
This article describes a set of experiments employing a kitchen microwave. These experiments were selected while developing an organic chemistry curriculum at the senior secondary high school level, which focuses on the different principles of green chemistry (Linkwitz & Eilks 2020). The experiments mostly involved esterification and sought to form either esters, fatty acid methyl esters or polyesters. Other experiments include the decomposition of polylactic acid (PLA) and the extraction of essential oils. All of the above experiments were carried out with the help of a standard household microwave oven. It is recommended that students use a microwave with a split-second timer and a power setting function in watts which is as graduated as possible. We used the OMW3332DM from the German brand OK, which costs about 90€.
When carrying out experiments with kitchen microwaves, certain specific safety issues need to be taken into account (Mitra et al. 2010). Most organic syntheses in laboratory microwave chemistry employ aqueous or other liquid solvents. These solvents tend to be contained in sealed vessels so that reactions can be completed efficiently and quickly. However, pressure measurement is not possible with typical kitchen microwaves. This can lead to unexpected vessel explosions when using sealed vessels. Therefore, school experiments need to take place either at more moderate temperatures or in open or only loosely covered vessels, several of which are described below.
Hazards
In order to prevent chemicals from coming into contact with students’ skin, eyes, and clothing, participants should wear personal protective equipment such as disposable gloves and eye goggles. All of the carboxylic acids in the experiments can be corrosive in concentrated solution and are irritants in the case of prolonged exposure. All of the alcohols used are flammable. Therefore, they should avoid contact with any ignition sources. 1-octanol may also be absorbed through the skin. Prolonged or repeated contact may dry the skin and cause irritation. The thermal decomposition of Amberlyst 15 may release irritating gases and vapors. Lipase may be harmful if inhaled and may cause eye, skin, and respiratory tract irritation. Potassium hydroxide and sodium hydroxide are highly corrosive and skin contact can cause severely irritability and chemical burns. Glycerine is slightly hazardous in case of ingestion, inhalation, or skin and eye contact. It is also slightly flammable in the presence of heat, open flames, and sparks, as well as oxidizing materials. Ethylene glycol is unlikely to be inhaled at room temperature, but can be toxic if heated, agitate, or sprayed. Polylactic acid may cause eye irritation and may also be harmful if inhaled, absorbed through the skin, or swallowed.
Disposal
The solutions have to be disposed as organic waste.
Green organic chemistry experiments with a kitchen microwave oven
Experiment 1.
Ester synthesis catalyzed by an acidic ion-exchange resin (Amberlyst 15) (inspired by Reilly et al. 2014).
Chemicals: acetic acid, 1-octanol, Amberlyst 15 (A15).
Materials: kitchen microwave, PTFE beaker (100 mL) with top cover (Figure 1).
Time required: approx. 5 min.
Methods: A mixture of 1 mL acetic acid, 2 mL 1-octanol and 0.2 g of A15 are combined in a PTFE beaker (100 mL). The beaker is covered and placed in the household microwave at 400 W for 60 s. The beaker is then removed from the microwave. The presence of 1-octyl acetate can be detected by the moderately intense odor of citrus fruit, which is still strongly masked by acetic acid. The condensed water formed at the cover of the beaker is removed. The reaction is repeated twice under the same parameters (400 W, 60 s). After the third sequence, a pure and intense fruity scent can be confirmed. Other carboxylic acids (e.g. propanoic acid) or alcohols (e.g. 1-butanol) can be used as well. A blank test may be added to see the effect of Amberlyst 15.
Result: An intense fruity sense can be smelled. The carboxylic acid reacts with the alcohol to form a fruit ester: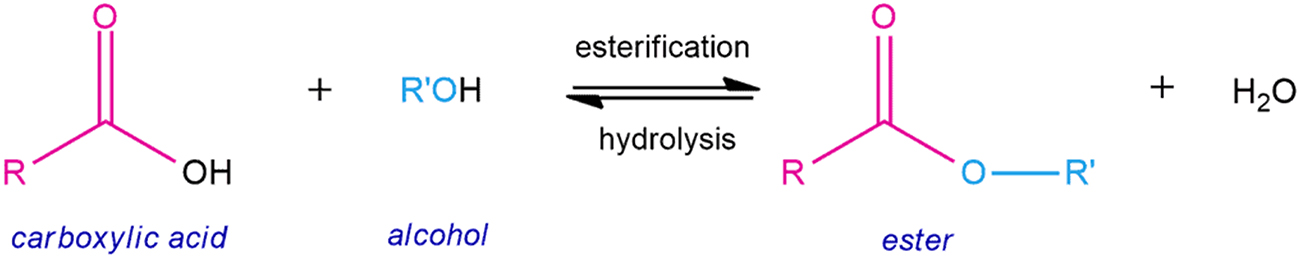 (Source: https://chemistryscore.com/esterification/)
(Source: https://chemistryscore.com/esterification/)

PTFE beaker.
Experiment 2.
Ester synthesis catalyzed by lipase (inspired by Yadav & Thorat, 2012)
Chemicals: acetic acid, 1-octanol, lipase (CalB, Novozym N435)
Materials: kitchen microwave, PTFE beaker (100 mL) with top cover.
Time required: approx. 5 min.
Method: A combination of 1 mL acetic acid, 2 mL 1-octanol and a micro-spoon spatula portion of lipase are mixed in a PTFE beaker (100 mL). The beaker is covered and heated in the microwave at 80 W for 60 s. The beaker is then removed from the microwave. 1-octyl acetate can be identified by an intense fruity odor, which is still slightly masked by acetic acid. After a second sequence (80 W, 60 s) of heating, 1-octyl acetate can be clearly identified by a pure fruity odor. If the time interval is shortened to 30 s, a total of three sequences must be run before the pure, intense odor is noticeable. Other carboxylic acids (e.g. propanoic acid) or alcohols (e.g. 1-butanol) can be used as well. A blank test may be added to see the effect of lipase.
Result: An intense fruity sense can be smelled. The carboxylic acid reacts with the alcohol to form a fruit ester (see above).
Experiment 3.
Biodiesel synthesis catalyzed by an acidic ion-exchange resin (Amberlyst 15) or potassium hydroxide (inspired by Mazo et al. 2011, and Miller & Leadbeater 2009).
Chemicals: ethanol, rapeseed oil, Amberlyst 15 (A15), potassium hydroxide, ice.
Materials: kitchen microwave, Erlenmeyer flask, evaporating dish, test tube, spatula, graduated pipette, graduated cylinder.
Time required: approx. 5 min.
Method: A total of 1 g of A15 and 5.64 g of rapeseed oil are placed in an Erlenmeyer flask. Then 2.5 mL of ethanol is added. Ethanol is used here instead of methanol because of the higher health risks associated with methanol. The Erlenmeyer flask is covered with a watch glass and some ice is placed on top. Then the Erlenmeyer flask is heated in the microwave at 640 W for 1 min. Afterward the flask is then allowed to cool to room temperature in the microwave. The liquid phase is separated from the Amberlyst 15 and placed in a test tube.
Result: The synthesis of biodiesel is indicated by the formation of two layers, the lower layer (gel-like) is the glycerol phase and the upper layer is the biodiesel. A viscosity test of the product compared with biodiesel and rapeseed oil indicates that biodiesel has been formed. When carrying out the experiment using potassium hydroxide, 0.02 g of potassium hydroxide replaces the Amberlyst 15.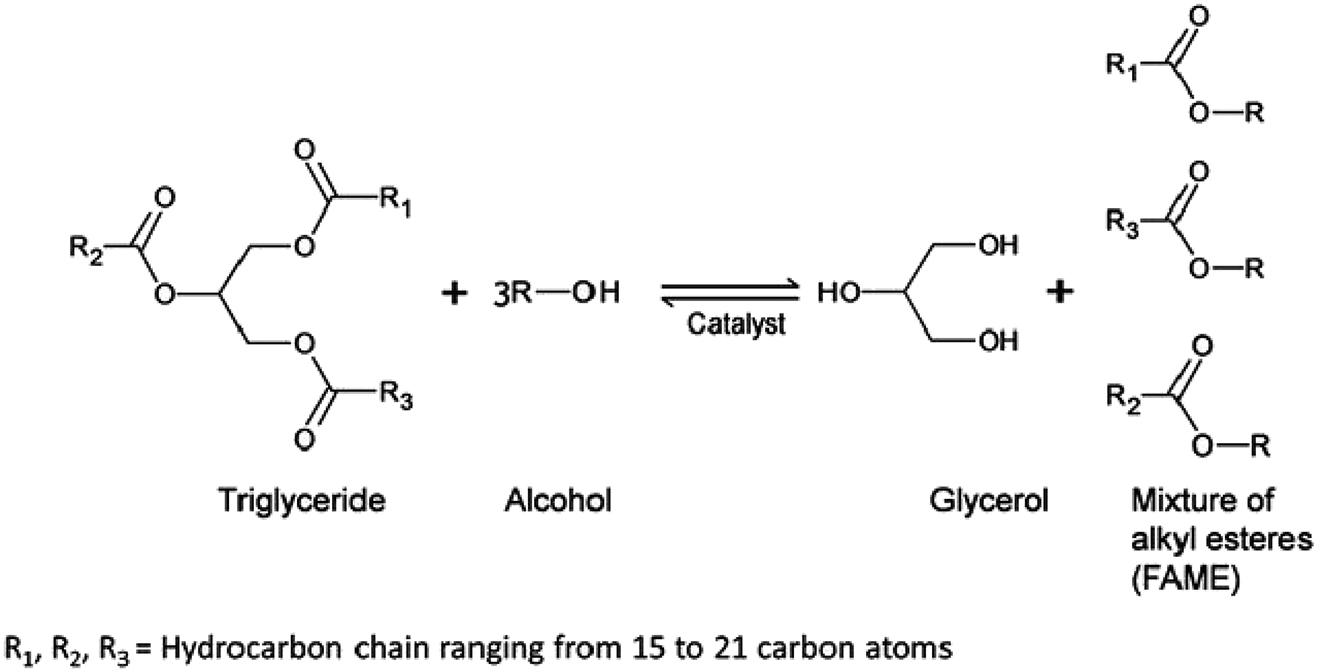 (source: https://www.researchgate.net/publication/323338927_Ultrasound_as_a_Metrological_Tool_for_Monitoring_Transesterification_Kinetics/figures?lo=1).
(source: https://www.researchgate.net/publication/323338927_Ultrasound_as_a_Metrological_Tool_for_Monitoring_Transesterification_Kinetics/figures?lo=1).

Result of the synthesis of biodiesel in the microwave oven: biodiesel (upper phase of b and c) compared to oil (a).
Experiment 4.
Synthesis of polyesters
Chemicals: glycerine, citric acid, ethylene glycol or castor oil.
Materials: kitchen microwave, watch glass, glass rod, 100 mL beaker.
Time required: approx. 5 min.
Method: A total of 1.7 g of glycerine and 7 g of citric acid are combined in the beaker. The beaker is then placed on the edge of the glass turntable in the microwave oven. The mixture is heated at 800 W for 1 min. After waiting for another minute, the microwave is opened and checked to see if the citric acid has completely dissolved. If not, the mixture is heated again until a clear, transparent solution is formed. The solution is poured onto a watch glass.
Result: After the reaction mixture cools down, threads can be drawn using the glass rod. After a complete cool down, a solid, transparent mass is obtained. Glycerine reacts with citric acid to form a polyester: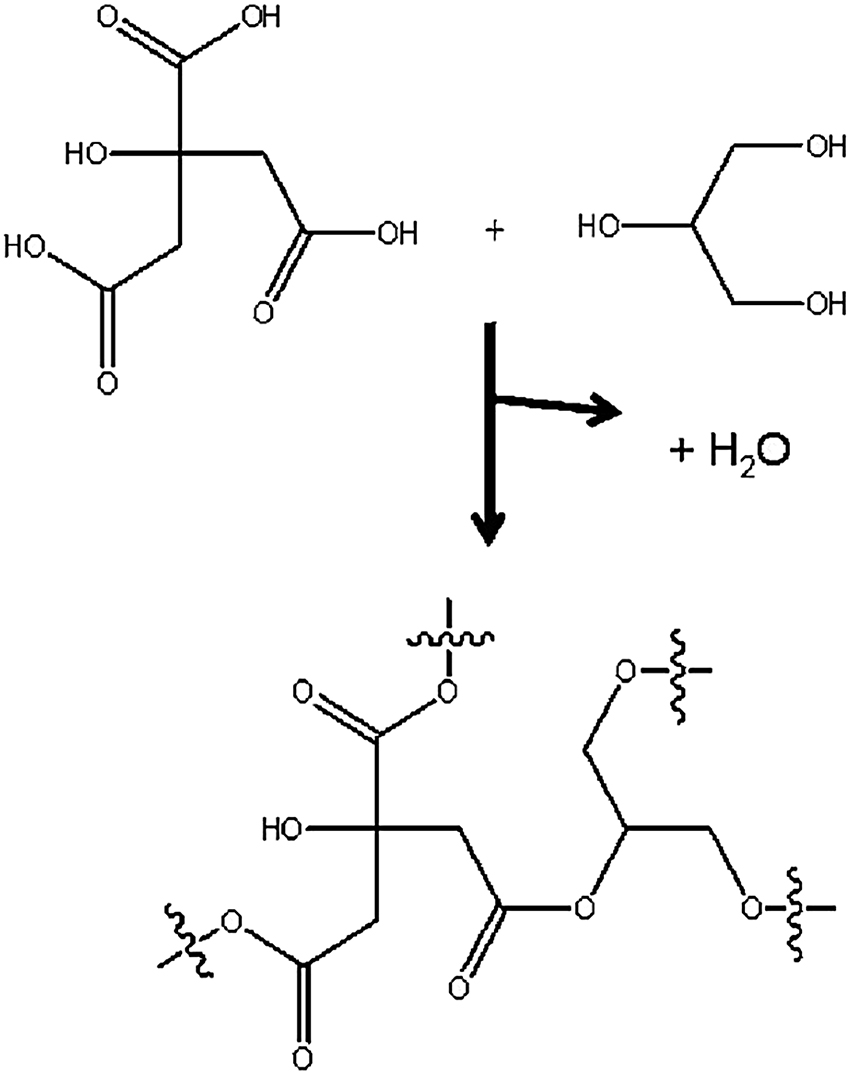
Experiment 5.
Decomposition of polylactic acid (PLA).
Chemicals: PLA granulate (crushed), sodium hydroxide, ethanol (50%).
Materials: kitchen microwave, watch glass, 250 mL Erlenmeyer flask, pH-meter.
Time required: approx. 5 min.
Method: A total of 5 g of shredded PLA and 5.6 g of sodium hydroxide are put into a 250 mL Erlenmeyer flask. Ethanol is then added until the mixture is covered. The flask is covered with the watch glass. The reaction mixture is heated in a microwave oven at 650 W for no longer than 4 min. It is then allowed to cool for 4 min before repeating the procedure. At 650 W, a total of 4 to 5 cycles are usually required. The mixture is then cooled in an ice bath.
Result: A pH-meter is then used to examine the pH-value of the mixture and show the appearance of lactic acid. Due to the cleavage of the ester groups a change in pH from 7 to a value of 6-6,5 should occur, which can be detected with a digital pH-meter. pH-paper cannot be used because the pH change can only be measured in tenths. The pH-change results from the cleavage of the polymer to lactic acid which is acidic due to the carboxyl group.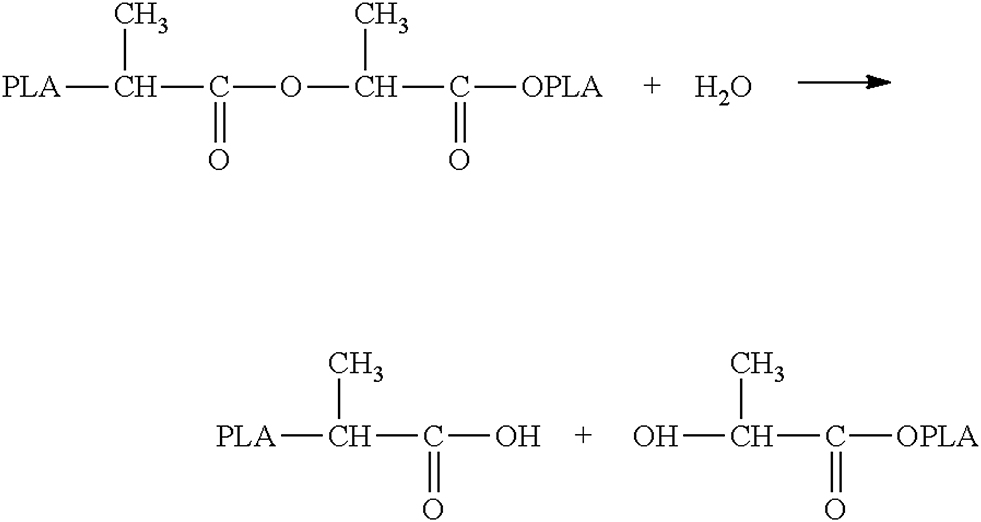
Experiment 6.
Extraction of an essential oil (inspired by Attard et al. 2014, Chemat et al. 2006, and Hackleman 2016)
Chemicals: orange or pomelo peels, ice, water.
Materials: kitchen microwave, grater, beaker (1 L), two beakers (100 mL), plastic funnel with holes, watch glass, volumetric flask (25 mL), pipette.
Time required: approx. 20 min.
Method: The oranges or grapefruits are washed and chopped up in a grater or food processor (Figure 2). The experiment is set up in the microwave as shown in Figure 3. Heating occurs for 7 min at 600 W. The mixture is allowed to cool for 5–10 min, until the steam has completely condensed in the glass, but not so long that the oil evaporates. The extract is then decanted into the volumetric flask, where the oily phase settles out after a short time. Oranges or pomelos are particularly suitable for this extraction, as you can recognize the oil by its color.
Result: The oily phase settles out after a short time.
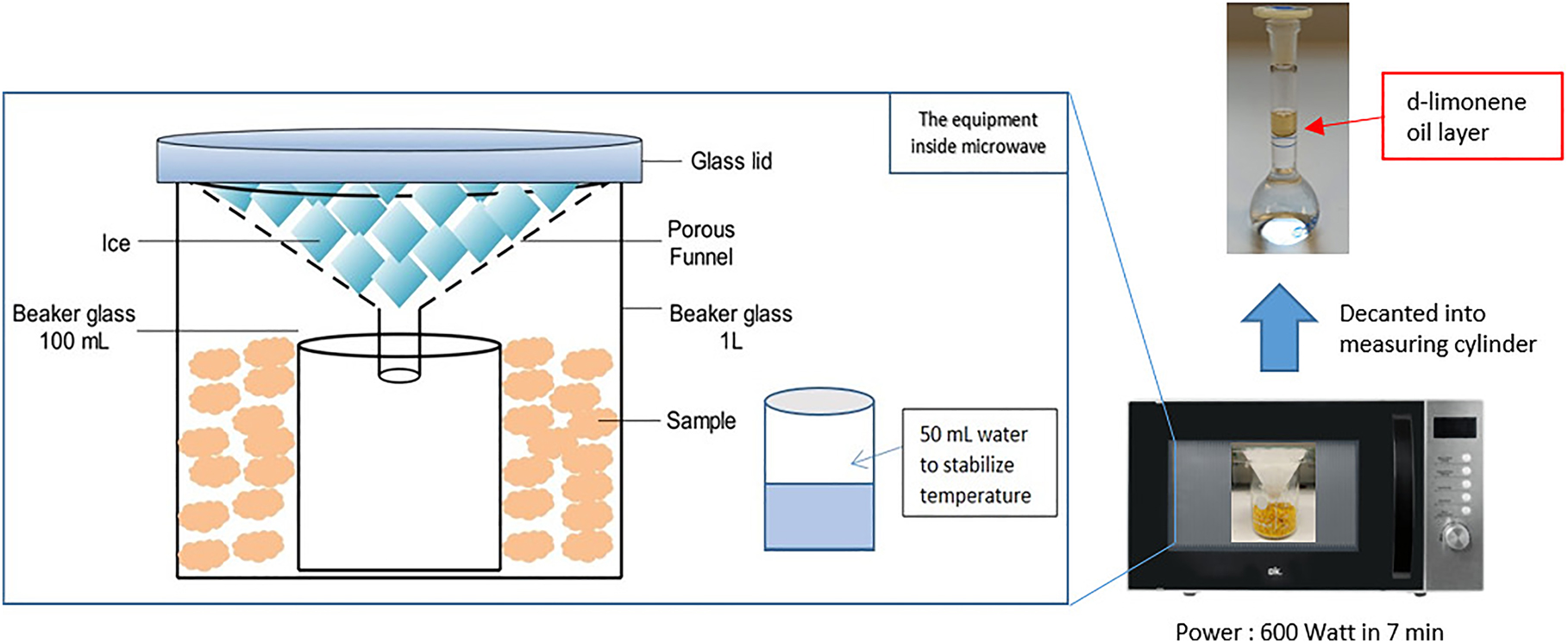
Experimental set-up for the extraction of essential oils in the kitchen microwave.
Conclusions
The experiments described above were developed and repeatedly tested while creating a new organic chemistry curriculum at the senior secondary high school level, which focused on the different principles of green chemistry (Linkwitz & Eilks 2020). Our experience with students of age 15–16 shows that the experiments can easily be applied, either as demonstrations or as student activities. Generally, the students were surprised to perform chemical experiments using a kitchen microwave. Reflections after the experiments revealed that the learners had started to see green chemistry as a different approach to doing chemistry. They also recognized that alternative syntheses with the use of microwave radiation can be a part of it.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Research funding: None declared.
-
Conflict of interest statement: Authors state no conflict of interest.
References
Anastas, P., & Warner, J. C. (1998). Green chemistry: Theory and practice. New York: Oxford University Press.Search in Google Scholar
Attard, T. M., Watterson, B., Budarin, V. L., Clark, J. H., & Hunt, A. J. (2014). Microwave assisted extraction as an important technology for valorising orange waste. New Journal of Chemistry, 38(6), 2278–2283. https://doi.org/10.1039/c4nj00043a.Search in Google Scholar
Chemat, F., Lucchesi, M. E., Smadja, J., Favretto, L., Colnaghi, G., & Visinoni, F. (2006). Microwave accelerated steam distillation of essential oil from lavender: A rapid, clean and environmentally friendly approach. Analytica Chimica Acta, 555, 157–160. https://doi.org/10.1016/j.aca.2005.08.071.Search in Google Scholar
de la Hoz, A., Díaz-Ortiz, A., & Prieto, P. (2016). Microwave-assisted green organic synthesis. In G. Stefanidis & A. Stankiewicz (Eds.), Alternative Energy Sources for Green Chemistry (pp. 1–33). Cambridge: RSC.10.1039/9781782623632-00001Search in Google Scholar
Hackleman, D. (2016). Chemistry around us. https://oilextech.com/wp-content/uploads/2016/09/Chemistry-Around-Us.pdf [Accessed 01 Jul 2021].Search in Google Scholar
Leadbeater, N. E. (2010). Microwave heating as a tool for sustainable chemistry. Boca Raton: CRC.10.1201/9781439812709Search in Google Scholar
Lindström, P., Tierney, J., Walthey, B., & Westman, J. (2001). Microwave assisted organic synthesis—a review. Tetrahedron, 57(45), 9225–9283.10.1016/S0040-4020(01)00906-1Search in Google Scholar
Linkwitz, M., & Eilks, I. (2020). Greening the senior high school chemistry curriculum: An action research initiative. In S. Obare, K. Peterman & C. Middlecamp (Eds.), Chemistry education for a sustainable society volume 1: High school, outreach, & global perspectives (pp. 55–68). Washington: ACS.10.1021/bk-2020-1344.ch005Search in Google Scholar
Mazo, P., Restrepo, G. M., & Rios, L. (2011). Alternative methods for fatty acid alkyl-esters production: Microwaves, radio-frequency and ultrasound. In M. Stoytcheva & G. Montero (Eds.), Biodiesel – Feedstocks and processing technologies (pp. 269–288). https://www.intechopen.com/books/biodiesel-feedstocks-and-processing-technologies. IntechOpen [Accessed 01 Jul 2021].10.5772/25289Search in Google Scholar
McGiwan, C. B., & Leadbeater, N. (2006). Clean, Fast. Organic chemistry: Microwave-assisted laboratory experiments. Matthews: CEM.Search in Google Scholar
Miller, T. A., & Leadbeater, N. (2009). Microwave assisted synthesis of biodiesel in an undergraduate organic chemistry laboratory course. Chemical Educator, 14, 98–104.Search in Google Scholar
Mitra, S., Ragunath, S.Mitra, A., & Khow, O. S. (2010). Green Chemistry in Teaching Laboratory - Microwave Induced Reactions. https://web.njit.edu/∼mitra/green_chemistry/Content/Manual-april-2010.pdf [Accessed 25 May 2022].Search in Google Scholar
Reilly, M. K., King, R. P., Wagner, A. J., & King, S. M. (2014). Microwave-assisted esterification: A discovery-based microscale laboratory experiment. Journal of Chemical Education, 91, 1706–1709. https://doi.org/10.1021/ed400721p.Search in Google Scholar
Wagner, R. (2006). Microwave-assisted synthesis in the pharmaceutical industry - a current perspective and future prospects. Summer 2006. In Drug Discovery World (pp. 59–66). https://www.ddw-online.com/media/32/06.sum.microwave-assisted-synthesis-in-the-pharmaceutical-industry.pdf [Accessed 01 Jul 2021].Search in Google Scholar
Yadav, G. D., & Thorat, P. A. (2012). Microwave assisted lipase catalyzed synthesis of isoamyl myristate in solvent-free system. Journal of Molecular Catalysis B: Enzymatic, 83, 16–22. https://doi.org/10.1016/j.molcatb.2012.06.011.Search in Google Scholar
© 2022 Michael Linkwitz et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Articles in the same Issue
- Frontmatter
- Guest Editorial
- Introduction to the special issue on Green Chemistry
- Special Issue Papers
- Simple experiments with immobilized enzymes as a contribution to green and sustainable chemistry education in the high school laboratory
- Learning Green Chemistry and its principles from Nature’s process and development of green procedures mimicking nature
- SpottingScience – a digital learning environment to introduce Green Chemistry to secondary students and the public
- Student explorations of calcium alginate bead formation by varying pH and concentration of acidic beverage juices
- Good Practice Report
- Simple green organic chemistry experiments with the kitchen microwave for high school chemistry classrooms
- Invited Paper
- Basic concept and application of conducting polymers for environmental protection
- Good Practice Report
- Distance learning: an interdisciplinary experiment on Rayleigh scattering
- Special Issue Paper
- Development of teaching material for green and sustainable chemistry in Japan
Articles in the same Issue
- Frontmatter
- Guest Editorial
- Introduction to the special issue on Green Chemistry
- Special Issue Papers
- Simple experiments with immobilized enzymes as a contribution to green and sustainable chemistry education in the high school laboratory
- Learning Green Chemistry and its principles from Nature’s process and development of green procedures mimicking nature
- SpottingScience – a digital learning environment to introduce Green Chemistry to secondary students and the public
- Student explorations of calcium alginate bead formation by varying pH and concentration of acidic beverage juices
- Good Practice Report
- Simple green organic chemistry experiments with the kitchen microwave for high school chemistry classrooms
- Invited Paper
- Basic concept and application of conducting polymers for environmental protection
- Good Practice Report
- Distance learning: an interdisciplinary experiment on Rayleigh scattering
- Special Issue Paper
- Development of teaching material for green and sustainable chemistry in Japan

